Abstract
Hydrogen sulfide (H2S) is a biologically-active molecule that exhibits protective effects in a variety of physiological and pathological processes. Although a number of H2S-related biological effects have been discovered by using H2S donors, knowing how much H2S has been released from donors under different conditions remains a significant challenge. Aligned with this need, we report here a series of γ-ketothiocarbamate (γ-KetoTCM) compounds that provide the first examples of colorimetric H2S donors and enable direct quantification of H2S release. These compounds are activated through a pH-dependent deprotonation/β-elimination sequence to release carbonyl sulfide (COS), which is quickly converted to H2S by carbonic anhydrase. The p-nitroaniline released upon donor activation provides an optical readout, which we demonstrate correlates directly with COS/H2S release. We also establish that γ-KetoTCM-1 releases COS/H2S in live cells and reduces LPS-induced NO generation, which is consistent with anti-inflammatory activity. Taken together, γ-KetoTCM compounds provide a promising new platform for H2S donation and readily enable colorimetric measurement of H2S donation.
Keywords: hydrogen sulfide, γ-ketothiocarbamate, carbonyl sulfide, colorimetric, anti-inflammation
Graphical Abstract
H2S is a significant biomolecule and H2S donors are key research tools for H2S study. We report the design and evaluation of a colorimetric H2S donor that is triggered to release H2S by a deprotonation/β-elimination sequence. Importantly, the p-nitroaniline generated upon donor activation provides an optical readout, which allows for the H2S release to be monitored and quantified directly during the course of an experiment.

Since its first recognition as a neuromodulator in 1996,[1] hydrogen sulfide (H2S) has gained significant attention and has been shown to provide promising protective effects in different physiological and pathological processes ranging from anti-inflammation to vasodilation.[2] Endogenous H2S is produced primarily from cysteine and homocysteine from the four main enzymes cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), 3-mercaptopyruvate sulfurtransferase (3-MST), and cysteine aminotransferase (CAT).[3] Due to its critical regulatory and signaling roles in mammals, H2S has been recognized as an important cellular signaling molecule, much like nitric oxide (NO) and carbon monoxide (CO).[4]
Despite this importance, many biological roles of H2S remain elusive, in part due to the lack of refined research tools to modulate cellular H2S levels. Therefore, significant efforts have focused on developing H2S-releasing agents (H2S donors).[5] Although the inorganic sulfide salts NaSH and Na2S are used most commonly, they provide a rapid and uncontrollable bolus of H2S in aqueous media, which fails to mimic well-regulated in vivo H2S generation. Synthetic donors, such as garlic-derived diallyl trisulfide, release H2S in the presence of glutathione (GSH) and exhibit H2S-related protective functions in animal models.[6] Although a variety of H2S-related activities have been discovered by using common H2S donors, including GYY4137 and dithiolethione derivatives, low H2S releasing efficiency remains a challenge.[5b] Building from these scaffolds, the library of available H2S donors has expanded in the last decade and now includes donors that are activated by different triggers, such as cellular thiols,[7] enzymes,[8] pH modulation,[9] and photo activation.[10]
Aligned with the need for new donor strategies, we recently reported H2S delivery from caged-carbonyl sulfide (COS) molecules.[11] In this approach, we utilized self-immolative thiocarbamates that undergo cascade reactions to release COS, which is quickly converted to H2S by the ubiquitous enzyme carbonic anhydrase (CA) (Scheme 1a).[12] Following our initial report, we, as well as others, have expanded this strategy to include COS-based H2S donors that are activated by different triggers, such as reactive oxygen species (ROS),[13] esterases,[14] glycine,[15] click chemistry,[16] light,[17] and cysteine.[18] Although these molecules exhibit promising H2S-related functions, most of these donor scaffolds require a 1,6-elimination system, which has limited the expansion of this platform.
Scheme 1.
(a) Previous design of COS-based H2S donors; (b) colorimetric COS-based H2S donors and proposed COS/H2S releasing pathway; (c) control compound activation; (d) activation of CO2-releasing control compounds; (e) synthesis of γ-KetoTCM-based COS/H2S donors and control compounds.
Building from this need, we report here a class of γ-ketothiocarbamate compounds (γ-KetoTCM-1 and γ-KetoTCM-2) as controllable and colorimetric COS/H2S donors, which are activated by a deprotonation/elimination reaction. The resultant enol intermediate undergoes a β-elimination to release COS, which is quickly converted to H2S by CA. Importantly, these γ-KetoTCM-based donors not only release COS but also release p-nitroaniline (PNA), which provides a convenient optical readout to monitor COS/H2S release (Scheme 1b). These donors also address a key challenge in the field: namely that the amount of H2S released from synthetic donors can vary depending on the local environment, thus making it difficult to correlate release rates in different environments. The colorimetric readout in this family of donors enables the real-time tracking of H2S release using UV-vis spectroscopy. In addition, this donor scaffold provides access to key control compounds, including γ-KetoTCM-3 and n-butylthiocarbamate (n-BuTCM-1), which are triggerless control compounds that do not release COS/H2S (Scheme 1c), and also the corresponding γ-ketocarbamate molecule (γ-KetoCM-1), which releases CO2 instead of COS (Scheme 1d). We anticipate that the γ-KetoTCM-based COS/H2S donors, together with control molecules, will serve as key research tools for H2S investigation.
To test our hypothesis that γ-ketothiocarbamates could serve as colorimetric COS/H2S donors, we prepared four thiocarbamates (γ-KetoTCM-1 – 3 and n-BuTCM-1) by reacting 4-hydroxy-2-butanone or its derivatives with 4-nitrophenyl isothiocyanate. The carbamate control compound γ-KetoCM-1 was obtained by reacting 4-hydroxy-2-butanone with p-nitrophenyl isocyanate (Scheme 1e) and n-BuTCM-1 was prepared by reacting n-butanol with 4-nitrophenyl isothiocyanate.
With these compounds in hand, we first evaluated the colorimetric response from γ-KetoTCM-1 and then quantified H2S release using the methylene blue (MB) assay (vide infra). γ-KetoTCM-1 (50 μM) was incubated in PBS buffer (pH 7.4, 10 mM) containing cellularly-relevant concentrations of CA (25 μg/mL) at 37 °C, and PNA formation was monitored by UV-Vis spectroscopy. As expected, a time-dependent decrease of γ-KetoTCM-1 absorbance at 333 nm was observed, with a concomitant increase of the PNA absorbance at 381 nm. The well-anchored isosbestic point at 358 nm supports the clean conversion of γ-KetoTCM-1 to PNA upon donor activation and is consistent with the proposed deprotonation/elimination sequence (Figures 1a and 1b).
Figure 1.
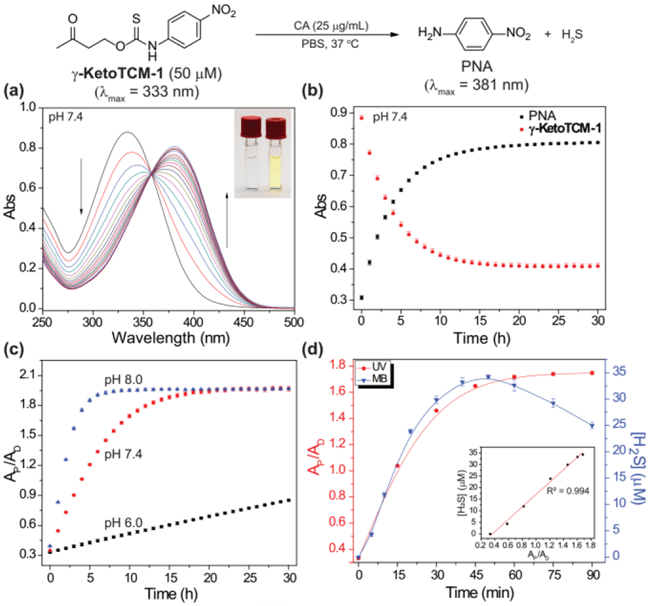
(a) Time-dependent UV-Vis spectra of γ-KetoTCM-1 and PNA. (10 mM PBS, pH 7.4, 25 μg CA/mL, 37 ºC). Inset: color of the solution at t = 0 (left) and t = 24 h (right). (b) Time-dependent absorbance of γ-KetoTCM-1 (333 nm) and PNA (381 nm) (10 mM PBS, pH 7.4). (c) pH dependence of PNA formation from of γ-KetoTCM-1 in PBS at pH 8.0 (blue), pH 7.4 (red), and pH 6.0 (black) at 37 ºC. (d) PNA formation (red) and H2S release (blue) upon γ-KetoTCM-1 (50 μM) activation in PBS (pH 7.4, 10 mM) containing BSA (5 mg/mL) at 37 ºC. Inset: Correlation between measured [H2S] and PNA formation. AP: PNA absorbance (381 nm); AD: γ-KetoTCM-1 absorbance (333 nm). Experiments were performed in triplicate. Results are expressed as mean ± SD (n=3).
Because the activation of γ-KetoTCM-1 was triggered by β-deprotonation, we expected that the COS/H2S release from γ-KetoTCM-1 would be pH-dependent. To test this hypothesis, we measured PNA formation from γ-KetoTCM-1 (50 μM) incubated in PBS buffers (pH 6.0 – 8.0, 10 mM) containing CA (25 μg/mL) at 37 °C. At physiological pH 7.4, γ-KetoTCM-1 was slowly activated to release COS/H2S (pseudo 1st-order rate constant (kobs) = (4.52 ± 0.02) × 10−5 s−1, relative rate (krel.) = 1.00, and half-life (t1/2) = 4.26 ± 0.02 h). The rate of PNA formation was significantly enhanced under basic conditions (krel. = 2.79 at pH 8.0), indicating a faster COS/H2S release. As expected, PNA formation under acidic conditions was significantly slower (krel. = 0.084 at pH 6.0) (Figures 1c and S1 and Table 1). Taken together, these results demonstrate that COS/H2S release from γ-KetoTCM-1 is pH dependent and support the proposed mechanism of activation.
Table 1.
Summary of COS/H2S releasing kinetics of γ-KetoTCM donor motifs.
| Donors | λmax (nm) |
ε (M−1 cm−1) | pH |
kobs (× 10−5) (s−1) |
krel | t1/2 (h) |
|---|---|---|---|---|---|---|
| γ-KetoTCM-1 | 333 | 17,600 ± 350 | 6.0 | 0.380 ± 0.006 | 0.0840 | 50.7 ± 0.8 |
| 7.4 | 4.52 ± 0.02 | 1.00 | 4.26 ± 0.02 | |||
| 7.4a | 81.0 ± 3.0 | 18.0 | 0.24 ± 0.01 | |||
| 8.0 | 12.6 ± 0.2 | 2.79 | 1.53 ± 0.03 | |||
| γ-KetoTCM-2 | 335 | 14,000 ± 270 | 7.4 | 0.82 ±0.02 | 0.188 | 23.6 ± 0.7 |
| γ-KetoTCM-3 | 331 | 11,100 ± 220 | 7.4 | N/A | N/A | N/A |
| n-BuTCM-1 | 335 | 13,200 ± 300 | 7.4 | N/A | N/A | N/A |
| γ-KetoCM-1 | 335 | 13,200 ± 300 | 7.4 | 1.44 ± 0.03 | 0.32 | 13.4 ± 0.3 |
PBS contains BSA (5 mg/mL)
We also investigated donor activation and subsequent COS/H2S release in PBS containing bovine serum albumin (BSA), to mimic a complex biological environment. In these experiments, γ-KetoTCM-1 (50 μM) was incubated in PBS (pH 7.4, 10 mM) containing BSA (5 mg/mL) and CA (25 μg/mL) at 37 °C. Under these conditions, we observed a significantly faster activation of γ-KetoTCM-1 (krel. = 18.0), which is consistent with prior reports of BSA-catalyzed β-elimination reactions (Figures 1d red curve and S2 and Table 1).[19] These results suggest that the COS/H2S releasing kinetics of γ-KetoTCM-1 may be faster under biological conditions than that in simple aqueous buffers, but also highlights the benefit of a colorimetric response upon donor activation.
Using the same conditions, we also measured H2S production at different time points using the MB assay (Figure 1d blue curve). The MB assay allows for H2S quantification from the reaction of H2S with N,N-dimethyl-p-phenylenediamine in the presence of FeCl3 and acid to produce methylene blue (MB, λmax = 670). Using this assay, we observed a 70% H2S release efficiency. We attribute the slight decrease in H2S at extended time points to aerobic H2S oxidation (Figure S4). We confirmed that the reaction byproducts PNA and methyl vinyl ketone (MVK) did not provide a positive response on their own, nor did they react further with H2S (Figure S5). Importantly, fitting the MB data up to the plateau point (~60 min), afforded a peudo first-order rate constant of kobs = (8.6 ± 0.9) × 10−4 s−1, which matches the (8.1 ± 0.3) × 10−4 s−1 value obtained from fitting the UV-vis data, thus confirming that the UV-vis signal correlates directly with H2S release. In addition, the strong correlation between these two methods suggests that PNA can serve as a reliable optical tool to profile the COS/H2S releasing capacities of γ-KetoTCM donors (Figure 1d inset).
After demonstrating COS/H2S release from γ-KetoTCM-1, we next investigated COS/H2S release from other thiocarbamate-based donors. Compared to γ-KetoTCM-1, incubation of γ-KetoTCM-2 at pH 7.4 resulted in slower COS/H2S release (krel. = 0.188), which we attribute to the formation of a more stable enol intermediate upon donor activation. As expected, the triggerless controls γ-KetoTCM-3 and n-BuTCM-1 were stable in pH 7.4 aqueous buffer and minimum PNA formation was observed, (Figure 2, Table 1). These studies demonstrated that controllable COS/H2S release from γ-KetoTCM-based donors can be achieved by donor structure modifications (e.g. tuning the pKa of β-protons by introducing R groups at β-position) and the γ-ketone trigger with at least one β-proton is required to initiate COS/H2S release. In addition, PNA formation was not observed from γ-KetoTCM-3 in PBS at pH 8.0, indicating that the thiocarbamate group is not hydrolyzed under the reaction conditions (Figure S6).
Figure 2.
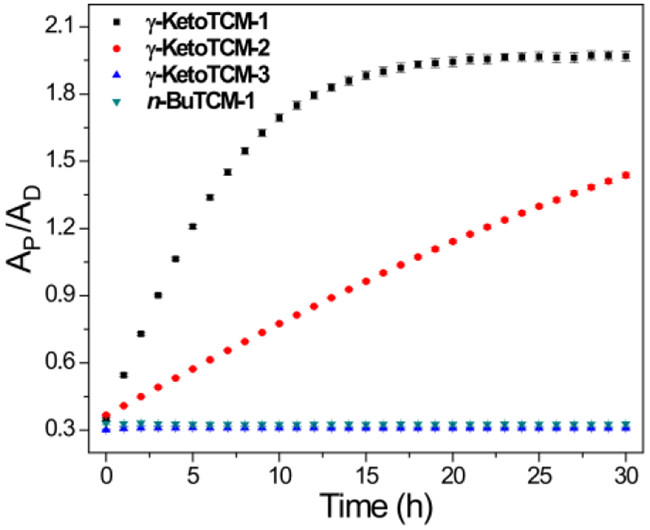
Measurement of PNA formation after compound activation. AP: PNA absorbance (381 nm); AD: compound absorbance (at λmax). Experiments were performed in triplicate. Results are expressed as mean ± SD (n = 3)
Because the thiocarbamate functional group is potentially electrophilic, we evaluated the effects of different nucleophiles on COS/H2S release. In these experiments, γ-KetoTCM-1 (50 μM) was incubated in PBS buffer (pH 7.4, 10 mM) containing 250 μM of Cys, N-acetyl cysteine (NAC), homocysteine (Hcy), GSH (1.0 mM), lysine (Lys), serine (Ser), glycine (Gly), or oxidized glutathione (GSSG) at 37 °C. PNA formation was monitored and recorded after a 4-h incubation using UV-vis spectroscopy. None of the tested nucleophiles resulted in substantial PNA formation by comparison to the background reaction, demonstrating that COS/H2S release from γ-KetoTCM-1 is solely pH-dependent and not facilitated directly by common cellular nucleophiles (Figure 3).
Figure 3.
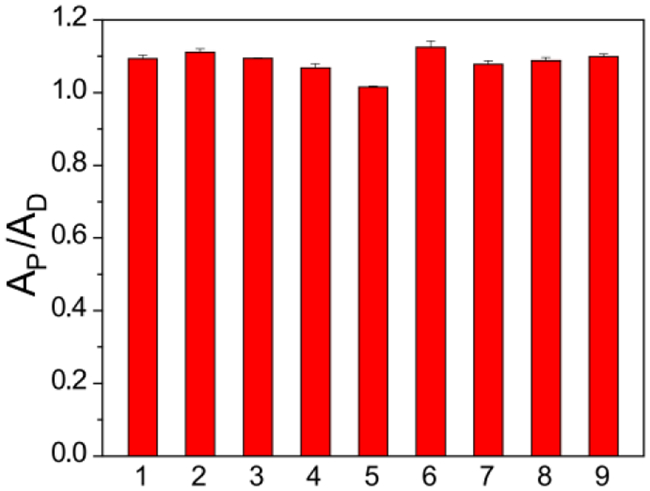
PNA formation after incubating γ-KetoTCM-1 (50 μM) in PBS (pH 7.4, 10 mM) only (1), or PBS containing 250 μM of Cys (2), NAC (3), Hcy (4), GSH (1.0 mM, 5), Lys (6), Ser (7), Gly (8), or GSSG (9) for 4 h at 37 ºC. Experiments were performed in triplicate. Results are expressed as mean ± SD (n = 3).
Before investigating potential biological applications of the γ-KetoTCM compounds, we investigated whether γ-KetoTCM-based donors can be activated to deliver H2S in cellular environments. We used SF7-AM, a cell-trappable H2S fluorescent probe,[20] to monitor H2S accumulation from γ-KetoTCM-1 in HeLa cells. In the absence of γ-KetoTCM-1, no fluorescent signal from SF7-AM was observed, indicating negligible endogenous H2S (Figure 4, left column). Treating cells with carbamate control γ-KetoCM-1 also failed to provide a SF7-AM signal, suggesting that the MVK and PNA byproducts did not provide a false-positive or upregulate H2S generation pathways (Figure 4, middle column). By contrast, addition of γ-KetoTCM-1 resulted in a significant increase in SF7-AM fluorescence, demonstrating that γ-KetoTCM-1 can be successfully activated in a cellular environment and that the released H2S can be visualized using an H2S-responsive fluorescent probe (Figure 4 right column).
Figure 4.
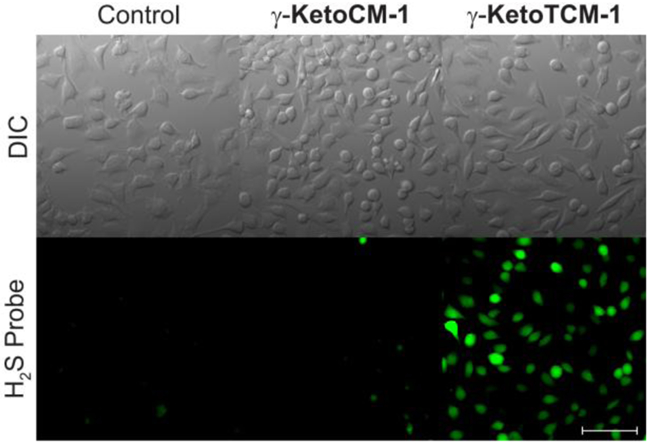
H2S Delivery from γ-KetoTCM-1 in HeLa cells. HeLa cells were treated with SF7-AM (5 μM) for 30 min, washed, and incubated with FBS-free DMEM only (left), with 100 μM γ-KetoCM-1 (middle), or with γ-KetoTCM-1 (right) for 2 h. Cells were then washed and imaged in PBS. Scale bar: 100 μm.
Prior reports have demonstrated that H2S donors can often provide anti-inflammatory activity. To investigate the potential protective effects of the developed donors, we pretreated macrophage RAW 264.7 cells with γ-KetoTCM-1 (25 μM) for 6 h, followed by an 18-h incubation with lipopolysaccharide (LPS, 1.0 μg/mL) to induce an inflammatory response. This response is accompanied by an increase in NO production, which we monitored by measuring nitrite (NO2–) accumulation. Our expectation was that the COS/H2S donor would decrease LPS-induced NO2– formation, indicating anti-inflammatory activity of the donor. To determine whether the observed effects were due to COS/H2S release, we also performed control experiments with γ-KetoTCM-3, γ-KetoCM-1, and GYY4137 under the identical condition. We used GYY4137 as a positive control because it has shown anti-inflammatory effects previously,[21] and because it generates a slow, continuous release of H2S. We chose to use 25 μM of each compound in this study because this concentration did not provide significant cytotoxicity (Figure S7).
In comparison to the control group, in which cells were only incubated in FBS-free DMEM, the LPS-treated cells showed a significant NO2– increase. γ-KetoTCM-1 pretreatment, however, significantly reduced LPS-induced NO2– production. Control experiments using γ-KetoCM-1 also reduced NO2– levels, although to a lesser extent than γ-KetoTCM-1. We attribute the observed effects from KetoCM-1 to MVK release, which we confirmed independently (Figure S8). We also observed a modest reduction of LPS-induced NO2– production from γ-KetoTCM-3, although this effect was significantly attenuated from that of γ-KetoTCM-1. GYY4137 exhibited a less pronounced effect on LPS-induced NO2– production at the same concentration (25 μM), which supports the increased efficiency of γ-KetoTCM-1 (Figure 5). Taken together, these investigations demonstrate that γ-KetoTCM-1 can deliver H2S in complex environment and provide protection against LPS-induced inflammation, suggesting potential therapeutic applications of γ-KetoTCM-based H2S donors. In addition these experiments highlight the benefits of having access to key control compounds that enable specific contributions to cellular protections to be analysed.
Figure 5.
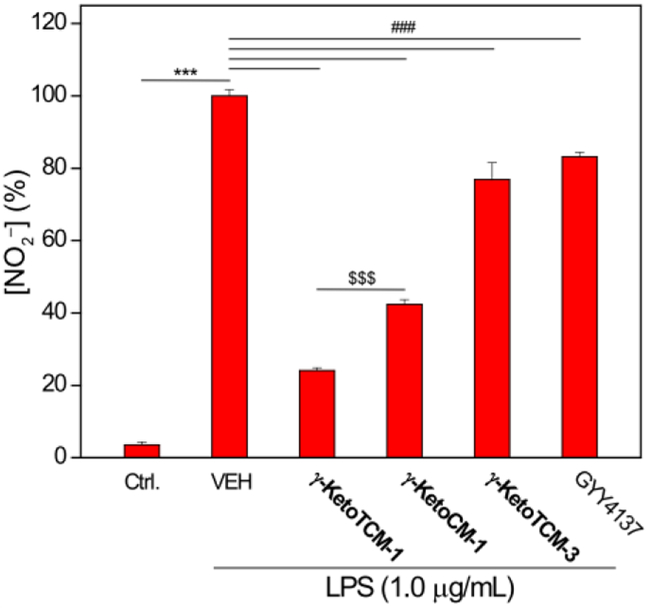
Effects of γ-KetoTCM-1 on LPS-induced NO2– formation. RAW 264.7 cells were pretreated with γ-KetoTCM-1 (25 μM) or control compounds for 6 h, followed by LPS (1.0 μg/mL, 18-h). Results are expressed as mean ± SD (n = 4). ***P < 0.001 vs the control group; ###P < 0.001 vs vehicle-treated group; and $$$P < 0.001 between γ-KetoTCM-1-treated and γ-KetoCM-1-treated groups.
In summary, we prepared and evaluated a series of γ-ketothiocarbamate compounds that function as COS/H2S donors and provide a colorimetric response upon donor activation. The PNA generated upon donor activation provides an optical readout, which allows for the COS/H2S release to be monitored and quantified directly during the course of an experiment. In addition, we also demonstrate that γ-KetoTCM-1 releases COS/H2S in live cells and reduces LPS-induced NO formation, which is consistent with anti-inflammatory activities. Taken together, γ-KetoTCM compounds provide a promising new platform for H2S donation and readily enables colorimetric measurement of H2S donation, making them as key research tools in H2S investigations.
Supplementary Material
Acknowledgements
We acknowledge financial support from the NIH (R01GM113030), Dreyfus Foundation, and NSF (AKS; DGE-1309047). NMR, MS, microscopy instrumentation in the CAMCOR facility is supported by the NSF (CHE-1427987, CHE-1531189, CHE-1625529).
References
- [1].Abe K, Kimura H, J. Neurosci. 1996, 16, 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2] (a).Wang R, Physiol. Rev. 2012, 92, 791–896; [DOI] [PubMed] [Google Scholar]; (b) Szabo C, Nat. Rev. Drug. Discov. 2007, 6, 917–935. [DOI] [PubMed] [Google Scholar]
- [3] (a).Fukuto JM, Carrington SJ, Tantillo DJ, Harrison JG, Ignarro LJ, Freeman BA, Chen A, Wink DA, Chem. Res. Toxicol. 2012, 25, 769–793; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kabil O, Banerjee R, Antioxid. Redox. Signal. 2014, 20, 770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4] (a).Li L, Rose P, Moore PK, Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187; [DOI] [PubMed] [Google Scholar]; (b) Olson KR, Antioxid. Redox. Signal. 2012, 17, 32–44; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Szabo C, Antioxid. Redox. Signal. 2012, 17, 68–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5] (a).Pluth MD, Bailey TS, Hammers MD, Hartle MD, Henthorn HA, Steiger AK, Synlett 2015, 26, 2633–2643; [Google Scholar]; (b) Zhao Y, Biggs TD, Xian M, Chem. Commun. 2014, 50, 11788–11805; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao Y, Pacheco A, Xian M, Handb. Exp. Pharmacol. 2015, 230, 365–388; [DOI] [PubMed] [Google Scholar]; (d) Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B, Med. Res. Rev. 2018, 38, 57–100; [DOI] [PubMed] [Google Scholar]; (e) Szabo C, Papapetropoulos A, Pharmacol. Rev. 2017, 69, 497–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW, Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17977–17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7] (a).Zhao Y, Wang H, Xian M, J. Am. Chem. Soc. 2011, 133, 15–17; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao Y, Bhushan S, Yang C, Otsuka H, Stein JD, Pacheco A, Peng B, Devarie-Baez NO, Aguilar HC, Lefer DJ, Xian M, ACS Chem. Biol. 2013, 8, 1283–1290; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhao Y, Kang J, Park CM, Bagdon PE, Peng B, Xian M, Org. Lett. 2014, 16, 4536–4539; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zhao Y, Yang C, Organ C, Li Z, Bhushan S, Otsuka H, Pacheco A, Kang J, Aguilar HC, Lefer DJ, Xian M, J. Med. Chem. 2015, 58, 7501–7511; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Foster JC, Powell CR, Radzinski SC, Matson JB, Org. Lett. 2014, 16, 1558–1561; [DOI] [PubMed] [Google Scholar]; (f) Martelli A, Testai L, Citi V, Marino A, Pugliesi I, Barresi E, Nesi G, Rapposelli S, Taliani S, Da Settimo F, Breschi MC, Calderone V, ACS Med. Chem. Lett. 2013, 4, 904–908; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Roger T, Raynaud F, Bouillaud F, Ransy C, Simonet S, Crespo C, Bourguignon MP, Villeneuve N, Vilaine JP, Artaud I, Galardon E, Chembiochem 2013, 14, 2268–2271. [DOI] [PubMed] [Google Scholar]
- [8] (a).Zheng Y, Yu B, Ji K, Pan Z, Chittavong V, Wang B, Angew. Chem. Int. Ed. 2016, 55, 4514–4518; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shukla P, Khodade VS, SharathChandra M, Chauhan P, Mishra S, Siddaramappa S, Pradeep BE, Singh A, Chakrapani H, Chem Sci 2017, 8, 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kang J, Li Z, Organ CL, Park CM, Yang CT, Pacheco A, Wang D, Lefer DJ, Xian M, J. Am. Chem. Soc. 2016, 138, 6336–6339. [DOI] [PubMed] [Google Scholar]
- [10] (a).Devarie-Baez NO, Bagdon PE, Peng B, Zhao Y, Park CM, Xian M, Org. Lett. 2013, 15, 2786–2789; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fukushima N, Ieda N, Sasakura K, Nagano T, Hanaoka K, Suzuki T, Miyata N, Nakagawa H, Chem. Commun. 2014, 50, 587–589. [DOI] [PubMed] [Google Scholar]
- [11].Steiger AK, Zhao Y, Pluth MD, Antioxid. Redox. Signal. 2018, 28, 1516–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Steiger AK, Pardue S, Kevil CG, Pluth MD, J. Am. Chem. Soc. 2016, 138, 7256–7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13] (a).Zhao Y, Pluth MD, Angew. Chem. Int. Ed. 2016, 55, 14638–14642; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao Y, Henthorn HA, Pluth MD, J. Am. Chem. Soc. 2017, 139, 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chauhan P, Jos S, Chakrapani H, Org. Lett., 2018, 20, 3766–3770. [DOI] [PubMed] [Google Scholar]
- [14] (a).Steiger AK, Marcatti M, Szabo C, Szczesny B, Pluth MD, ACS Chem. Biol. 2017, 12, 2117–2123; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chauhan P, Bora P, Ravikumar G, Jos S, Chakrapani H, Org. Lett. 2017, 19, 62–65. [DOI] [PubMed] [Google Scholar]
- [15].Powell CR, Foster JC, Okyere B, Theus MH, Matson JB, J. Am. Chem. Soc. 2016, 138, 13477–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Steiger AK, Yang Y, Royzen M, Pluth MD, Chem. Commun. 2017, 53, 1378–1380. [DOI] [PubMed] [Google Scholar]
- [17] (a).Zhao Y, Bolton SG, Pluth MD, Org. Lett. 2017, 19, 2278–2281; [DOI] [PubMed] [Google Scholar]; (b) Sharma AK, Nair M, Chauhan P, Gupta K, Saini DK, Chakrapani H, Org. Lett. 2017, 19, 4822–4825. [DOI] [PubMed] [Google Scholar]
- [18].Zhao Y, Steiger AK, Pluth MD, Chem. Commun. 2018, 54, 4951–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19] (a).Jourdain N, Carlon RP, Reymond JL, Tetrahedron Lett. 1998, 39, 9415–9418; [Google Scholar]; (b) Sagi A, Weinstain R, Karton N, Shabat D, J. Am. Chem. Soc. 2008, 130, 5434–5435. [DOI] [PubMed] [Google Scholar]
- [20].Lin VS, Lippert AR, Chang CJ, Proc. Natl. Acad. Sci. U S A 2013, 110, 7131–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, Moore PK, Antioxid. Redox. Signal. 2010, 12, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.