Abstract
In this issue of Science Signaling, Larrieu et al. show that an acetyltransferase inhibitor that rescues many dominant nuclear phenotypes caused by progerin, a truncated form of lamin A, does so by releasing the specialized nuclear import receptor TNPO1 from sequestration by microtubules. This release enables TNPO1-dependent import of specific cargoes, including the nuclear pore protein Nup153 and the heterogeneous nuclear ribonucleoprotein hnRNPA1, thus restoring the functionality of nuclear pore complexes, rebalancing the nucleocytoplasmic Ran gradient, and normalizing gene expression.
Healthy aging is a shared dream of humanity that motivates scientists to understand aging at the molecular and cellular level. A rare condition, Hutchinson-Gilford progeria syndrome (HGPS), is providing key insights. HGPS is caused by a single base pair change in the LMNA gene. LMNA encodes two nuclear intermediate filament proteins, lamin C and lamin A. Lamin A is first made as a precursor, prelamin A, which is C-terminally modified and proteolytically cleaved to generate mature lamin A. The HGPS mutation favors mis-splicing of the precursor mRNA. The resulting protein, progerin, differs from lamin A in that it lacks 50 residues near the C terminus and is permanently farnesylated, which enhances its association with the inner membrane of the nuclear envelope. Progerin accumulates at the nuclear envelope and dominantly disrupts nuclear shape, nuclear lamina organization, nuclear pore complexes (NPCs), nucleocytoplasmic transport, and chromatin silencing (1). Altered nuclear structure and genome organization, in turn, cause a plethora of downstream problems, including inefficient DNA repair, tissue-inappropriate gene expression, and premature entry into senescence. Similar phenotypes also arise in normal aging, associated with the accumulation of wild-type prelamin A (2). Identification of the primary molecular causes of nuclear dysfunction in HGPS and aged cells is critical for understanding the toxicity of progerin and prelamin A.
A small molecule named Remodelin improves many progerin phenotypes and specifically restores nuclear accumulation of the small guanosine triphosphatase (GTPase) Ran, which is disrupted in cells from HGPS patients (3). High amounts of RanGTP in the nucleoplasm (the “Ran gradient”) are especially important for large cargoes to be imported through NPCs by a family of receptors known as karyopherins, which include importins, exportins, and transportins. Reduced nuclear import of one particular large cargo in HGPS cells, a ~265-kDa nucleoporin called Tpr (4), is devastating because Tpr dimers are required to finalize NPC assembly. Tpr is a major component of NPC “basket” and filament structures needed for nucleoporins to associate with transcriptionally active chromatin inside the nucleus (4–6). However, it was a mystery how Remodelin restored NPCs or nuclear import. This molecule specifically blocks the acetyltransferase activity of NAT10, an enzyme with a few known substrates, including ribosomal RNA and tubulin. Tubulin appeared to be the biologically relevant target in this context because treatment with Remodelin (or loss or inactivation of NAT10) rescued the overly stable microtubule phenotype in HGPS cells (3).
To explore potential mechanisms of rescue by Remodelin, Larrieu et al. (1) focused on NPCs and nuclear import. They found that Nup153, an NPC basket protein that directly binds to and anchors Tpr, was depleted from the nuclear envelope of HGPS cells. Remodelin rescued nuclear Nup153 import and the accumulation of Tpr at the envelope, indicating that Tpr deficiency at the nuclear envelope of HGPS cells is a consequence of the failure to import Nup153. Although Ran localization was aberrant in HGPS cells and rescued by Remodelin, the authors noticed that its dedicated nuclear import partner, nuclear transport factor 2 (NTF2), localized normally in HGPS cells, and knocking down NTF2 did not further exacerbate HGPS cellular phenotypes. Furthermore, nuclear Ran abundance was also reduced in control cells when Nup153 was knocked down (1, 2). This result, and evidence that Nup153 directly binds guanosine diphosphate (RanGDP) and RanGTP (7), suggested that deregulation of the Ran gradient was secondary to the loss of Nup153 import and not a consequence of NTF2 deficiency or mislocalization.
Work from Vollmer et al. (8) provided an important clue about the connection between Nup153 nuclear import and the Ran gradient, particularly in nondividing cells where ongoing import of Nup153 is important for the formation of new NPCs. During interphase, Nup153 is imported by a specific receptor, Transportin-1 (TNPO1). TNPO1 is unusual because it recognizes only a small subset of proteins as cargo, including Nup153 and the mRNA splicing protein hnRNPA1. Once inside the nucleus, however, TNPO1 behaves like other import receptors and releases its cargo in response to binding RanGTP (Fig. 1). Larrieu et al. (1) found that TNPO1 was present in approximately equal amounts in the nucleus and cytoplasm of control cells but was significantly depleted from the nuclei of HGPS cells and normal cells from aged individuals. They found that TNPO1 was retained in the cytoplasm of HGPS cells by microtubules, based on proximity ligation assays that showed significantly greater interaction between TNPO1 and α-tubulin in HGPS cells. Consistent with this, TNPO1 was retained in the cytoplasm when normal fibroblasts were treated with a microtubule-stabilizing drug and, conversely, was released when fibroblasts were treated with a microtubule-destabilizing drug.
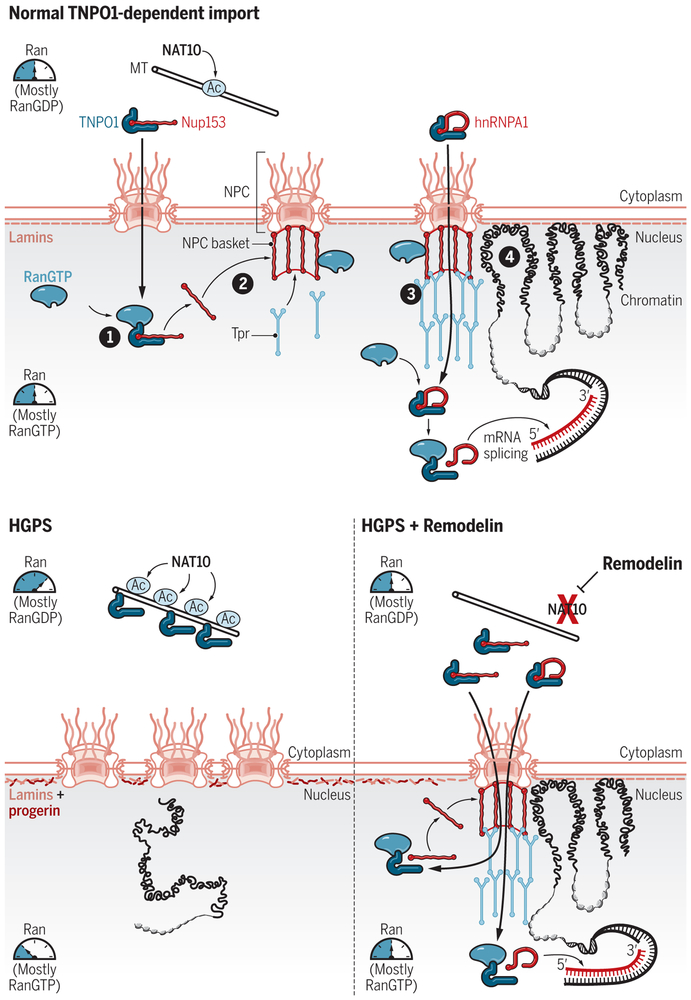
Fig. 1. Proposed mechanism by which Remodelin rescues NPC structure, nuclear import, and downstream functions in HGPS cells.
In normal cells, NPC assembly during interphase requires TNPO1-dependent import of the nucleoporin Nup153. Most Ran in the cytoplasm is bound to RanGDP, whereas most Ran in the nucleus is bound to RanGTP. RanGTP is critical for nuclear import because it binds to importins to stimulate the release of cargo proteins such as Nup153 (1). Nup153 forms the NPC basket, binds to lamin A, recruits Tpr to the basket (2), and anchors Tpr oligomers that extend into the nucleoplasm (3). These extended networks are necessary for Nup153 and other mobile nucleoporins to associate with transcriptionally active chromatin in the nuclear interior (4). Transcriptionally silent chromatin associates with lamins near the inner membrane of the nuclear envelope. TNPO1 is also required to import the mRNA-splicing protein hnRNPA1, which is released in the nucleus upon RanGTP binding to TNPO1. The acetyltransferase NAT10 covalently attaches acetyl groups (Ac) to many target proteins, including microtubules (MT). In cells from HGPS patients, progerin accumulates at the inner membrane of the nuclear envelope and disrupts normal nuclear lamina organization; other phenotypes include reduced accumulation of nuclear RanGTP, loss of heterochromatin positioning near the nuclear envelope, transcriptional dysregulation, and premature cellular senescence. Because NAT10 activity and microtubule stability are increased in HGPS cells, NAT10-mediated hyperacetylation of tubulin is proposed to explain the increased association of TNPO1 with microtubules in HGPS cells. Sequestration of TNPO1 by microtubules reduces TNPO1-dependent import of cargoes, such as Nup153 and hnRNPA1, leading to defective NPC assembly and transcriptional dysregulation. Treating HGPS cells with Remodelin, which inhibits NAT10 activity, releases TNPO1 from microtubules, thereby restoring TNPO1-dependent import of Nup153, hnRNPA1, and other cargoes. This, in turn, restores NPCs, rebalances the nucleocytoplasmic Ran gradient, and normalizes gene expression.
NAT10 depletion rescued HGPS phenotypes only when TNPO1 and Nup153 were both present (Fig. 1). Nup153 is a fascinatingly mobile and multifunctional nucleoporin; its partners are not limited to Tpr, lamin A, RanGDP, and RanGTP (7) but also include the transcription factor Sox2 during neuron-specific gene expression (6). This new report by Larrieu and colleagues (1) identifies TNPO1-dependent import as a pathway that is critically affected in both progeria and normal aging, and further shows that this pathway, and to a large extent proper gene expression, is restored in cultured cells by inhibiting a specific enzyme, NAT10. This study paints a vivid picture of NPCs and their impacts on human biology, providing important new pieces of this complex puzzle and raising even more questions. How and why does NAT10 activity increase in progeria and normal aging, and why does this drive TNPO1 association with microtubules? Might other TNPO1-dependent cargoes such as PER1, which regulates circadian rhythm (9), or TNPO1-dependent protein targeting to primary cilia (10) also contribute to HGPS or normal aging?
Acknowledgments:
I thank K. S. Ullman for insightful comments on the manuscript.
Funding: I acknowledge funding from the Progeria Research Foundation, and the Johns Hopkins University Claude D. Pepper Older Americans Independence Center of the National Institute on Aging (grant no. P30AG021334).
Footnotes
Competing interests: The author declares that she has no competing interests.
REFERENCES AND NOTES
- 1.Larrieu D, Viré E, Robson S, Breusegem SY, Kouzarides T, Jackson SP, Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway. Sci. Signal 11, eaar5401 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cobb AM, Larrieu D, Warren DT, Liu Y, Srivastava S, Smith AJO, Bowater RP, Jackson SP, Shanahan CM, Prelamin A impairs 53BP1 nuclear entry by mislocalizing NUP153 and disrupting the Ran gradient. Aging Cell 15, 1039–1050 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP, Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 344, 527–532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snow CJ, Dar A, Dutta A, Kehlenbach RH, Pascal BM, Defective nuclear import of Tpr in progeria reflects the Ran sensitivity of large cargo transport. J. Cell Biol 201, 541–557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krull S, Dörries J, Boysen B, Reidenbach S, Magnius L, Norder H, Thyberg J, Cordes VC, Protein Tpr is required for establishing nuclear pore-associated zones of heterochromatin exclusion. EMBO J. 29, 1659–1673 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitazawa T, Rijli FM, Nuclear pore protein meets transcriptional factor in neural fate. Neuron 96, 259–261 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Higa MM, Alam SL, Sundquist WI, Ullman KS, Molecular characterization of the Ran-binding Zinc finger domain of Nup153. J. Biol. Chem 282, 17090–17100 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Vollmer B, Lorenz M, Moreno-Andrés D, Bodenhöfer M, De Magistris P, Astrinidis SA, Schooley AM, Flotenmeyer M, Leptihn S, Antonin W, Nup153 recruits the Nup107-160 complex to the inner nuclear membrane for interphasic nuclear pore complex assembly. Dev. Cell 33, 717–728 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Korge S, Maier B, Brüning F, Ehrhardt L, Korte T, Mann M, Herrmann A, Robles MS, Kramer A, The non-classical nuclear import carrier Transportin 1 modulates circadian rhythms through its effect on PER1 nuclear localization. PLOS Genet. 14, e1007189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madugula V, Lu L, A ternary complex comprising transportin1, Rab8 and the ciliary targeting signal directs proteins to ciliary membranes. J. Cell Sci 129, 3922–3934 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]