Abstract
BACKGROUND
Interleukin-2 (IL2) is a growth factor for T and NK cells, promotes pro-inflammatory cytokines and can lead to durable responses in melanoma. VEGF promotes angiogenesis and modulates host innate and adaptive immunity. High VEGF levels were associated with nonresponse to IL2. Ziv- aflibercept may deplete VEGF and thereby enhance antitumor T cell responses, supporting a combination immunotherapeutic strategy with IL2.
METHODS
NCI 8628 was a phase II trial of ziv-aflibercept and IL2 (arm A) versus IL2 alone (arm B) randomized 2:1 respectively. Eligible patients had inoperable Stage III or IV melanoma. The primary endpoint was progression-free survival (PFS).
RESULTS
89 patients were enrolled and 84 treated. Median follow up was 41.4 months. Among treated patients (55 in A, 29 in B), PFS was significantly improved in favor of A: median (95% CI) of 6.9 (4.1–8.7) months versus 2.3 (1.6–3.5) months, p <0.001. No significant difference in OS: median (95% CI) of 26.9 (14.4–63.6) months (A) and 24.2 (11.3–36.4) months (B). Response rate (RECIST) was 22% in A (4CR, 8PR) and 17% in B (1CR, 4PR). Stable disease or PR or CR was seen in 65% in A and 48% in B. The combination was superior to monotherapy in patients with high and low levels of serum VEGF and VEGFR2. Adverse events were consistent with the expected profiles of monotherapy with IL2 and ziv- aflibercept.
CONCLUSION
Ziv-aflibercept and IL2 significantly improved PFS over IL2 alone, meeting the study’s primary endpoint. Our findings support further study of immunotherapeutic combination strategies involving VEGF inhibitors.
Keywords: Ziv-aflibercept, Interleukin-2, Immunotherapy, Melanoma, angiogenesis, vascular endothelial growth factor
Precis
Since high VEGF levels were associated with nonresponse to IL2, ziv-aflibercept combined with IL2 may have immune enhancing and antiangiogenic activity via capturing free VEGF. This phase II study demonstrated significantly improved PFS with the combination versus IL2 alone in patients with inoperable Stage III or IV melanoma, meeting the study’s primary endpoint.
INTRODUCTION
The prior lack of progress in the systemic management of metastatic melanoma for several decades has recently changed dramatically, driven by a deepening understanding of melanoma biology and host immunology 1,2. This progress at the molecular level has been translated into the clinic with the advent of multiple new molecularly targeted agents (BRAF and MEK kinase inhibitors) and immune checkpoint modulators (CTLA4 and PD-1 blocking antibodies) that have made major improvements in disease control and the survival of patients with metastatic melanoma. First line therapy in patients with metastatic melanoma in practice currently consists primarily of immune checkpoint inhibitor or targeted kinase inhibitor therapy for BRAF mutant melanoma.
IL2 plays a central role in immune regulation as it affects the survival of key cells of the immune system that are responsible for the antitumor cytotoxicity of T-lymphocytes and natural-killer (NK) cells, and it has a cofactor role in the activation of B cells and macrophages 3. Initial studies with high dose bolus (HD) IL2 utilized doses of 600,000–720,000 units/kg every 8 hours from days 1–5 (cycle 1) and days 15–19 (cycle 2) with a maximum of 14 doses per cycle or 28 doses per course (1 course = 2 cycles). A review of eight clinical trials (270 patients) conducted between 1985 and 1993 reported an objective response rate of 16% with durable responses in 4% of patients 4. The median response duration was 8.9 months (range 4 to 106+ months). Among responding patients, 28% including 59% of those patients who had achieved a complete response (CR), remained progression free at a median follow-up of 62 months. Furthermore, no patient who had responses longer than 30 months has relapsed, suggesting the possibility that these patients may be “cured” 5. A proteomic analysis of the serum of patients with metastatic melanoma and renal cell carcinoma who were treated with HD IL2, identified VEGF as a predictor of response to IL2 therapy 6. Patients with serum VEGF levels greater than 125 pg/ml did not respond to HD IL2 and elevated levels were also associated with a significantly worse overall survival.
The VEGF family plays a critical role in mediating angiogenesis, lymphangiogenesis, and vasculogenesis and has an impact on host innate and adaptive immunity 7,8. The role of elevated VEGF levels on tumor angiogenesis is well documented and recently, high circulating levels of VEGF were reported to be associated with poor prognosis in patients with metastatic melanoma 9. VEGF has been shown to block maturation of dendritic cells and inhibit effective priming of T cell responses 10,11. These data support an important role for VEGF in the progression of cancer and evasion of anti-tumor immunity. A therapeutic strategy designed to deplete high serum VEGF levels prior to HD IL2 administration may reverse the negative impact of high serum VEGF on dendritic cell maturation and T cell priming allowing more effective antitumor T cell cytotoxicity induced by HD IL2. As a potent VEGF inhibitor, ziv-aflibercept is a fusion protein of human IgG1 Fc portion and extracellular ligand-binding domains of VEGFR1 and VEGFR2 12. It acts a high-affinity soluble decoy VEGF receptor. We previously reported a phase II study of ziv-aflibercept in patients with inoperable stage III or IV melanoma that demonstrated evidence of clinical activity including a median overall survival of 16.3 months (95% CI, 9.2 - not reached) and median PFS of 3.7 months (95% CI, 2.8–6.8) 13. We hypothesized that sequential biotherapy with ziv-aflibercept and HD IL2 will lead to improved anti-tumor efficacy compared to HD IL2 alone.
PATIENTS AND METHODS
Eligibility
Eligibility criteria included patients with histologically confirmed inoperable Stage III or IV metastatic melanoma with measurable disease (RECIST v.1.1). Age >16; ECOG performance status of 0–1; adequate hematological and biochemical parameters; and no major comorbidity or concurrent malignancy. Up to 2 prior regimens for metastatic melanoma and stable treated brain metastases were permitted.
Treatment
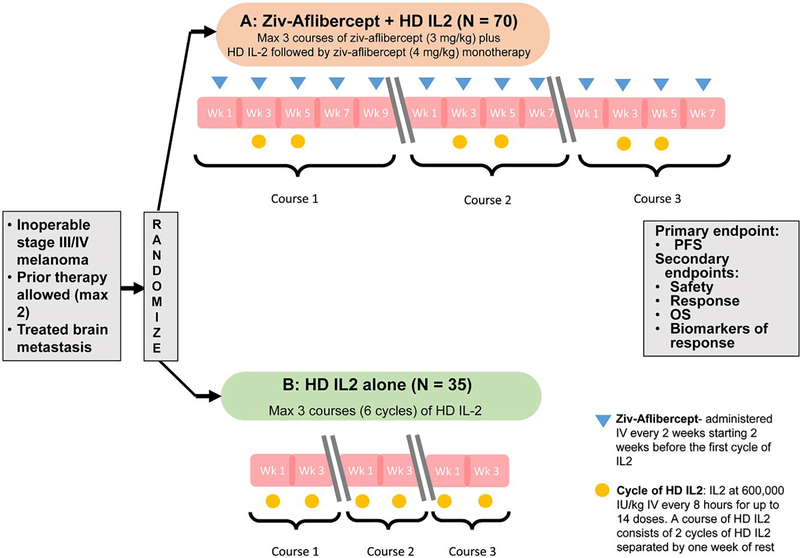
This therapeutic protocol was approved by the ethics committees at participating sites. All patients provided written informed consent. Patients were randomly assigned to the combination of ziv-aflibercept and HD IL2 or HD IL2 alone, stratified by (1) the presence or absence of visceral disease, (2) ECOG 0 versus 1, (3) male versus female and (4) prior systemic therapy for melanoma ≤ 2 versus > 2 prior regimens. Study schema is shown in Figure 1.
Figure 1.
NCI 8628 Study Schema. A phase II randomized trial of the combination of HD IL-2 and ziv- aflibercept versus HD IL2 alone. (HD IL 2: High dose interleukin 2; Max: maximum; OS: Overall survival; PFS: Progression free survival; Wk: week)
Patients randomized to the combination arm received up to 3 courses of immunotherapy. Each course consisted of 2 cycles of HD IL2 at 600,000 IU/kg IV every 8 hours for up to 14 doses (1st cycle), followed by a period of 1 week rest and readmission for treatment with HD IL2 (2nd cycle). Ziv-aflibercept was given concurrently at 3 mg/kg IV every 2 weeks, starting 2 weeks prior to the initial administration of IL2 in course 1. In the absence of disease progression, maintenance ziv-aflibercept was given at 4 mg/kg every 2 weeks after completion of IL2. In the HD IL2 alone arm, patients received HD IL2 for a maximum of 3 courses (6 cycles).
Patients who experienced toxicity were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 and were managed in accordance to toxicity specific management and dose modification guidelines provided in the study protocol.
Endpoints
The primary objective was to compare progression free survival (PFS) between the combination and HD IL2 alone. Secondary objectives were to compare the response rate (RR), overall survival (OS) and assess toxicity. PFS was defined as time from randomization to disease progression or death without progression. RR was assessed utilizing RECIST criteria v.1.1 14. OS was defined as time from randomization to death from any cause. Adverse events (AEs) were coded and graded according to CTCAE version 4.0.
Statistical Design and Analysis
This was a randomized Phase II study of HD IL2 plus ziv-aflibercept versus HD IL2 alone. The randomization was 2:1 in favor of the combination, using blocked randomization. The randomization was carried out by the central data coordination center. Follow-up time was reported based on the reverse Kaplan-Meier method. The primary endpoint of PFS was measured from date of randomization until date of progression, death or censored at last contact. The primary comparison was based on the log-rank test for comparison of PFS. The accrual goal was 105 (70 patients on the combination arm, 35 on the monotherapy arm). The study design (as planned) had 89% power (with 91 events) to detect a 75% increase in the median PFS (7 versus 4 months), at the 1-sided 0.10 significance level. There was no planned interim analysis other than for toxicity considerations.
Laboratory Correlative Studies
Peripheral blood was collected at each clinical site, shipped overnight to the central lab in Pittsburgh to be processed. Serum, drawn into red top tubes lacking anticoagulant, was centrifuged to remove the cell clot, and the serum was aliquoted and frozen at −80°C until testing in batch.
Serum VEGF (baseline and post treatment) and VEGFR2 (baseline only) levels were measured in both study arms using standardized (R&D Systems) ELISA kits. Assays were performed at UPMC Hillman Cancer Center Immunologic Monitoring and Cellular Products Laboratory (IMCPL). The IMCPL is College of American Pathologists (CAP) accredited and CLIA certified.
RESULTS
This United States (U.S.) National Cancer Institute (NCI)-sponsored study was initiated by the California Cancer Consortium with Pittsburgh (CCCP) under N01 contract in January 2011 with participation from multiple sites across the U.S. The study was terminated on 2/½016 short of the originally planned target accrual of 105, due to factors related to slow accrual and NCI N01 grants contract termination.
A total of 89 patients were enrolled. Baseline demographics and characteristics are listed in Table 1.
Table 1.
Patient demographics and baseline disease characteristics
| Arm A (N=55) | Arm B (N=29) | |
|---|---|---|
| Age (median and range), years | 55 (26–73) | 55 (31–74) |
| Gender | ||
| Female | 22 (40%) | 11 (38%) |
| Male | 33 (60%) | 18 (62%) |
| Performance status (ECOG) | ||
| 0 | 31 (56%) | 17 (59%) |
| 1 | 24 (44%) | 12 (41%) |
| Primary | ||
| Cutaneous | 36 (65%) | 16 (55%) |
| Mucosal | 5 (9%) | 4 (14%) |
| Uveal | 6 (11%) | 4 (14%) |
| Unknown | 8 (15%) | 5 (17%) |
| AJCC Stage | ||
| III (N3) | 9 (16%) | 3 (10%) |
| M1a | 10 (18%) | 5 (17%) |
| M1b | 11 (20%) | 6 (21%) |
| M1c | 25 (45%) | 15 (52%) |
| Duration of Treatment (median no. of cycles, range) |
3 (1–31) | 2 (1–3) |
A total of 89 pts were enrolled, but 5 who never started study treatment were excluded
Arm A: Patients receiving combination of HD IL-2 and ziv-aflibercept. Arm B: Patients receiving HD IL2 alone
Abbreviations: AJCC: American Joint Committee on Cancer; ECOG: Eastern Cooperative Oncology Group; IL2: Interleukin 2; no.: number
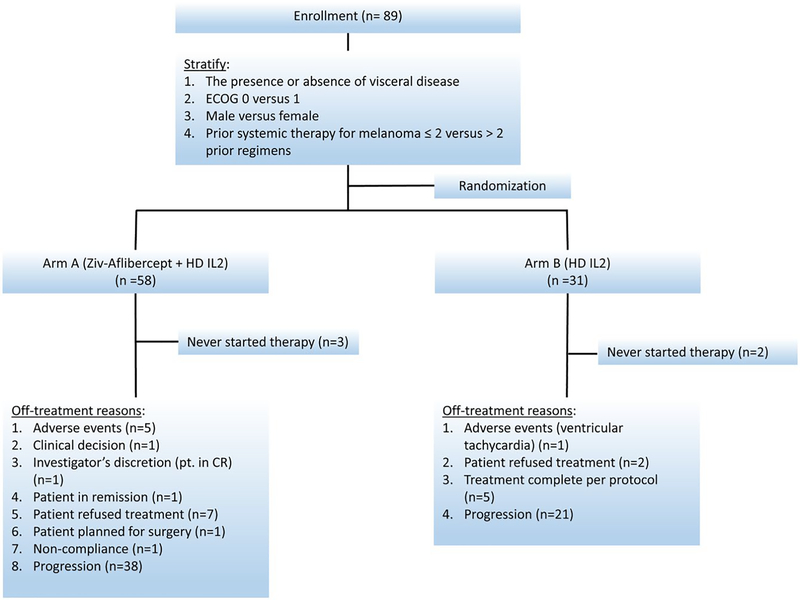
The data as of a cutoff date of February 16, 2018 was used, with median (95% CI) follow-up time of 41.4 (27.9, 49.4) months for all patients, 34.5 (21.3, 51.7) months for patients on the combination arm and 41.9 (39.4, 57.7) months for HD IL2 alone patients. As described in the consort diagram (Figure 2), 5 who never started study treatment were excluded. Seven patients (5 in the combination arm and 2 in IL2 alone arm) who were treated but withdrew early without a response assessment were considered non- responders in this analysis. The median (range) number of cycles for patients on the combination arm was 3 (1–31), and 2 (1–3) for patients on HD IL2 arm.
Figure 2.
NCI 8628 Consort diagram.
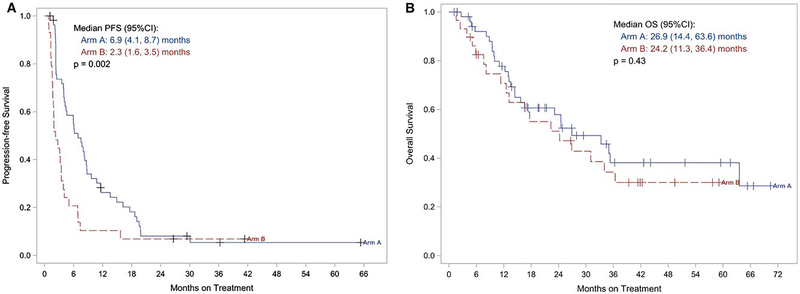
Among the 84 treated patients (55 and 29), there was significant improvement in PFS in favor of the combination. The median (95% CI) was 6.9 (4.1 – 8.7) months versus 2.3 (1.6 – 3.5) months, log rank p<0.001. Figure 3A displays the Kaplan-Meier plot of PFS. No significant difference in OS was seen. The median (95% CI) was 26.9 (14.4 – 63.6) months (combination) versus 24.2 (11.3 – 36.4) months. Figure 3B displays the Kaplan-Meier plots for OS. Response rate (RECIST) was 22% in the combination arm (4CR, 8PR) versus 17% (1CR, 4PR). Disease control (stable disease, PR or CR) was 65% with the combination versus 48% in the single agent arm.
Figure 3A/B.
Kaplan-Meier plots of (3A) progression-free survival (PFS) and (3B) overall survival (OS) by treatment arm. Arm A: The combination of HD IL-2 and ziv-aflibercept. Arm B: HD IL2 alone.
Adverse events (AEs) were consistent with the AE profiles of monotherapy with IL2 and ziv-aflibercept. Grade 4 events in the combination arm included decreased lymphocytes (41 patients) and platelets (6), renal failure (1), neutropenia (2), hypertension (2) and thromboembolism (1). Toxicity is summarized in Table 2.
Table 2.
Treatment related toxicities recorded on the trial
| Representative Grade 2 (≥ 5%) - 4 AEs | Arm A (N = 55) | Arm B (N = 29) | |||||
|---|---|---|---|---|---|---|---|
| System | Adverse Event | Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 |
| Blood | Anemia | 8 | 2 | 6 | |||
| Leukocytosis | 1 | ||||||
| Lymphocyte count decreased | 41 | 1 | 19 | ||||
| Lymphocyte count increased | 13 | 2 | |||||
| Neutrophil count decreased | 5 | 2 | 5 | ||||
| Platelet count decreased | 13 | 11 | 6 | 8 | 9 | ||
| Cardiovascular | Atrial fibrillation | 1 | 3 | ||||
| Left ventricular systolic dysfunction | 1 | ||||||
| Sinus tachycardia | 7 | 5 | 1 | ||||
| Supraventricular tachycardia | 1 | 1 | 1 | ||||
| Ventricular tachycardia | 3 | 2 | |||||
| Hypertension | 14 | 12 | 2 | 1 | |||
| Hypotension | 10 | 1 | 11 | 3 | 1 | ||
| Thromboembolic event | 2 | 1 | 1 | 1 | |||
| Constitutional / General | Fatigue | 17 | 5 | 10 | 3 | ||
| Fever & Chills | 30 | 1 | 16 | 1 | |||
| Infusion related reaction | 4 | ||||||
| Edema limbs | 7 | 6 | |||||
| Weight gain | 11 | 10 | |||||
| Gastrointestinal / Liver / Pancreas | Diarrhea | 13 | 2 | 1 | |||
| Mucositis oral | 7 | 3 | 1 | ||||
| Nausea & Vomiting | 23 | 2 | 8 | 2 | |||
| LFTs increased | 20 | 13 | 10 | 9 | |||
| Lipase increased | 2 | ||||||
| Neurologic | Anxiety | 10 | 5 | ||||
| Confusion | 3 | 3 | 1 | ||||
| Renal | Creatinine increased | 8 | 2 | 3 | |||
| Proteinuria | 14 | 6 | |||||
| Urine output decreased | 7 | 1 | 7 | ||||
| Respiratory | Dyspnea | 10 | 1 | 4 | 4 | ||
| Skin | Rash maculo- papular | 3 | 1 | 2 | |||
Arm A: Patients receiving combination of HD IL-2 and ziv-aflibercept. Arm B: Patients receiving HD IL2 alone
Abbreviations: AE: Adverse events;
Among 29 treated patients in arm B, salvage medications received included: 13 (45%) ipilimumab, 9 (31%) anti-PD1 monotherapy, 1 (3%) ipilimumab + nivolumab, 7 (24%) BRAF inhibitor with or without MEK inhibitor, 3 (10%) dendritic cell vaccine, 5 (17%) chemotherapy and 1 (3%) talimogene laherparepvec. In arm A: 17 (31%) ipilimumab, 15 (27%) anti-PD1 monotherapy, 4 (7%) ipilimumab + nivolumab, 8 (15%) BRAF inhibitor with or without MEK inhibitor, 4 (7%) dendritic cell vaccine, 16 (29%) chemotherapy, 5 (9%) other agents.
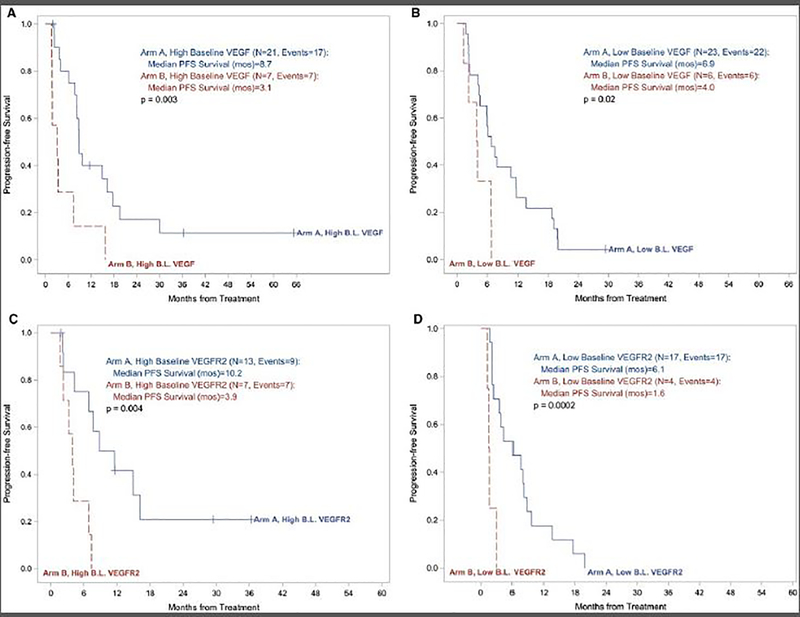
Longer PFS among patients with high baseline serum VEGF and VEGFR2 in favor of the combination arm
To further investigate the impact of VEGF blockade, we next assessed serum VEGF in the combination arm as compared to the IL2 alone arm. We used the median baseline measures of VEGF and VEGFR2 to establish the cut point for “high” and “low”. As expected, there was significant reduction in VEGF levels on-treatment (end of course 2) compared to baseline on arm A versus arm B (p<0.0001). Median PFS was significantly longer in the combination arm as compared to the IL2 alone arm including the high baseline VEGF groups (8.7 versus 3.1 months; p=0.003) and the low baseline VEGF groups (6.9 versus 4.0 months; p=0.02) (Figure 4A). Similarly, in our assessment of baseline serum VEGFR2, median PFS was significantly longer in the combination arm including the high VEGFR2 groups (10.2 versus 3.9 months; p=0.004) and the low VEGFR2 groups (6.1 versus 1.6 months; p=0.0002) (Figure 4B).
Figure 4A/B.
Kaplan-Meier plots of progression free survival (PFS) by baseline serum VEGF (4A) and VEGFR2 (4B) levels in arm A (the combination of HD IL-2 and ziv-aflibercept) versus arm B (HD IL2 alone).
DISCUSSION
This study tested the primary hypothesis that combination biotherapy with ziv-aflibercept, as a high-affinity soluble VEGF receptor and potent angiogenesis inhibitor, and HD IL2 would lead to improved anti- tumor efficacy compared to HD IL2 alone. The study met its primary endpoint of significantly improving PFS, but there were no significant differences in the secondary endpoints of OS or RR undermining the primary endpoint outcome seen. The lack of significant differences in overall survival may be understood taking into account the limited phase II sample size as well as the therapeutic salvage patterns observed in patients who eventually progressed as summarized in the results section. Therefore, we expect survival benefits from active agents received as salvage in both study arms. Response rates were numerically higher for the combination (22% compared to 17%) but were not significantly different which may also be a limitation of the phase II sample size in this study.
Systemic treatment of metastatic melanoma has undergone a major transformation over the 7 years with the development of novel molecularly targeted kinase inhibitors and immune checkpoint blockers.15–18 However, most patients still do not achieve long-term disease remission and control and continue to have a need for salvage systemic therapy. Overall, recent 5-year OS data from a phase 2 trial of dabrafenib and trametinib for patients with BRAF V600–mutant metastatic melanoma reported a median OS of 25.0 months and a 5-year OS of 28% in patients receiving the approved label dose.20 Similarly, recent OS analysis of the phase 3 KEYNOTE-006 trial that tested pembrolizumab monotherapy reported a 33-month OS of 50%.21 In the phase 3 CheckMate 067 trial, the 3 year OS was 52% and 58% in patients treated with nivolumab monotherapy or nivolumab plus ipilimumab, respectively.16 Therefore, at least 50% of patients with metastatic melanoma treated with kinase inhibitors and/or immune checkpoint inhibitors will require second line or subsequent salvage systemic therapy where HD IL2 in combination with ziv- aflibercept would be worth considering in candidate patients.
Baseline serum VEGF as a marker of immune resistance was associated with non-response to HD IL2 6. In our analysis, patients with high levels of VEGF and VEGFR2 had significantly better clinical outcome, as measured by PFS, when treated on the combination arm compared to HD IL2 monotherapy. Similarly, improved PFS was also observed with the combination among patients with low VEGF and VEGFR2 groups. These findings support the initial hypothesis related to reversing immune suppression mediated by VEGF, and probably also suggests an additive antiangiogenic clinical impact with ziv-aflibercept, a known clinically active agent in melanoma, as we previously have reported 13. VEGF-A, typically referred to as VEGF, is present in a variety of different splice isoforms, 2 of which are freely circulating (VEGF121 and VEGF165)7. VEGF can signal through a number of different receptors: VEGFR-1, VEGFR-2, and VEGFR-3. The major signaling receptor for VEGF-mediated angiogenesis is thought to be VEGFR-2, also called kinase-insert domain–containing receptor in humans or fms-like tyrosine kinase (flt) 1 in mice 7. VEGFR-3 is mainly involved in lymphangiogenesis, although there is growing evidence that it can be also involved in angiogenesis. The role of VEGFR-1 is still unknown. However, there is growing interest in VEGFR-1, in part because of its role in the mobilization of a number of the bone marrow–derived circulating cell populations 7. High-circulating VEGF levels are prognostic for poor PFS and OS regardless of treatment, but they are not predictive of clinical outcomes for therapy with VEGF inhibitors in various solid tumors 22. Similarly, in melanoma, there is increased expression of VEGF in metastatic melanoma biopsies 23, correlation between VEGF levels and tumor burden and a correlation between increased serum concentration of angiogenic factors and disease progression and survival 24. In this study, clinical benefit was seen regardless of the baseline VEGF or VEGFR2 levels supporting a prognostic rather than a predictive value.
The enhanced antitumor activity observed with the combination of antiangiogenic and immunotherapeutic agents has been reported with other agents in patients with metastatic melanoma. A phase I study combining anti-VEGF blockade with bevacizumab and CTLA4 blockade with ipilimumab reported a response rate of approximately 20%, and a disease control rate of 67.4% 25. Median survival was 25.1 months. On-treatment tumor biopsies demonstrated significant infiltration by CD8+ T cells and CD163+ dendritic macrophages within tumor vessel endothelium with enhanced CD31 staining at the interendothelial junctions. These data suggested that vessels adapted for efficient lymphocyte trafficking where CD31 may impact adhesive and signaling functions for vascular cellular extravasation 26. Peripheral blood flow cytometry showed increased CD4+ and CD8+ T cells that are CCR7(+/−)/CD45RO(+) and increased circulating memory cell phenotypes compared to ipilimumab alone, further supporting the immune modulator impact of VEGF inhibition 25. This study led to a national cooperative group randomized trial testing ipilimumab and bevacizumab versus ipilimumab alone (E3611; NCT01950390). Similarly, a phase 2 study of bevacizumab and high-dose interferon-α2b in metastatic melanoma reported a response rate of 24% 27. More recently, PD-L1 blockade with atezolizumab in combination with VEGF inhibition with bevacizumab has shown promising activity in phase II-III trial testing in metastatic renal cell carcinoma (mRCC) supporting the use of atezolizumab + bevacizumab as a first line treatment option in mRCC 28. This phase III data in mRCC is consistent with our data in this study and support a role for VEGF inhibition as an important component of a combination immunotherapeutic strategy.
Efforts to overcome the limitations of the short half-life and pleiotropic systemic effects of systemic IL2 led to the development of interesting molecules such as ALKS-423029 and NKTR-214 30. ALKS 4230, currently in phase I development, consists of a circularly permuted IL-2 and IL-2 Receptor (IL-2R)α designed to selectively target intermediate-affinity IL-2R (comprised of IL-2Rβ and γc) which is primarily expressed on effector lymphocytes29. NKTR-214 consists of the IL2 protein bound by multiple slowly releasable polyethylene glycol chains, acting as a kinetically-controlled IL2 receptor agonist with evidence that it favors activation of CD8+ T cells over regulatory T cells. It is currently in clinical development as both a monotherapy and in combination with anti-PD-1 antibodies 30. Our data may support a future combination strategy of NKTR-214 or ALKS 4230 and VEGF inhibition in the second or subsequent line treatment setting in advanced melanoma as an outpatient and potentially less toxic regimen.
CONCLUSION
The combination of ziv-aflibercept and HD IL-2 significantly improved PFS over IL-2 alone, meeting the study’s primary endpoint. The regimen was relatively safe and manageable. The combination was superior to monotherapy in patients with high and low levels of serum VEGF and VEGFR2. Our findings provide support for further study of combination strategies of VEGF inhibitors and immunotherapy.
Acknowledgement
This United States (U.S.) National Cancer Institute (NCI)-sponsored study was initiated by the (California Cancer Consortium with Pittsburgh (CCCP) under N01 contract: NO1-CM-2011–00038. This study utilized the UPMC Hillman Cancer Center’s Immunologic Monitoring and Cellular Products Laboratory shared facility, supported in part by award P30 CA047904
FUNDING SUPPORT
This United States (U.S.) National Cancer Institute (NCI)-sponsored study was initiated by the (California Cancer Consortium with Pittsburgh (CCCP) under N01 contract: NO1-CM-2011–00038. This study utilized the UPMC Hillman Cancer Center’s Immunologic Monitoring and Cellular Products Laboratory shared facility, supported in part by award P30 CA047904
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Ahmad A Tarhini declared consulting role (advisory board participation) to Sanofi-aventis Pharmaceuticals. Timothy M. Kuzel declared speaker role for Sanofi-aventis Pharmaceuticals. Theodore F. Logan declared consulting or advisory role to Prometheus. The remaining co-authors declared that they have no conflict of interest relevant to this study.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. [DOI] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA: a cancer journal for clinicians. 2012;62(5):309–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240(4856):1169–1176. [DOI] [PubMed] [Google Scholar]
- 4.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(7):2105–2116. [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6 Suppl 1:S11–14. [PubMed] [Google Scholar]
- 6.Sabatino M, Kim-Schulze S, Panelli MC, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(16):2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. The New England journal of medicine. 2008;358(19):2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285(21):1182–1186. [DOI] [PubMed] [Google Scholar]
- 9.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma research. 2006;16(5):405–411. [DOI] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature medicine. 1996;2(10):1096–1103. [DOI] [PubMed] [Google Scholar]
- 11.Ohm JE, Gabrilovich DI, Sempowski GD, et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood. 2003;101(12):4878–4886. [DOI] [PubMed] [Google Scholar]
- 12.Investigator’sBrochure. [AFLIBERCEPT (VEGF Trap, AVE0005 )] (2009). Regeneron Pharmaceuticals,Inc., Tarrytown, NY: and Aventis Pharmaceuticals Inc., Bridgewater, NJ: Edition 9 May 1,. 2009. [Google Scholar]
- 13.Tarhini AA, Frankel P, Margolin KA, et al. Aflibercept (VEGF Trap) in inoperable stage III or stage iv melanoma of cutaneous or uveal origin. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(20):6574–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 15.Schadendorf DHF, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in metastatic or locally advanced, unresectable melanoma.. Paper presented at: European Cancer Congress 2013 (ECCO-ESMO-ESTRO)2013. [Google Scholar]
- 16.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. The New England journal of medicine. 2015;372(1):30–39. [DOI] [PubMed] [Google Scholar]
- 18.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. The New England journal of medicine. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 19.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. The New England journal of medicine. 2014;371(20):1867–1876. [DOI] [PubMed] [Google Scholar]
- 20.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF- mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. The lancet oncology. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 21.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017. [DOI] [PubMed] [Google Scholar]
- 22.Hegde PS, Jubb AM, Chen D, et al. Predictive impact of circulating vascular endothelial growth factor in four phase III trials evaluating bevacizumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(4):929–937. [DOI] [PubMed] [Google Scholar]
- 23.Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. British journal of cancer. 1997;76(7):930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(2):577–583. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer immunology research. 2014;2(7):632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57(4):523–528. [DOI] [PubMed] [Google Scholar]
- 27.Grignol VP, Olencki T, Relekar K, et al. A phase 2 trial of bevacizumab and high-dose interferon alpha 2B in metastatic melanoma. Journal of immunotherapy. 2011;34(6):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). Paper presented at: Journal of Clinical Oncology 36, no. 6_suppl (February 20 2018) 578–578.2018. [Google Scholar]
- 29.Vaishampayan UN, Ernstoff MS, Velcheti V, et al. A phase I trial of ALKS 4230, an engineered cytokine activator of NK and effector T cells, in patients with advanced solid tumors. American Society of Clinical Oncology; 2017. [Google Scholar]
- 30.Charych D, Khalili S, Dixit V, et al. Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. PloS one. 2017;12(7):e0179431. [DOI] [PMC free article] [PubMed] [Google Scholar]