Abstract
In the elderly, intact motor functions of the upper extremity are critical for the completion of activities of daily living. Many studies have provided insight into age-related changes in motor function. However, the precise nature and extent of motor impairments of the upper extremity remains unclear. In the current study we have modified two tasks to assess hand/digit function in both young and aged rhesus monkeys. We tested monkeys from 9 to 26 years of age on these tasks to determine the level of fine motor performance across the adult age range. Compared to young monkeys (9–12 years of age), aged monkeys (15–26 years of age) were mildly impaired on fine motor control of the digits. These findings are consistent with previous studies that have found age-related impairment in fine motor function. However, the magnitude and extent of impairment in the current study does differ from previous findings and is likely due to methodological differences in the degree of task complexity.
Keywords: motor function, hand, rhesus monkey, aging
Introduction
A key factor in the successful completion of activities of daily living and independent living in the elderly is the intact motor function of the upper extremity. However, it is established that motor function of the upper extremity, particularly fine motor movements of the hand such as manual dexterity and digit strength, decreases during the normal aging process in both humans (Desrosiers et al. 1999; Ranganathan et al. 2001; Carmeli et al. 2002, 2003) and monkeys (Zhang et al. 2000; Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007; Walton et al. 2006).
In humans, specific age-related changes in the motor function of the hand are common and can have a serious impact on an individual’s daily living and their ability for independent living (Shiffman 1992; Desrosiers et al. 1999; Ranganathan et al. 2001; Wiesendanger and Serrien 2001; Nichols Larsen et al. 2005). Throughout the aging process decreased muscle strength, muscle mass, and bone density are among the most prevalent changes to occur in the hand (Mathiowetz et al. 1985; Kallman et al. 1990; Shiffman 1992; Ranganathan et al. 2001; Wiesendanger and Serrien 2001; Carmeli et al. 2002; Livshits et al. 2002). A longitudinal study investigating the effects of age on upper extremity abilities in community-dwelling individuals over the age of 60 years demonstrated significant declines in fine manual dexterity, motor coordination, and grip strength of the hand (Desrosiers et al. 1999). In addition, significant nerve changes also occur with age including loss of motor neurons and ventral root axon functioning, reduction of the number of mye- linated nerve fibers from cervical nerve roots, and decreased sensation of the hands (Warabi et al. 1986; Mittal and Logmani 1987; Kallman et al. 1990; Metter et al. 1998; Laidlaw et al. 2000; Carmeli et al. 2002). These changes result in reductions in preci sion dexterity, grip strength, and speed of movements (Mathiowetz et al. 1985; Shiffman 1992; Desrosiers et al. 1999; Carmeli et al. 2003). These age-related changes in the motor function of the hand become most notable in the 60s and 70s and occur in the absence of significant neurological disease or cortical damage (Desrosiers et al. 1999; Ranganathan et al. 2001; Carmeli et al. 2003).
In non-human primates, use of the digits of the upper extremity is also critical to daily function. Similar to age-related changes in motor function of the hand in humans several studies have demonstrated an overall age-related motor slowing, impaired ability for complex movements, and increased bradykinetic movements in the hand of non-human primates (Zhang et al. 2000; Carmeli et al. 2003; Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007; Walton et al. 2006).
Several research groups have examined the effects of age on motor function of the hand in non-human primates using a variety of tasks that quantify changes in the dexterity and strength of the hands. In the rhesus monkey, investigators (Zhang et al. 2000; Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007; Walton et al. 2006) have demonstrated age- related decreases in bimanual motor function, fine motor dexterity, and movement speed of the hand. In addition, male rhesus monkeys appear to have greater declines in motor function of the hand than females and hormone status does not appear to affect performance (Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007).
In the current study, we modified two commonly used tasks of fine motor function of the hand in order to quantitatively assess this function in both young and aged rhesus monkeys and further explore the precise nature of age-related changes in motor function of the hand. We have designed these tasks to require varying degrees of complexity in order to assess the range of fine motor function of the hand across the lifespan in our non-human primate model of normal aging. The first task is a modified version of the Kluver test (Lawrence and Kuyper 1968) requiring the monkeys to retrieve food rewards from wells of various depths and diameters. The second task is a simplified version of the visuospatial motor task (Bachevalier et al. 1991; Gash et al. 1999). The data from this group of animals will provide baseline values for monkeys across the lifespan on these tests and will establish the degree of age-related impairment observed on specific components of each test.
Methods
Subjects
The subjects in this cross-sectional study were from our non-human primate models of normal aging and of ischemic stroke that were established in our laboratory. Data from animals from the non-human primate model of ischemic stroke was either control data or pre-operative data. Sixteen young and aged adult female and male rhesus monkeys (Macaca mulatta) between the ages of 9 and 26 years of age were used. The young group consisted of five animals (3 males and 2 females) aged 9–12 years old and the aged group consisted of 11 animals (3 females and 8 males) aged 15–26 years (Table I). Monkeys attain sexual maturity at about 5 years of age and the oldest rarely live beyond 30, suggesting an approximate relationship to humans of 1:3 (Tigges et al. 1988). Hence the monkeys between 9 and 12 years of age correspond roughly to humans between 27 and 36 years of age and those between 15 and 26 years of age to humans between 45 and 78 years of age. All of the monkeys were obtained from a national primate research facility or breeding facility and had known birth dates and complete health records. Before entering the study, monkeys received medical examinations that included serum chemistry, hematology, urine analysis, and fecal analysis. In addition, explicit criteria were used to exclude monkeys with a history of any of the following: splenectomy, thymectomy, exposure to radiation, cancer, organ transplantation, malnutrition, chronic illness including viral or parasitic infections, neurological diseases, or chronic drug administration. Prior to entering the study, monkeys were housed in a variety of group housing facilities. Once entered into the study, all monkeys were individually housed in colony rooms where they were in constant auditory and visual range of other monkeys in the Laboratory Animal Science Center (LASC) of Boston University School of Medicine. This facility is fully AAALAC approved and animal maintenance and research were conducted in accordance with the guidelines of the National Institutes of Health Committee on Laboratory Animal Resources and according to procedures approved by the Institutional Animal Use and Care Committee of the Boston University Medical Campus. Diet consisted of Purina Monkey Chow (Purina Mills Inc., St Louis, MO, USA) supplemented by fruit with feeding taking place once per day, immediately following behavioral testing. Water was available continuously. The monkeys were housed under a 12-hour light/dark cycle with cycle changes occurring in a graded fashion over the course of an hour.
Table I.
List of animals used in this study, including sex and age at testing for each subject.
| Monkey | Sex | Age |
|---|---|---|
| Young | ||
| SM 004 | M | 9 |
| AM 255 | F | 9 |
| AM 244H | M | 10 |
| AM 254 | F | 11 |
| SM 005 | M | 12 |
| Aged | ||
| SM 007 | M | 15 |
| AM 257 | F | 17 |
| SM 006 | M | 17 |
| AM 253 | F | 18 |
| AM 252 | F | 19 |
| SM 008 | M | 20 |
| SM 009 | M | 20 |
| SM 010 | M | 20 |
| AM 242 | M | 23 |
| AM 243 | M | 24 |
| AM 034 | M | 26 |
AM = aging monkey; SM stroke monkey (control animals from a =larger study of stroke).
Behavioral testing procedures
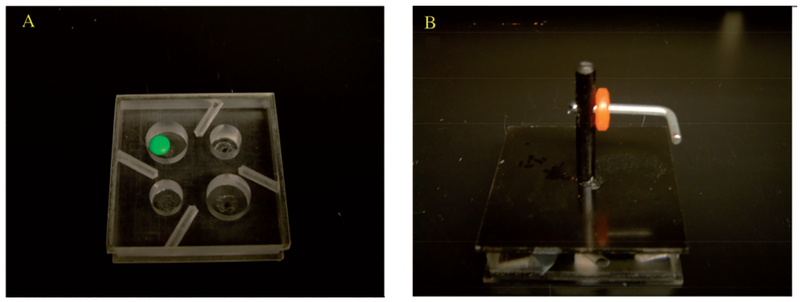
Monkeys were tested on two tasks of fine motor function of the hand. First, the hand dexterity task (HDT) is a modified version of the Kluver board that required the monkeys to retrieve small food rewards (M&M’s and Reece’s Pieces) from wells of various diameters and depths with each hand (Figure 1A). Second, a visuospatial motor task (VMT), modified from Bachevalier’s visuospatial orientation task (Bachevalier et al. 1991) and the Gash Movement Assessment Panel (Gash et al. 1999) required the monkeys to retrieve a ring-shaped candy (Life Saver) from a horizontal L-shaped metal rod with each hand independently (Figure 1B).
Figure 1.
Plexiglas testing trays used for HDT (A) and VMT (B) tasks.
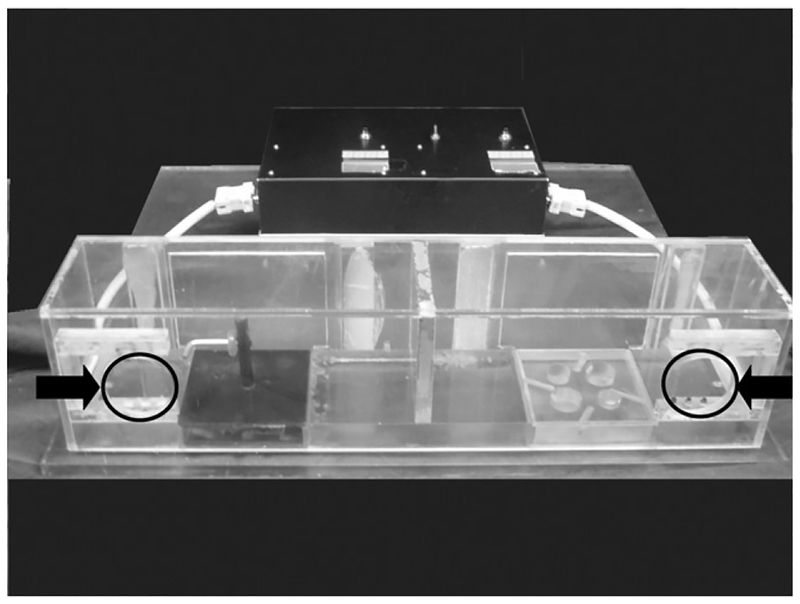
Both tasks were administered in a Wisconsin General Testing Apparatus (WGTA) that has been modified to accommodate a specially designed apparatus used for administering both tasks. Inside the WGTA, the testing apparatus consists of a clear Plexiglas box (Figure 2) that is divided into right and left sides. The box is placed directly in front of the monkey with access limited to a small opening located on each side that forces the use of the right or left hand for the right and left openings, respectively. Each side of the box is fitted with a square tray that contains either four wells for the HDT task or a horizontal L-shaped rod for the VMT task. The openings on either side of the apparatus were fitted with a photocell that triggers a timer to begin recording when the animal’s hand enters the box and stops when the hand is removed from the box (with or without the reward) establishing a reward retrieval latency that provides a quantitative measure of the efficiency of digit use for reward retrieval. In addition to this latency measure of fine motor control the success or failure on retrieval was recorded independently by the tester.
Figure 2.
Plexiglas testing apparatus with trays from HDT and VMT tasks. Openings for monkey’s hands marked with black circles and arrows on figure.
Hand dexterity task (HDT)
All monkeys were trained to retrieve a food reward (M&M’s and Reece’s Pieces) from four wells of various depths and diameters located in a tray on the left or right side of the apparatus. The most efficient performance is attained utilizing precise control of the digits in a finger–thumb pincer fashion. The type of food reward remained constant for all trials for all monkeys. Each tray contained the following four wells that varied by depth and diameter: well 1—diameter = 2.5 cm and depth=1.59 cm; well 2—diameter = 2.5 cm and depth = 0.95 cm; well 3—diameter=1.9 cm and depth = 1.59 cm; and well 4—diameter = 1.9 cm and depth = 0.95 cm. These differences required the monkey to retrieve the food reward from the larger diameter wells (1 & 2) using several fingers and the thumb together but required the use of the thumb and one finger to retrieve the reward from the smaller diameter wells (3 & 4). An opaque screen occluded access to and sight of the box between trials when one of the four wells in either the right or the left side box was baited with a food reward out of view of the monkey. The centers of each well were symmetrically placed in a square block that was rotated so that each well that was baited was in the same location relative to the opening. Once the appropriate well was baited and located, the opaque screen was then raised initiating a trial as it revealed the location of the baited well. The monkey then was allowed to retrieve the reward from the baited tray and the time required to retrieve the reward (i.e., while the hand was inside the box) was recorded.
A total of 32 trials were presented in a pseudo- random and counterbalanced fashion each day and were equally divided into 16 trials for each hand (4 trials per well). If an animal was unsuccessful at retrieving the food reward because the food reward was dropped inside the box, the latency data for that trial was discarded, the failure noted, and the trial was repeated. All monkeys were trained on this task for 20 days.
Visuospatial motor task (VMT)
Following the completion of the training on the HDT, the monkeys were then tested on the VMT. The identical testing apparatus was used for this task but the trays with wells were replaced with a solid block with a horizontal, inverted L-shaped metal rod mounted in the center (Figure 1B). A subset of monkeys (3 young and 5 aged; all males) were trained to retrieve a ring-shaped candy (Life Saver) from the horizontal arm of the rod using each hand. The opaque screen occluded access to and sight of the box between trials. With the occluding screen in place, a ring-shaped candy (Life Saver) was placed on the horizontal rod against the face of the vertical arm of the inverted L. The trial was initiated when the opaque screen was raised to reveal the location of the reward. The monkey then was allowed to retrieve the reward by manipulating it off of the rod and the time required to retrieve the reward was recorded. A total of 8 trials were presented each day and were equally divided for each hand. All monkeys were trained on this task for 25 days.
Determination of hand preference
At the completion of each day of testing, the experimenter placed several food rewards on the top and directly in the center of the Plexiglas apparatus and then opened the occluding screen to allow the monkey access to the apparatus. The hand that the monkey used to retrieve these rewards was recorded each day and this data was used to determine the hand preference for each monkey. All monkeys in this study demonstrated a left-handed preference for retrieval of food reward.
Data analysis
Hand dexterity task (HDT)
Data collected included the latency to retrieve a reward from each of the four wells with each hand for a total of 32 trials each for 20 days of testing (4 trials to each well for each hand). If a trial was not successfully completed (i.e., the monkey dropped the food reward before removing their hand from the apparatus) the trial was repeated. Unsuccessful trials occurred very infrequently and did not account for a significant increase in the number of trials administered to each animal (i.e.,<10 times over the 20 days of testing). The data were divided into four blocks of 5 days of testing with mean latency to retrieve a reward over the 5 days in each block for each hand recorded (e.g., days 1–5, 6–10, 11–15, and 16–20).
A four-way repeated measures ANOVA with age as a between subject variable and hand, block, and well as within subjects variables was used to compare the performance of the two groups of monkeys in terms of the mean time to retrieve the food reward from each of the wells with each hand across the 5 days of testing in each of the four blocks of days. All analyses were followed by Scheffe Post-Hoc tests when appropriate.
Finally, a Pearson’s r correlation was used to determine the presence of a relationship between age and performance on the hand dexterity task.
Visuospatial motor task (VMT)
Data collected included the latency to retrieve a reward from the L-shaped horizontal rod with each hand for a total of 8 trials a day for 25 days. If a trial was not successfully completed (i.e., the monkey dropped the food reward before removing their hand from the apparatus) the trial was repeated. Unsuccessful trials occurred very infrequently and did not account for a significant increase in the number of trials administered to each animal (i.e., <10 times over the 25 days of testing). The data were divided into five blocks of 5 days of testing with mean latency to retrieve a reward over the 5 days in each block for each hand (e.g., days 1–5, 6–10, 11–15, 16–20, and 21–25).
A three-way repeated measures ANOVA with age as a between subject variable and hand and block as within subjects variables was used to compare the performance of the two groups of monkeys in terms of the mean time to retrieve the food reward from the L-shaped horizontal rod with each hand across the 25 days of testing. All analyses were followed by Scheffe Post-Hoc tests when appropriate.
Following this analysis, a Pearson’s r correlation was used to determine the presence of a relationship between age and performance on the Life Saver task.
Results
Hand dexterity task (HDT)
A four-way repeated measures ANOVA with age as a between subject variable and hand, block, and well as within subjects variables revealed no overall effect of age group [F(1,13) = 2.20, p = 0.162] or hand F(1,13) = 0.252, p = 0.624] and no age group by hand [F(1,13) = 1.30, p = 0.274], age group by well [F(3,39) = 0.473, p = 0.703], or age group by block interaction [F(3,39) = 0.457, p = 0.714] for the mean latency to retrieve a food reward across the four blocks of testing. There was a significant effect of well [F(3,39) = 14.392, p.≤ 0.0001] block [F(3,39) = 10.99, and p ≤ 0.0001].
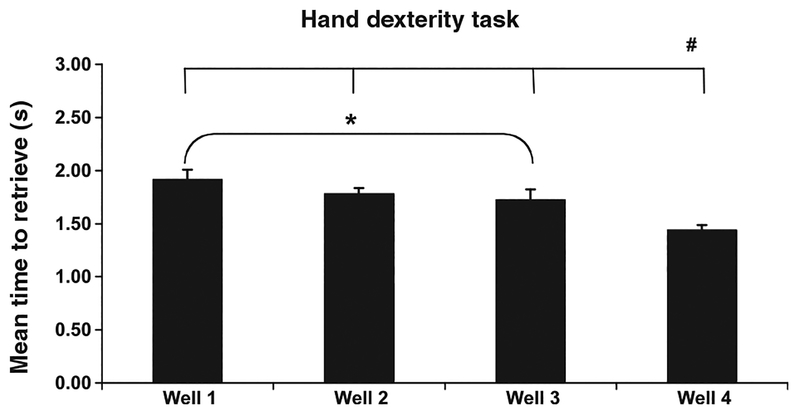
To further explore the significant effect of well on overall performance, Scheffe Post-Hoc tests were conducted and revealed a significant difference between performance of all animals between well 1 which had the longest latency of all four wells and well 3 ( p ≤ 0.05; Figure 3). These wells were both the same depth (1.59 cm) but differed in diameter with well 1 being 2.5 cm wide compared with the narrow diameter of well 3 at only 1.9 cm. This suggests that for a given depth, the narrow well is easier. This is confirmed by the fact that the other narrow well, well 4 which was also the most shallow (0.95 cm deep) had significantly shorter latency than all of the other three wells, making it the easiest of the four ( p ≤ 0.01; Figure 3).
Figure 3.
Performance of all animals on each well for both hands on the HDT task. Errors bars = standard error of the mean (SEM). *Significant difference between performance of all animals on well 1 and well 3 (p ≤ 0.05). #Significant difference between performance of all animals on well 4 and the other three wells (p ≤ 0.01).
To further explore the significant effect of block on overall performance, Scheffe Post-Hoc tests were conducted and there was a significant difference between performance of all animals on block 1 (days 1–5) of testing and the other three blocks of testing (days 6–10, 11–15, and 16–20). This finding suggests a period of performance improvement in the later days of testing and likely does not reflect a true difference in overall performance.
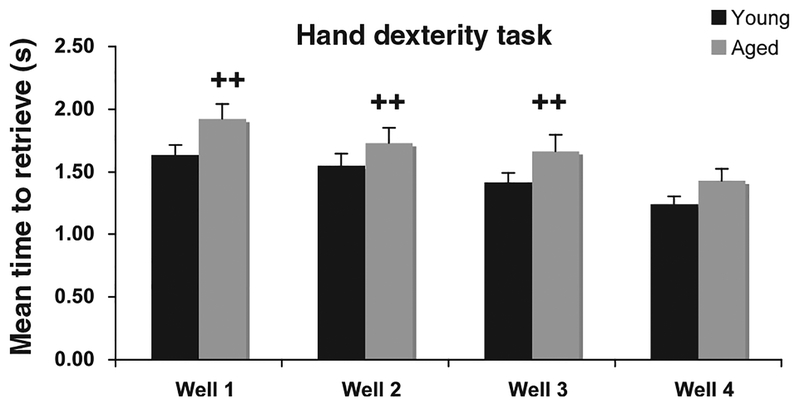
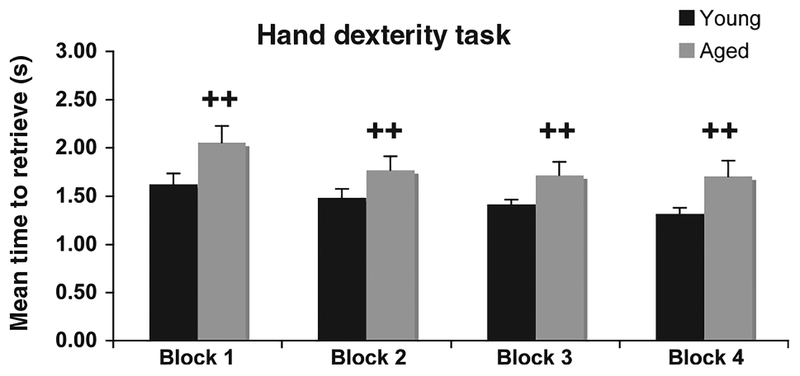
Due to the complex nature of this analysis and the number of variables we chose to further explore with the conservative Scheffe Post-Hoc test, the interactions between age group and the different wells and age group and the different blocks, even though age alone was not statistically significant across either variable. As shown in Figure 4, these analyses revealed a significantly superior performance of the young animals on all wells ( p ≤ 0.02) except well 4 (the easiest). Furthermore, performance of the young animals was significantly better than the aged animals across all blocks of testing days ( p ≤ 0.02) (Figure 5).
Figure 4.
Group differences for performance on wells 1–3 of the HDT task. ++Significant difference between young and aged animals on wells 1, 2, and 3 (p ≤ 0.02). Errors bars = standard error of the mean (SEM).
Figure 5.
Group differences for performance on the HDT task across four blocks of 5 days of testing. ++p≤0.02. Errors bars = standard error of the mean (SEM).
A Pearson’s r correlation revealed no significant linear relationship between age and performance on the hand dexterity task ( p = 0.45).
Visuospatial motor task (VMT)
A three-way repeated measures ANOVA with age as a between subject variable and hand and block as within subjects variables revealed no overall effect of age group [F(1,4) = 2.13, p = 0.139] or hand [F(1,13) = 0.278, p = 0.617] and no age group by hand [F(1,13) = 0.172, p = 0.692], age group by block interactions [F(3,39) = 1.06, p = 0.399] for the mean latency to retrieve a food reward from the horizontal L-shaped rod across the five blocks of 5 days of testing. There was a significant effect of block [F(3,39) = 2.89, p ≤ 0.04].
To further explore the significant effect of block on overall performance, Scheffe Post-Hoc tests were conducted but revealed no significant difference between the age groups on any of the blocks of testing days. This lack of a significant effect of age on the performance on the VMT may be related to extensive training on the HDT. In future studies, the order of tasks should be varied across young and aged animals.
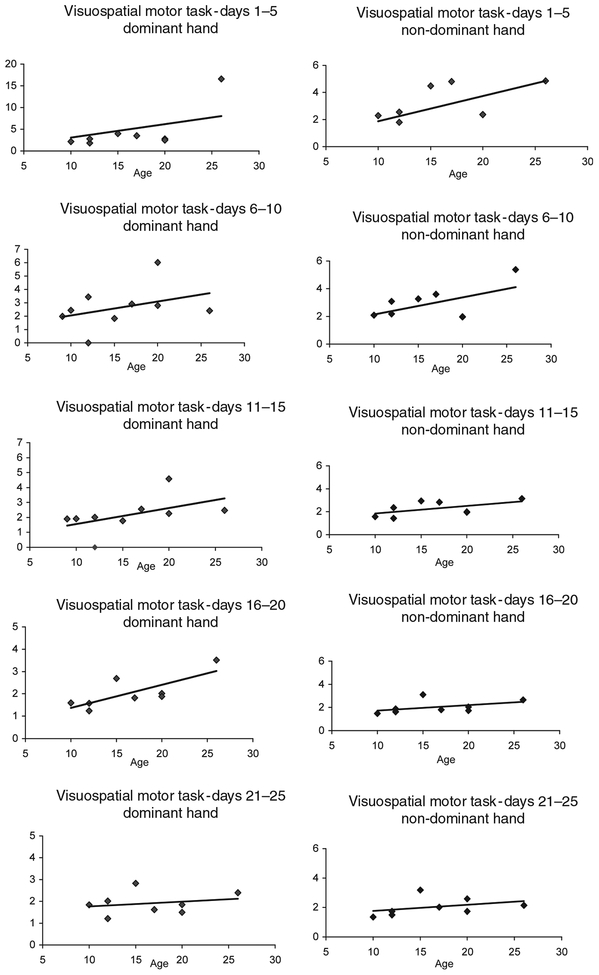
In contrast, a Pearson’s r correlation revealed a significant linear relationship between age and performance with the dominant hand on the VMT task during testing on days 1–5 (r = 0.744, p ≤ 0.05), days 6–10 (r = 0.627,p ≤= 0.05), days 11–15 (r = 0.790,p ≤ 0.05), and days 16–20 (r = 0.763, p ≤ 0.05) but not for days 21–25 (r = 0.235, p ≤ 0.575) (Figure 6 and Table II). There was no significant linear relationship between age and performance with the non-dominant hand on this task.
Figure 6.
Linear relationship between age and performance on the VMT for the dominant and non-dominant hands for each block. There was a significant linear relationship between age and performance with the dominant hand on days 1–5 (r = 0.744, p ≤ 0.05), days 6–10 (r = 0.627, p ≤ 0.05), days 11–15 (r = 0.790, p ≤ 0.05), and days 16–20 (r = 0.763, p ≤ 0.05) but not for days 21–25 (r = 0.235, p = 0.575). There was no significant linear relationship between age and the non-dominant hand on this task.
Table II.
Correlations between age and performance on the VMT task and mean latency to retrieve food reward on VMT for young and aged group (dominant and non-dominant hand).
| Correlation with age | Mean latency for young group | Mean latency for aged group | |
|---|---|---|---|
| Block 1 | |||
| Dominant | 0.744 | 2.26 | 5.87 |
| Non-dominant | 0.496 | 2.21 | 3.77 |
| Block 2 | |||
| Dominant | 0.627 | 1.94 | 3.22 |
| Non-dominant | 0.588 | 1.93 | 3.23 |
| Block 3 | |||
| Dominant | 0.790 | 1.82 | 2.75 |
| Non-dominant | 0.558 | 1.54 | 2.57 |
| Block 4 | |||
| Dominant | 0.763 | 1.47 | 2.38 |
| Non-dominant | 0.446 | 1.66 | 2.27 |
| Block 5 | |||
| Dominant | 0.235 | 1.69 | 2.04 |
| Non-dominant | 0.370 | 1.53 | 2.34 |
Significant correlations (r) are in italics. p ≤ 0.05.
Discussion
Summary
The major finding of this study was that the HDT is sufficiently sensitive to detect improvements in performance over multiple days of testing as well as mild impairments in fine motor function of the hands and digits of aged monkeys who required longer to retrieve a small food reward from wells 1–3 across all blocks of testing days. In addition, performance on well 4 (easiest well) differed from performance on any of the other three wells for all animals regardless of their age group. In contrast, on the VMT the performance of aged animals as a group was not significantly different from that of the group of young animals. However, there was a significant linear relationship between age and performance on this test within the first four blocks for the dominant hand. These findings demonstrate that aged animals are mildly impaired on the HDT task and perform within normal ranges on this version of VMT.
Published motor testing procedures
A commonly used task of motor function with non- human primates is the Kluver Testing Board. The Kluver Testing Board consists of a wood or Plexiglas board with numerous wells of varying diameters. The well diameters typically vary from 1.0 to 2.5 cm and the depth of the wells are usually approximately 1 cm. Formal testing consists of retrieval of food pellets placed in the wells. The level of fine motor function of the digits required to retrieve the reward varies depending on the diameter of the well. The assessment of performance on the Kluver Testing Board is determined by the number of food rewards retrieved from the various size wells in a pre-determined amount of time and/or the time required to retrieve the food reward from individual wells.
Emborg et al. (1998) used a modified Kluver test, the food pick-up test, to assess fine motor function in young and aged rhesus monkeys. The apparatus consisted of a 3 × 3 matrix of recessed wells embedded in Plexiglas that was mounted on the monkey’s home cage. Pieces of apple were used in this apparatus, one piece in each of six wells, and the amount of time required to retrieve all six pieces of apple was recorded. This was repeated for 10 trials for each arm for each testing session. The apparatus was positioned so that the animal could only use the arm being evaluated.
Using this apparatus, Emborg et al. (1998) demonstrated that there was no difference between young and aged animals learning this task. However, when performance latency was assessed, it was shown that aged animals required significantly more time to retrieve food rewards from the wells than young animals. Performance by aged monkeys on our fine motor task, the HDT, is similar to that demonstrated by Emborg et al. (1998) though the level of impairment on our task was less as the aged animals were significantly impaired on retrieving food rewards from only three of the four wells.
There are three major differences between these two tasks. First, our version is administered in a WGTA while in the other study the apparatus is mounted directly on the home cage. While there was this difference in the task apparatus, WGTA vs home cage, both testing set-ups allow for restriction of which arm is used while still allowing considerable movement by the monkeys and this likely does not account for the difference in performance levels.
Second, we require the monkey to retrieve a single food reward per trial for 32 trials across both hands in each testing session (total 32 retrievals), whereas Emborg et al. (1998) required the animal to retrieve one food reward from six wells for 10 trials per hand (total 120 retrievals). The number of trials administered each day could impact the performance of the monkeys. A significantly higher number of trials may cause fatigue or decreased motivation by the animals. While this issue of changes in motivation is addressed by Emborg et al. (1998) in that all animals completed every trial, they do not examine whether scores increase across trials or days of testing. A comparison of specific latencies would be needed to determine if this difference in test administration accounts for the differing results, however, this data was not available in the published paper.
The third factor that may have affected performance is the exact dimensions of the wells used in each testing apparatus board but the precise dimensions of the wells used by Emborg et al. (1998) are not described. However, the well dimensions typically used for a Kluver type testing board vary in diameter from 2.0 to 1.0 cm (our narrowest is 1.9 cm) and a depth of 1 cm while our deepest was 1.59 cm. If the well dimensions used by Emborg et al. (1998) are similar to those typically used for a Kluver board then their task may be much more difficult than our apparatus. They report that as a group young animals require an average of 7.88 ± 0.96 s and aged animals an average of 11.32 ± 1.47 s to retrieve the food reward from six wells. While the young animals in our study required a mean of 1.30 ± 0.25 s and the aged animals required a mean of 1.55 ± 0.44 s to retrieve a single food reward from one well (averaged across the four wells) for the dominant hand. In order to directly compare their findings to ours, we assume that an equal amount of time is required for the animals in Emborg et al.’s study to retrieve a single reward from each well. Under this assumption, their animals, both young and aged, do in fact require more time to retrieve food rewards from the individual wells than the animals in our study. In order to determine the precise nature of this difference in results it would be necessary to know the precise dimensions of the wells used in their apparatus and the time required for their animals to retrieve a food reward from a single well. It is likely though that their well dimensions were more different than those used in the current study and therefore represent a greater degree of difficulty. This is supported by data from Fukushima et al. (2007) that reported increasing latencies for retrieval of a food reward from wells of increasing depths in young animals. This study demonstrates that animals require 0.67 ± 0.27 s to retrieve a food reward from a well of 30 mm diameter and 10 mm depth but 2.16 ± 1.68 s to retrieve a food reward from a well of 30 mm diameter and 50 mm depth. While this study only included two young monkeys it does support the notion that wells of increased depths are more difficult for retrieving food rewards. In this regard it is important to point out that the presence in our task of several easy measures was planned in order to ensure that animals with greater age-related or neuropathological changes, would still be able to perform the task.
A second commonly used task of motor function of the hand with the non-human primate is the visuospatial motor task, commonly referred to as the Life Saver task. This is a test of fine motor function of the hand that requires the monkey to retrieve a Life Saver candy from metal rods of various shapes and complexities (e.g., straight, question mark, and S-shaped rods). The monkey must learn to thread the Life Saver from the beginning of the rod to the end over the various curves in the shape of the rod. The initial phases of this task usually consist of a vertical straight rod. Then, once the monkey successfully retrieves the Life Saver from this rod over a series of trials, the more difficult rods are used. This task requires considerable fine motor coordination and dexterity but there is also a cognitive component to the task as the monkey must develop a strategy for retrieving the candy from the more complex rods. The assessment of performance is based on the time to retrieve the reward from each of the rods with each hand across successive sessions.
Consistent findings from studies using the Life Saver task have demonstrated that aged animals are as efficient as young animals in retrieving the reward from the straight vertical rod but that their level of performance is impaired with the more complex rods (Gash et al. 1999; Zhang et al. 2000; Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007). Specifically, one study (Lacreuse et al. 2005) reported that the age-related impairment in performance on the complex rods of this test was limited to aged male rhesus monkeys, while another study demonstrated that estrogen replacement therapy did not improve the performance of aged ovariectomized female rhesus monkeys (Lacreuse and Herndon 2003).
Zhang et al. (2000) tested young, middle-aged, and aged animals on a modified Life Saver task that included retrieving a Life Saver from a flat platform, a straight rod, and a question-mark-shaped rod to assess motor function. They found that young animals were significantly faster at retrieving the food reward from the flat platform and the two rods than the middle-aged and the aged monkeys and the middle-aged animals were faster than the aged animals on the flat platform and the straight rod. Of note in all of these studies, the aged animals were not significantly different from young animals on successful retrieval of food rewards but differed on the length of time required to retrieve the food reward. Performance on this test is negatively correlated with age (Zhang et al. 2000; Lacreuse and Herndon 2003; Lacreuse et al. 2005, 2007).
The version of the Life Saver task used in the present study includes one horizontal L-shaped rod rather than the straight vertical rod or the rods with two or more curves. The rod used in this task requires less fine motor function of the hand and eliminates the cognitive component of the task. As would be predicted from the findings of other studies, aged animals are not impaired in retrieving the food reward from the straight horizontal rod compared to the performance of young animals. However, similar to other studies there was a significant liner correlation between age and time to retrieve the food reward from the horizontal L-shaped rod though all animals attained the same performance level by the final block of testing.
Effect of training
For the VMT task there was evidence that training impacted performance as shown by the lack of a group difference by the last block of testing days. Both young and aged animals showed improvement across the task and in fact the aged animals were performing at a similar level to the young animals by the last block. The lack of effect of age on the VMT may be related to extensive training on the HDT resulting in improved motor abilities in the aged animals allowing them to perform at similar levels to the young animals. This finding is consistent with studies that show improvements in motor function after stroke with regular physical and/or occupational therapy (French et al. 2007, 2008; Forster et al. 2009). Winstein et al. (1999) showed that repeated training on a motor task benefited both stroke patients and healthy controls on learning a goal directed programmed action and that the training consistently improved performance during acquisition and performance on the retention of the task. This notion supports the importance for rehabilitative therapy of the extremities not only in post-stroke individuals but also for older individuals experiencing declines in motor functions with age.
Application to assessment of motor system impairment
While the findings of this study are similar to the findings of other groups investigating age-related changes in motor function, our tasks vary in degree of complexity and therefore in the degree of fine motor function necessary for completion of different parts of each task. It has been demonstrated that age- related impairments in fine motor function are related to task complexity (Light and Spirduso 1990) and with increasing task difficulty, reaction time and response time of older individuals increases (Light and Spirduso 1990; Gorus et al. 2008; Ratcliff 2008; Sturnieks et al. 2008; Wild-Wall et al. 2009). This is supported by our finding that aged animals were impaired at retrieval of a food reward from all wells on the HDT except for the well requiring the least dexterity. Based on the literature and our findings, we can conclude that tasks that require varying degrees of levels of performance allow for the quantification of a broader range of intact and impaired performance in rhesus monkeys across the lifespan. In addition, the tasks used in this study, assessing a range of intact and impaired performance, will also be valuable for the assessment of fine motor impairments that can occur with age-related disease. They will be able to differentiate normal levels of performance, age-related impairments of motor function, and impairments related to specific disease processes. For example, these tasks could be used in non-human primate models of Parkinson’s disease and ischemic stroke, not only to assess the motor impairment that occurs with these diseases but to also differentiate this impairment from the level of impairment that is related to age alone. Further, the use of these tests requiring varying degrees of functionality could be applicable to the assessment of the efficacy of therapeutics and rehabilitation therapies on the enhancement of fine motor function of the hand in non-human primate models of aging and age-related disease.
Acknowledgements
The authors wish to thank Emily Hamlyn, Ana Amaral, Lindsay Schommer, and Penny Schultz for their invaluable assistance with this project. This research was supported by NIH-NIA grant P01- AG000001 and NIA grant R21AG028680–01A1.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. 1991. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging 12:99–111. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Coleman R, Reznick AZ. 2002. The biochemistry of aging muscle [Review]. Exp Gerontol 37(4):477–489. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Patish H, Coleman R. 2003. The aging hand [Review]. J Gerontol A Biol Sci Med Sci 58(2):146–152. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Hébert R, Bravo G, Rochette A. 1999. Age-related changes in upper extremity performance of elderly people: A longitudinal study. Exp Gerontol 34(3):393–405. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. 1998. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 401(2):253–65. [PubMed] [Google Scholar]
- Forster A, Lambley R, Hardy J, Young J, Smith J, Green J, Burns E. 2009. Rehabilitation for older people in long-term care [Review]. Cochrane Database Syst Rev (1):CD004294. [DOI] [PubMed] [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A, Langhorne P, Price CI, Walker A, Watkins CL. 2007. Repetitive task training for improving functional ability after stroke [Review]. Cochrane Database Syst Rev (4):CD006073. [DOI] [PubMed] [Google Scholar]
- French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, Langhorne P, Price C, Walker A, Watkins C. 2008. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness [Review]. Health Technol Assess 12(30):iii, ix,–x, 1–117. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Kasahara S, Tadayoshi T, Saito H, Yamanaka M. 2007. Behavioral findings during recovery after experimental stroke in monkeys—Assessment with modified hand performance test. J Phys Therapy Sci 19:33–40. [Google Scholar]
- Gash DM, Zhang Z, Umberger G, Mahood K, Smith M, Smith C, Gerhardt GA. 1999. An automated movement assessment panel for upper limb motor functions in rhesus monkeys and humans. J Neurosci Methods 89(2):111–117. [DOI] [PubMed] [Google Scholar]
- Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T. 2008. Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Geriatr Psychiatry Neurol 21(3):204–218. [DOI] [PubMed] [Google Scholar]
- Kallman DA, Plato CC, Tobin JD. 1990. The role of muscle loss in the age-related decline of grip strength: Cross-sectional and longitudinal perspectives. J Gerontol 45(3):M82–M88. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG. 2003. Effects of estradiol and aging on fine manual performance in female rhesus monkeys. Horm Behav 43(3):359–366. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Diehl MM, Goh MY, Hall MJ, Volk AM, Chhabra RK, Herndon JG. 2005. Sex differences in age-related motor slowing in the rhesus monkey: Behavioral and neuroimaging data. Neurobiol Aging 26(4):543–551. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Woods CE, Herndon JG. 2007. Effects of aging and hormonal status on bimanual motor coordination in the rhesus monkey. Neurobiol Aging 28(2):186–193. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. 2000. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23(4):600–612. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, HGJM Kuyper. 1968. The functional organization of the motor system in the monkey. The effects of bilateral pyramidal lesions. Brain 91:1–33. [DOI] [PubMed] [Google Scholar]
- Light KE, Spirduso WW. 1990. Effects of adult aging on the movement complexity factor of response programming. J Gerontol May 45(3):107–109. [DOI] [PubMed] [Google Scholar]
- Livshits G, Karasik D, Kobyliansky E. 2002. Complex segregation analysis of the radiographic phalanges bone mineral density and their age-related changes. J Bone Miner Res 17(1):152–161. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. 1985. Grip and pinch strength: Normative data for adults. Arch Phys Med Rehabil 66(2):69–74. [PubMed] [Google Scholar]
- Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. 1998. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano) 10(6):471–478. [DOI] [PubMed] [Google Scholar]
- Mittal KR, Logmani FH. 1987. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol 42(1):8–10. [DOI] [PubMed] [Google Scholar]
- Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. 2005. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke 36(7):1480–1484, Epub Jun 9. [DOI] [PubMed] [Google Scholar]
- Ranganathan VK, Siemionow V, Sahgal V, Yue GH. 2001. Effects of aging on hand function. J Am Geriatr Soc 49(11):1478–1484. [DOI] [PubMed] [Google Scholar]
- Ratcliff R 2008. Modeling aging effects on two-choice tasks: response signal and response time data. Psychol Aging 23(4):900–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman LM. 1992. Effects of aging on adult hand function [Review]. Am J Occup Ther 46(9):785–792. [DOI] [PubMed] [Google Scholar]
- Sturnieks DL, St George R, Fitzpatrick RC, Lord SR. 2008. Effects of spatial and nonspatial memory tasks on choice stepping reaction time in older people. J Gerontol A Biol Sci Med Sci Oct 63(10):1063–1068. [DOI] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. 1988. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol 15: 263–273. [DOI] [PubMed] [Google Scholar]
- Walton A, Branham A, Gash DM, Grondin R. 2006. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging 27(10):1477–1483. [DOI] [PubMed] [Google Scholar]
- Warabi T, Noda H, Kato T. 1986. Effect of aging on sensorimotor functions of eye and hand movements. Exp Neurol 92(3):686–697. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M, Serrien DJ. 2001. Neurological problems affecting hand dexterity [Review]. Brain Res Brain Res Rev Oct 36(2–3):161–168. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N, Willemssen R, Falkenstein M. 2009. Feedback- related processes during a time-production task in young and older adults. Clin Neurophysiol Feb 120(2):407–413, Epub (2008) Dec 23. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Merians AS, Sullivan KJ. 1999. Motor learning after unilateral brain damage. Neuropsychologia Jul 37(8): 975–987. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. 2000. Motor slowing and Parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol A Biol Sci 55(10): B473–B480. [DOI] [PubMed] [Google Scholar]