Abstract
Objective:
The contribution of individual immune response to Staphylococcus aureus bacteremia (SAB) on outcome has not been well studied. The objective was to relate the host cytokine response to outcome of SAB.
Design, Setting, and Participants:
Prospective observational study of adult patients infected with SAB hospitalized between July 2012 and August 2014 at three U.S. medical centers.
Exposure:
Blood specimens were obtained at SAB onset and 72h after therapy initiation. Levels of tissue necrosis factor (TNF) and interleukin-6 (IL-6), IL-8, IL-17A, and IL-10 were measured by ELISA at each time point and compared between those with persistent bacteremia (≥4d) (PB) and resolving bacteremia (RB). Primary outcome was PB after 4 days of effective therapy. Secondary outcomes were 30-day mortality and 30-day recurrence.
Measurements and Main Results:
A total of 196 patients were included (mean age 59y), of which 33% had MRSA bacteremia. 47% of the MRSA strains were SCCmec IV. PB occurred in 24% (47/196) of patients; they were more likely to die than RB group (28% vs. 5%, p<0.001). Compared to RB group, PB patients had higher initial median levels of TNF (44.73 vs. 21.68pg/ml, p<0.001), IL-8 (124.76 vs. 47.48pg/ml, p=0.028) and IL-10 (104.31 vs. 29.72pg/ml, p<0.001). Despite 72h of treatment, levels remained higher for PB than RB group (TNF: 26.95 vs. 18.38pg/ml, p=0.02; IL-8: 70.75 vs. 27.86pg/ml, p=0.002; IL-6: 67.50 vs. 21.81pg/ml, p=0.005; IL-10: 30.98 vs. 12.60 pg/ml, p<0.001). IL-17A levels were similar between groups at both time points. After controlling for confounding variables by multivariate analysis, IL-10/TNF ratio at 72h most significantly predicted persistence (OR 2.98, 95% CI 1.39–6.39, p=0.005) and mortality (OR 9.87, 95% CI 2.64–36.91; p<0.001) at values >1.00 and >2.56, respectively.
Conclusions:
Sustained elevation of IL-10/TNF ratio at 72h suggests a dysregulated immune response and may be used to guide management to improve outcomes.
Keywords: S. aureus, bacteremia, cytokines, persistence, mortality, immunoparalysis
INTRODUCTION
Staphylococcus aureus bacteremia (SAB) affects an estimated 50/100,000 people annually (1), with an overall mortality rate of 19%-57% in adults (2). Despite receipt of standard treatment, 1 in 3 patients experience persistent bacteremia (PB) in which growth of bacteria from the bloodstream persists beyond 7 days (3, 4). PB is significantly associated with metastatic complications, relapse, prolonged hospitalization, and increased mortality (4, 5). Adding to the challenge are the numerous reports of treatment failure in methicillin-resistant S. aureus (MRSA) associated with the development of resistance to the treatment standard (vancomycin) (6). To date, multiple studies have assessed both clinical and microbial variables that may be associated with PB (3–5). Reduced susceptibility to vancomycin, accessory gene regulator dysfunction, and lack of timely source control have been identified as risk factors for PB (4, 6, 7). However, studies have reported conflicting results with the risk factors identified so far, suggesting that differential host immune response may contribute to the heterogeneity in outcomes.
Recent studies have pointed to the patient’s immune response as a significant predictor of both persistence of infection and outcomes (8–11). Specifically in SAB, Rose et al. found that those who died had increased interleukin (IL)-10 levels at onset of infection, possibly conferring a state of “immunoparalysis” from an exaggerated anti-inflammatory response (8). The study did not evaluate the impact of treatment on host innate immune response. Elevated IL-10/TNF ratio at 48 h post-infection was predictive of mortality in a cohort of 65 patients with bacterial sepsis, of whom 7 had SA infections (12). The objective of our study was to establish a quantitative marker of host immune response to SAB that can identify patients at risk for poor outcomes, early during treatment to allow the clinician to make a prompt change in management approach. We assessed host immune response by measuring serum levels of pro-inflammatory [tissue necrosis factor (TNF), IL-6, IL-8, and IL-17A] and anti-inflammatory (IL-10) cytokines at onset of infection and at 72h following treatment. We hypothesize that an excess of anti-inflammatory to pro-inflammatory cytokine ratio early during S. aureus bacteremia is predictive of poor outcome.
MATERIALS AND METHODS
This was a prospective observational cohort study conducted at three university-affiliated medical centers (625-bed community, 411-bed academic, and 600-bed county) in Los Angeles County, CA, USA. The study protocol was approved by institutional review boards at each study site. Informed consent was waived as this was an observational study. Adult patients with SAB hospitalized between July 2012 and August 2014 were screened for eligibility. Patients were identified using the Microbiology Laboratory computer records for growth of S. aureus from a blood specimen. For patients with multiple admissions during the study period, only the initial admission was included. Patients were excluded if they had: polymicrobial growth from blood culture, unavailable medical charts, received ≤ 48h of effective antibiotic therapy, or delayed initiation (≥ 48h) of effective therapy from time of initial positive blood culture.
Bacterial Isolates
S. aureus isolates from blood cultures were collected and stored at -80°C until later testing. Antimicrobial susceptibility testing was performed by the Vitek System (bioMérieux, Durham, NC) and by Etest (bioMérieux) for vancomycin. Results were interpreted following CLSI guidelines (13). Bacterial isolates were cultured overnight in tryptic soy broth at 37°C. The staphylococcal cassette chromosome (SCC) mec type was determined on the initial isolate from each patient by polymerase chain reaction assay using previously published methods (14, 15).
Cytokine Analysis
Plasma or serum samples were collected from specimens drawn for routine labs once physician-ordered tests were completed. Samples were collected at onset of SAB (within 24h of the index positive blood culture) and at 72h after starting effective antibiotic therapy and stored at -80°C until analysis. Cytokine concentrations were determined by Luminex® multiplex assay according to manufacturer’s instructions (Millipore, Billerica, MA) for pro-inflammatory cytokines: TNF, IL-6, IL-8, IL-17A, and anti-inflammatory cytokine: IL-10. Individual ratio of IL-10 to each pro-inflammatory mediator was calculated for each patient at both time points. Samples were allowed to thaw to room temperature only once before testing. All assays were performed in duplicate.
Data Collection
Patients’ medical records were reviewed for pertinent clinical information: age, gender, comorbid conditions, presence of hardware, residence prior to admission, intravenous (IV) drug use history, history of S. aureus infection or vancomycin use (within 6 months), history of prior hardware infection, admission to the intensive care unit (ICU), daily vital signs, clinical response, white blood cell count, culture and sensitivities, echocardiographic findings, surgical interventions, infectious disease consultation. In addition, duration of intensive care and hospital stay, and 30-day mortality were recorded. Comorbid conditions included diabetes mellitus, cardiovascular, renal insufficiency (serum creatinine >2 mg/dL or 34.2 mmol/L), requirement for hemodialysis, and immunosuppression (receipt of chemotherapy or other immunosuppressive drugs). The Pitt bacteremia score (PBS), an illness severity scoring system previously validated in SAB (16), was calculated at onset of bacteremia based on the following variables: temperature, blood pressure, need for mechanical ventilation, cardiac arrest, and mental status. Details of antimicrobial therapy (agent, dose, duration, and serum trough levels for vancomycin) during hospital course were recorded. Study data were managed using the REDCap(17) electronic data capture software hosted at the University of Southern California.
Study Definitions
The source of bacteremia was divided into 3 categories relative to risk of mortality: low (<10%), intermediate (10–20%), and high (>20%) as previously defined (5). Sources of infection considered low risk were IV catheters, urinary tract infection, ear-nose-larynx, gynecologic, and several manipulation-related sources; intermediate risk were osteoarticular, soft-tissue, and unknown sources; and high risk were endovascular, low respiratory tract, intra-abdominal, and central nervous system foci. Antibiotic therapy was considered effective if sensitivity was documented. Early response was evaluated on day 4, 72–96 h after initiation of effective antibiotic therapy. Recurrence was defined as growth of S. aureus from the blood within 30 days of completion of effective antibiotic therapy.
Data Analysis
The primary outcome was response at day 4 after initiation of effective therapy, dichotomized as persistent bacteremia (PB) (positive blood culture despite effective treatment) or resolution (partial or complete clinical response with negative blood culture). Clinical response was based on resolution of fever, leukocytosis, local signs of infection, return of abnormal vital signs and altered mental status to baseline. Secondary outcomes were 30-day all-cause mortality and recurrence of SAB. Day 4 outcome groups were compared on host and microbiologic characteristics as well as cytokine profile. Univariate analysis was performed using Wilcoxon rank sum tests or Student t-test for continuous data and Fisher’s exact or chi-square test for categorical data where appropriate. Receiver operating characteristics (ROC) analysis was performed to determine the cytokine cutoff values that maximized sensitivity and specificity in the prediction of PB and 30-day mortality. Multivariate logistic regression analysis was conducted to determine independent predictors of each outcome after controlling for age and gender and adjusting for confounding variables such as infection source risk category, immunosuppression, and ICU admission. All statistical tests were 2-tailed and p<0.05 was considered significant. Statistical analyses were performed using GraphPad Prism v4.0 (San Diego, CA, USA) or SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Population
A total of 240 adult hospitalized patients with positive blood cultures for S. aureus were screened. Of those, 196 patients met inclusion criteria. Patients were excluded for the following: 17 received ≤48h of effective therapy, 5 received therapy ≤ 48h after initial positive blood culture, 10 had polymicrobial bacteremia, and 12 had unavailable medical charts. Study patients had mean (SD) age of 59 (6.6) years; more than half (61%) were male. Nosocomial SAB (onset >48h after admission) occurred in 14% of patients. PB on Day 4 after start of effective therapy occurred in 24% (47/196) of patients. Characteristics of patients were compared between those who had resolving vs PB on Day 4 (Table 1). The study groups did not differ on age, gender, comorbid conditions, history of S. aureus infection, prior vancomycin use, or presence of infected hardware. The most common infection source in the study cohort was skin and soft tissue infection (18%), followed by unknown source (14%), dialysis catheter (13%), line infection (11%), and pneumonia (8%). The majority of patients who died (52%, 101/196) had infection sources associated with intermediate risk of death. Notably, the median Pitt bacteremia score, with a possible range of 0 to 14 points, was low in both groups (1 point); however, twice as many patients with PB required ICU admission during bacteremia (51% vs 28%, p<0.01).
Table 1.
Comparison of Characteristics of Study Patients
| Characteristic | Resolving SAB (n=149) |
Persistent SAB (n=47) |
P value |
|---|---|---|---|
| Age, yearsa | 59.07 ± 16.58 | 61.26 ± 16.74 | 0.43 |
| Maleb | 93 (62%) | 26 (55%) | 0.40 |
| Residence PTA (Home)b | 105 (71%) | 36 (77%) | 0.46 |
| Comorbid Conditions | |||
| Dialysisb | 40 (27%) | 15 (32%) | 0.57 |
| Intravenous Drug Usec | 14 (9%) | 3 (6%) | 0.77 |
| Diabetes mellitusb | 58 (39%) | 23 (49%) | 0.24 |
| Cardiovascular diseaseb | 93 (62%) | 34 (72%) | 0.23 |
| Immunosuppressedb | 19 (13%) | 3 (6%) | 0.30 |
| Prior History of SA Infectionb, d | 15 (10%) | 6 (13%) | 0.59 |
| History of prior Vancomycin useb, d | 28 (19%) | 7 (15%) | 0.66 |
| Pitt Bacteremia Scoree | 1 (0, 2) | 1 (0, 4) | 0.23 |
| ICU stay during SABb | 41 (28%) | 24 (51%) | <0.01 |
| Source of SA Bacteremiab, f | |||
| Low Risk | 41 (28%) | 10 (21%) | 0.69 |
| Intermediate Risk | 75 (50%) | 26 (55%) | - |
| High Risk | 33 (22%) | 11 (23%) | - |
| Infected Hardwarec, g | 40 (27%) | 11 (23%) | 0.71 |
Abbreviations: PTA, prior to admission; SA, S. aureus;
mean ± standard deviation reported, Student’s t-test;
Chi- squared test;
Fisher's exact test;
within 6 months of 1st positive culture;
median (IQR) reported, Wilcoxon rank sum test;
Based on mortality risk (low <10%: intravenous catheter, urinary tract, ear-nose- larynx, gynecologic sources, and manipulation-related sources, 10–20%: osteoarticular, soft-tissue, and unknown sources, >20%: endovascular, lower respiratory tract, and abdominal sources, and CNS foci) (5);
Cardiac device, non-cardiac device, dialysis catheter, prosthetic hardware
Pathogen Characteristics
Methicillin-resistant S. aureus (MRSA) accounted for 33% of the bacteremic cases (64/196). Of those, 47% of the isolates had SCCmec IV type. No difference in Day 4 response was observed based on methicillin sensitivity of the infected S. aureus strain (34% [50/149] of patients with resolving bacteremia vs. 30% [14/47] of patients with persistent bacteremia; p=0.72) or mec type (SCCmec IV or not), 44% [19/43] resolving vs. 58% [7/12] persistent; p=0.52). Nearly all initial S. aureus isolates had vancomycin minimal inhibitory concentration (MIC) >1 ug/mL by Etest (44% had 1.5 ug/mL; 41% had 2 ug/mL); none had MIC >2 ug/mL.
Management of SAB and Outcome
Management of SAB was similar between those who had resolution vs PB on day 4 with respect to time to receipt of effective therapy and hardware removal. (Table 2) Infected hardware was removed within the first 4 days of onset of bacteremia with a trend towards earlier removal in the resolving group (median 2d vs 4d, p=0.06). Most patients received vancomycin-containing regimens empirically in both the persistent and resolving group (70% vs. 82%, p=0.10) (Table 2). Average overall vancomycin trough level was a median of 11.70 vs 11.95 mg/dL (p=0.32) and did not differ between groups.
Table 2.
Management and Outcomes of S. aureus bacteremia by Day 4 Outcome
| Characteristic | Resolving SAB (n=149) | Persistent SAB (n=47) | P value |
|---|---|---|---|
| Management | |||
| Received ID consult a | 77 (52%) | 34 (72%) | 0.02 |
| Time to receive consultation (days) b, c | 2 (1,4) | 1 (1,2) | 0.05 |
| Consult received prior to day 4 a, b | 67 (87%) | 29 (85%) | 0.77 |
| Time to receipt of effective Anti-SA therapy (days) c | 0 (0, 1) | 0 (0, 1) | 0.16 |
| Empiric Antibiotic therapy a | |||
| Vancomycin | 122 (82%) | 33 (70%) | 0.10 |
| Other anti-Staphylococcal | 27 (18%)f | 14 (30%)g | 0.10 |
| Hardware Removed a, d | 34 (85%) | 9 (90%) | 1.00 |
| Time to Hardware removal (days) c, d | 2 (1, 3.75) | 4 (2.5, 5) | 0.06 |
| Outcomes | |||
| 30-day mortality a | 7 (5%) | 13 (28%) | <0.0001 |
| 30-day recurrence a, e | 8 (6%) | 1 (3%) | 1.00 |
| Length of hospital stay (days)c, e | 10 (6, 17) | 16.5 (10.8,25) | 0.01 |
Abbreviations: ID, Infectious diseases; SA, S. aureus; MRSA, Methicillin-resistant S. aureus; MSSA, Methicillin-susceptible S. aureus;
Chi- squared test;
Of those who received ID consult;
median (IQR) reported, Wilcoxon rank sum test;
Of those with infected hardware (n=40, n=10);
Calculated from surviving patients only;
linezolid, clindamycin, trimethoprim/sulfamethoxazole, daptomycin, ceftriaxone, oxacillin, cefazolin, other;
clindaymycin, linezolid, daptomycin, ceftriaxone, cefazolin
Of the forty seven patients (24%) in the study cohort who had PB on day 4, 28% died; in contrast, less than 5% died in the resolving group (p<0.0001) (Table 2). The majority of those who survived in both groups were discharged home (61%, 86/142 vs 53%, 18/34; p=0.44). Among the survivors, recurrence of SAB within 30 days of completing therapy occurred in 8 patients in the resolving group and 1 in persistent group.
Host cytokine profile and outcome analysis
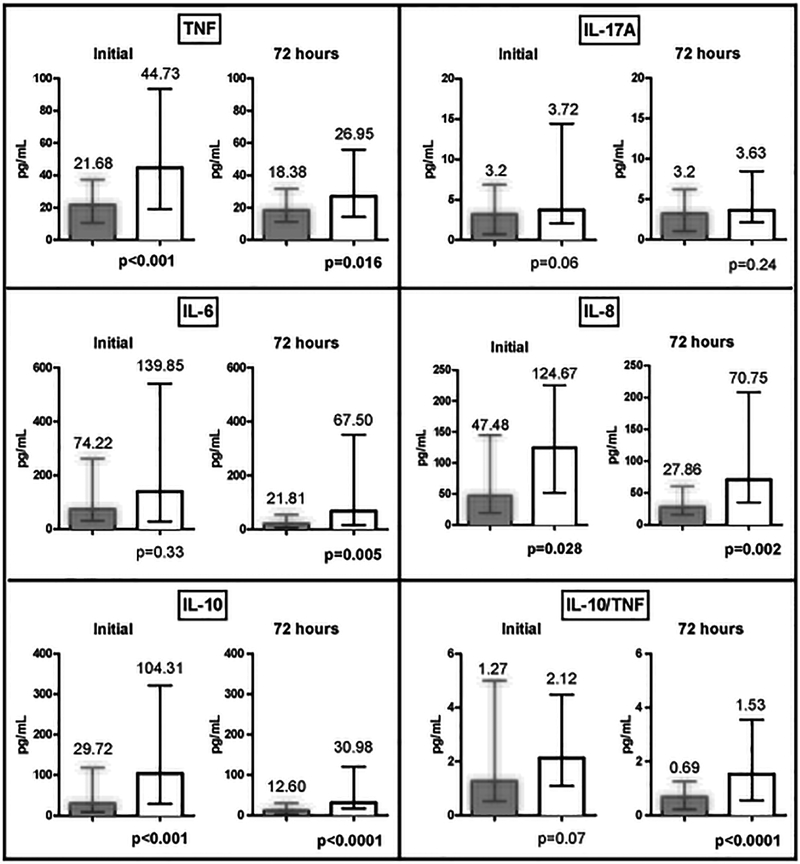
Pro-inflammatory and anti-inflammatory cytokine levels were higher in all patients at onset of SAB relative to the 72h time point post-treatment initiation (Figure 1). Notably, at initial presentation, the persistent group had higher pro-inflammatory response with significantly higher median TNF level (44.73 vs. 21.68 pg/mL, p<0.001), IL-6 (139.85 vs 74.22 pg/ml, p=0.33), and IL-8 (124.67 vs 47.48 pg/ml, p=0.028) compared to those with early resolution. Similarly, the persistent group had a 3.5 fold higher anti-inflammatory cytokine level (IL-10) vs the resolving group (median 104.31 vs. 29.72 pg/mL, p<0.001) at initial presentation. (Figure 1) As expected, after receipt of 72h of effective therapy, both the pro-inflammatory and anti-inflammatory cytokine levels decreased in both groups, yet all cytokine levels remained significantly higher in the persistent compared to the resolving group (median values): TNF (26.95 vs 18.38 pg/ml, p=0.016), IL-6 (67.50 vs 21.81 pg/ml, p=0.005), IL-8 (70.75 vs 27.86 pg/ml, p=0.002), IL-10 (30.98 vs 12.6 pg/ml, p<0.001). IL-17A levels did not significantly differ between the groups at both time points. When the balance of host cytokine response based on IL-10 to TNF ratio was examined for individual patients, those with PB at day 4 showed a predominance of anti-inflammatory response (IL-10/TNF ratio: 1.53 vs. 0.69, p<0.001).
Figure 1: Cytokine Profile at Initial Presentation of Bacteremia and 72 Hours After Receiving Effective Treatment Grouped by Day 4 Outcome.
Abbreviations: IL, interleukin; TNF, tumor necrosis factor; Median reported, error bars represent IQR, Wilcoxon rank sum test. Dark grey bars represent resolving SAB group (TNF initial n=138; TNF 72 hour n=146; IL-6 initial n=94; IL-6 72 hour n=100; IL-8 initial n=94; IL-8 72 hour n=104; IL-17A initial n=121; IL-17A 72 hour n=118; IL-10 initial n=131; IL-10 72 hour n=132). White bars represent persistent SAB group (TNF initial n=46; TNF 72 hour n=43; IL-6 initial n=25; IL-6 72 hour n=26; IL-8 initial n=26; IL-8 72 hour n=27; IL-17A initial n= 44; IL-17A 72 hour n= 41; IL-10 initial n= 45; IL-10 72 hour n= 42).
Similar findings were observed when host cytokine profile was analyzed in relation to 30-day mortality. Compared to survivors, those who died had a much more robust cytokine storm with higher initial cytokine levels (median values): TNF (65.80 vs 22.60 pg/mL, p<0.001), IL-8 (130.3 vs 58.50 pg/ml, p=0.043), and IL-10 (159.50 vs 30.80 pg/mL, p=0.002). Initial IL-6 (192.70 vs 75.90 pg/ml, p=0.096) and IL-17A (3.61 vs 3.30 pg/ml, p=0.55) levels were not significantly different. Even after 72h of effective therapy, those who died continued to have significantly higher pro-and anti-inflammatory levels than survivors: TNF (34.42 vs 18.56 pg/ml, p=0.002), IL-8 (112.10 vs 31.92 pg/ml, p=0.002), IL-6 (147.60 vs 22.11 pg/ml, p=0.002) and IL-10 (109.00 vs 14.81 pg/ml, p<0.001). Similarly, IL-10/TNF ratio at 72h was 4.3-fold higher in the non-survivors (3.10 vs. 0.70, p<0.001). Comparing the change in cytokine levels from initial time point to 72h after therapy in each patient, those who died vs. survivors had a significantly greater decrease in TNF levels (median (IQR): 17.55 (1.58, 88.21) pg/ml vs 2.47 (-4.65, 12.02) pg/ml, p=0.007; percent change: 44% vs 14%, p=0.015) but lower magnitude of decrease in IL-10 levels (median (IQR): 30.64 (-79.37, 111.3) pg/ml vs 12 (0, 108.8) pg/ml, p=0.51; percent change: 31% vs 60%, p=0.09) resulting in an overall 1% increase in IL-10/TNF ratio vs 50% decrease among survivors.
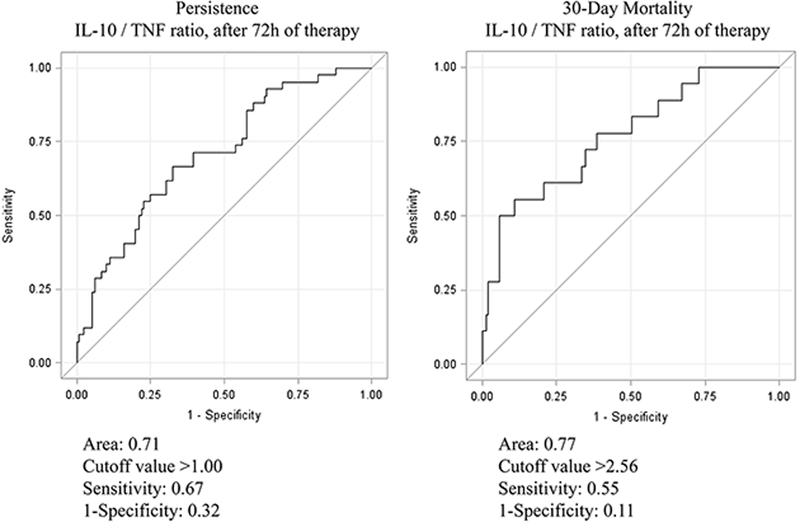
By univariate logistic regression, all cytokines measured in the study at onset and at 72h were analyzed (by themselves and as a ratio to IL-10) in relation to persistence or resolution of bacteremia at day 4 after initiation of effective therapy. IL-10/TNF ratio at 72h was found to be most significantly associated with day 4 outcome; the others did not significantly correlate with persistence and thus were not included in the multivariate analysis (IL-10/IL-8 [OR 1.07 (0.89–1.29), p=0.48]; IL-10/IL-6 [OR 0.92 (0.75–1.14), p=0.45)]; and IL-10/IL-17A [OR 1 (0.99–1.00), p=0.87]). ROC analysis was performed to determine the cutoff values for IL-10/TNF ratio after 72h of therapy that predicted the development of persistent bacteremia and 30-day mortality. The cutoff values for IL-10/TNF ratio were >1.00 (Sensitivity, 1-specificity: 0.67, 0.32) for persistent bacteremia and >2.56 (Sensitivity, 1-specificity: 0.55, 0.11) for 30-day mortality. (Figure 2) Of note, 42% (66/156) of the study cohort had elevated initial IL-10/TNF levels >1.00 but less than half of those (48%, 32/66) had sustained elevation at 72h meeting the IL-10/TNF ratio cutoff value (>1.00) predictive of PB. Conversely, among the 58% (90/156) of the study cohort whose initial IL-10/TNF ratio was below the cutoff value of 1.00 predictive of persistence, 5 patients had the ratio increase past the cutoff value at 72h after treatment was initiated. Additionally, the cutoff values for IL-10/TNF ratio were included in a multivariate logistic regression analysis to identify independent predictors of persistent bacteremia and mortality controlling for potential confounders (age, gender, risk of death by source of infection, immunosuppression, ICU admission). (Table 3) Elevated IL-10/TNF ratio at 72h of therapy above the cutoff values was the most significant predictor of both persistent bacteremia (OR 2.98, 95% CI 1.39–6.39) and mortality (OR 9.87, 95% CI 2.64–36.91).
Figure 2:
ROC analysis of IL-10/TNF Ratio at 72 hours of Therapy in Relation to Day 4 Outcomes
Table 3.
Multivariate Logistic Regression model With Independent Predictors of Day 4 Persistence and Mortality
| Variable | Persistencec OR (95% CI) | p-value | Mortalityd OR (95% CI) | p-value |
|---|---|---|---|---|
| 72h IL-10/TNF ratioa | 2.98 (1.39 − 6.39) | 0.005 | 9.87 (2.64 − 36.91) | <0.001 |
| Source risk categoryb | ||||
| High vs. Low Risk | 0.83 (0.27 − 2.59) | 0.64 | 0.62 (0.12 − 3.12) | 0.91 |
| Intermediate vs. Low | 1.08 (0.41 − 2.87) | 0.67 | 0.32 (0.07 − 1.56) | 0.17 |
| Age | 1.01 (0.98 − 1.03) | 0.68 | 1.02 (0.98 − 1.07) | 0.29 |
| Female | 1.22 (0.56 − 2.64) | 0.62 | 3.09 (0.86 − 11.15) | 0.09 |
| Immunosuppressed | 0.31 (0.06 − 1.54) | 0.15 | 0.76 (0.12 − 4.88) | 0.77 |
| ICU admission | 2.87 (1.33 − 6.18) | <0.01 | 9.48 (2.39 − 37.62) | <0.01 |
Abbreviations: CI, confidence interval; IL, interleukin; TNF, tumor necrosis factor; OR, odds
ratio
IL 10/TNF ratio cutoff value used in model was 1.00 for persistence and 2.56 for mortality;
Based on mortality risk (low <10%: intravenous catheter, urinary tract, ear-nose- larynx, gynecologic sources, and manipulation-related sources, 10–20%: osteoarticular, soft-tissue, and unknown sources, >20%: endovascular, lower respiratory tract, and abdominal sources, and CNS foci) (5);
r2 = 0.19;
r2 = 0.42
DISCUSSION
Staphylococcus aureus bacteremia presents a significant challenge to clinicians. SAB affects a large population, and persistence of infection despite receipt of effective therapy occurs in 20%-40% of patients (18). Prior studies have identified several microbial and clinical characteristics associated with PB but such findings are inconsistent (3, 19–21). Specifically, some investigators have found that susceptible strains with high vancomycin MIC or hVISA phenotype are associated with PB while others did not find these associations (4, 16, 22). These inconsistent findings point to the need to better characterize the host immune response during infection in order to address the individual heterogeneity in observed outcome.
Our study is the largest study to date examining the relationship between host cytokine response at onset and at 72h following initial treatment and outcomes of SAB. Our study findings are consistent with those from a recently published study (8) which showed that elevated IL-10 level at initial presentation was associated with increased mortality. This relationship has been observed with other infections, such as candidemia (23), meningococcal disease (24), and Bartonella quintana bacteremia (25) in which an initial elevation of IL-10 was found to be associated with poor outcomes. An excess of the anti-inflammatory mediators is thought to result in a state of “immunosuppression” or “immunoparalysis” which thwarts the host’s ability to clear the primary infection and leads to the development of secondary infections. (26) Additionally, we observed that elevated initial TNF and IL-8 levels were also associated with poor outcomes.
We are the first to examine the impact of treatment on host immune response during SAB by investigating changes in cytokine levels at two time points early during the course of disease, at onset and at 72h after initiation of treatment, to allow for a prompt change in management approach if necessary. Our data support the 72h time point measurement in that initial cytokine levels as well as the balance of pro-inflammatory/anti-inflammatory cytokines changed in a bidirectional manner by Day 4 following initial treatment. Additionally, our data provide a biological explanation with respect to host immune response, corroborating earlier findings from our group that lack of early clinical response to treatment assessed at 72h is predictive of eventual treatment failure in patients receiving vancomycin therapy for SAB. (22)
Pro- and anti-inflammatory cytokine responses assessed on day 4 were significantly larger in persistent versus resolving bacteremia. Specifically, serum levels of TNF, IL-6, IL-8, and IL-10 levels were significantly elevated after 72h of therapy in the PB group. IL-17A is an important mediator of inflammation for neutrophil recruitment; however, it did not appear to differ between those with persistent versus resolving bacteremia in our population. Previous studies have shown that elevated IL-6 correlated with severity (27) and death (28) in sepsis. However these studies included patients with a variety of infection types and organisms. Similarly, IL-8 was shown to be significantly elevated in those with severe sepsis and was evaluated for its potential to be used as a clinical marker for sepsis. (29) While both IL-6 and IL-8 were found to be significantly elevated in our study, IL-10 had the strongest association with risk of PB and death. More importantly, we found that the ratio of anti-inflammatory to pro-inflammatory cytokines (IL-10/TNF) at 72h signifying an “immunoparalysis” state is the strongest predictor of early response after adjusting for confounding factors. Our findings are consistent with previously published literature which showed that non-survivors with severe sepsis due to various infection types had significantly higher IL10/TNF ratio than survivors.(12) This “immunoparalysis” state provides a biological explanation for the poor response observed in the subset of patients with SAB despite receipt of appropriate antimicrobial therapy. As the 72h cytokine profile points to the host immune response as a key determinant of eventual outcomes, this time point provides a quantifiable indicator that can help clinicians with treatment decisions early on during the course of therapy to positively affect outcome. The cutoff values determined for IL-10/TNF ratio at 72h predictive of PB on day 4 and 30-day mortality are >1.00 and >2.56, respectively, which should be validated in future studies. This elevation in IL-10/TNF ratio at 72h may be a marker indicative of a sustained dysregulated balance in host-specific immune response tipping towards a state of “immunoparalysis” despite effective antibiotic treatment.
Our study has several limitations. Due to its observational design, confounding factors were not matched for and management of infection was at the discretion of the treating physician. It is notable that potential confounders such as age, SAB source risk, and Pitt bacteremia score were evenly distributed in both persistent and resolving infection groups. We also adjusted for other potential confounding variables including gender, immunosuppression, and ICU admission in a multivariate regression model; IL-10/TNF ratio at 72h was the most significant covariate associated with PB even after adjustment. We acknowledge that a limited panel of cytokines was analyzed, in which the most robust markers were chosen based on previous published work from in vitro, experimental, and human studies. Plans to include a broader panel of cytokines for future investigations are underway.
CONCLUSIONS
Staphylococcus aureus bacteremia is associated with significant resource utilization and mortality. In this study, about 24% of patients with SAB developed persistent bacteremia despite receipt of “effective” therapy selected based on in vitro susceptibility testing. Of those, 50% required ICU admission and 28% died within 30 days of onset. While multiple factors (i.e. comorbid conditions, source control, drug dosing) contribute to persistence and poor outcomes, there is a lack of early markers predictive of poor outcome that clinicians can use to guide management approach at present. Here, we have shown promise with the measurement of host cytokine response early during treatment to identify those at risk for infection persistence and death. We focused on the measurement of cytokines for which there are standardized immunoassays currently performed in specialty labs that can be readily incorporated into routine bedside testing. Our results deserve confirmation in prospective studies to validate the cutpoints identified for the immune biomarkers from this study and to examine the effectiveness of immune biomarker-guided management of SAB. Future studies should determine the underlying mechanisms responsible for the dysregulated balance of cytokine response and design a target-specific intervention necessary to affect a positive outcome.
ACKNOWLEDGMENTS
We thank Anne Au, Fatima Vasquez-Beltran, Consuelo Hernandez, and Joan Hsu from Clinical Laboratory at Huntington Hospital for retrieving blood specimens; Nadine Musallam, Priyanka Rughwani, Edward Brauer, Joanna Wu, and Chong Fang for assisting with data collection; bioMerieux for providing Etests for MIC testing; USC-Norris Immune Monitoring Core Facility for technical assistance in cytokine measurements.
No financial support to disclose.
Copyright form disclosures: Dr. Wong-Beringer lectured for Cubist Pharmaceuticals. Her institution received grant support from Cubist Pharmaceuticals, Forest Pharmaceuticals, and Durata Pharmaceuticals. Dr. Ho received other support from the University of Southern California (Received 2013 Dean's Summer Research Fellowship from own institution). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No conflicts of interest for all authors.
Institutions where work was done: Huntington Hospital; Los Angeles County-University of Southern California Medical Center; Keck Hospital of University of Southern California
Contributor Information
Emi Minejima, University of Southern California, Los Angeles, 90089, USA.
Joyce Bensman, University of Southern California, Los Angeles, 90089, USA.
Rosemary C. She, Department of Pathology, Keck School of Medicine, Los Angeles, 90089, USA
Wendy J. Mack, Department of Preventative Medicine, Keck School of Medicine, Los Angeles, 90089, USA
Martin Tuan Tran, University of Southern California, Los Angeles, 90089, USA.
Pamela Ny, Huntington Hospital, Pasadena, 91105, USA.
Mimi Lou, University of Southern California, Los Angeles, 90089, USA.
Jason Yamaki, University of Southern California, Los Angeles, 90089, USA.
Paul Nieberg, Department of Medicine, Huntington Hospital, Pasadena, 91105, USA
Joyce Ho, University of Southern California, Los Angeles, 90089, USA.
Annie Wong-Beringer, University of Southern California, Los Angeles, 90089, USA.
REFERENCES
- 1.van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 2012;25(2):362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamagni TL, Potz N, Powell D, et al. Mortality in patients with meticillin-resistant Staphylococcus aureus bacteraemia, England 2004–2005. J Hosp Infect 2011;77(1):16–20. [DOI] [PubMed] [Google Scholar]
- 3.Yoon YK, Kim JY, Park DW, et al. Predictors of persistent methicillin-resistant Staphylococcus aureus bacteraemia in patients treated with vancomycin. J Antimicrob Chemother 2010;65(5):1015–1018. [DOI] [PubMed] [Google Scholar]
- 4.Howden BP, Johnson PD, Ward PB, et al. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006;50(9):3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008;46(2):193–200. [DOI] [PubMed] [Google Scholar]
- 6.Sakoulas G, Moellering RC, Eliopoulos GM. Adaptation of methicillin-resistant Staphylococcus aureus in the face of vancomycin therapy. Clin Infect Dis 2006;42 Suppl 1:S40–50. [DOI] [PubMed] [Google Scholar]
- 7.Chong YP, Park SJ, Kim HS, et al. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine (Baltimore) 2013;92(2):98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012;206(10):1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adhikari RP, Ajao AO, Aman MJ, et al. Lower antibody levels to Staphylococcus aureus exotoxins are associated with sepsis in hospitalized adults with invasive S. aureus infections. J Infect Dis 2012;206(6):915–923. [DOI] [PubMed] [Google Scholar]
- 10.Verkaik NJ, de Vogel CP, Boelens HA, et al. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J Infect Dis 2009;199(5):625–632. [DOI] [PubMed] [Google Scholar]
- 11.Johansson D, Shannon O, Rasmussen M. Platelet and neutrophil responses to Gram positive pathogens in patients with bacteremic infection. PLoS One 2011;6(11):e26928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181(1):176–180. [DOI] [PubMed] [Google Scholar]
- 13.Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. In. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 14.Bonnstetter KK, Wolter DJ, Tenover FC, et al. Rapid multiplex PCR assay for identification of USA300 community-associated methicillin-resistant Staphylococcus aureus isolates. J Clin Microbiol 2007;45(1):141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boye K, Bartels MD, Andersen IS, et al. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I-V. Clin Microbiol Infect 2007;13(7):725–727. [DOI] [PubMed] [Google Scholar]
- 16.Hill PC, Birch M, Chambers S, et al. Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001;31(2):97–103. [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis 2006;38(1):7–14. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins C, Huang J, Jin N, et al. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 2007;167(17):1861–1867. [DOI] [PubMed] [Google Scholar]
- 20.Lalani T, Federspiel JJ, Boucher HW, et al. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol 2008;46(9):2890–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong YQ, Fowler VG, Yeaman MR, et al. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 2009;199(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo J, Yamaki J, Lou M, et al. Early response assessment to guide management of methicillin-resistant Staphylococcus aureus bloodstream infections with vancomycin therapy. Clin Ther 2013;35(7):995–1004. [DOI] [PubMed] [Google Scholar]
- 23.Johnson MD, Plantinga TS, van de Vosse E, et al. Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study. Clin Infect Dis 2012;54(4):502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann AK, Halstensen A, Sørnes S, et al. High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun 1995;63(6):2109–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capo C, Amirayan-Chevillard N, Brouqui P, et al. Bartonella quintana bacteremia and overproduction of interleukin-10: model of bacterial persistence in homeless people. J Infect Dis 2003;187(5):837–844. [DOI] [PubMed] [Google Scholar]
- 26.Monneret G, Venet F, Pachot A, et al. Monitoring immune dysfunctions in the septic patient: a new skin for the old ceremony. Mol Med 2008;14(1–2):64–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 1992;215(4):356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jekarl DW, Lee SY, Lee J, et al. Procalcitonin as a diagnostic marker and IL-6 as a prognostic marker for sepsis. Diagn Microbiol Infect Dis 2013;75(4):342–347. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald SP, Stone SF, Neil CL, et al. Sustained elevation of resistin, NGAL and IL-8 are associated with severe sepsis/septic shock in the emergency department. PLoS One 2014;9(10):e110678. [DOI] [PMC free article] [PubMed] [Google Scholar]