Abstract
Atherosclerosis is an inflammatory arterial pathogenic condition, which leads to ischemic cardiovascular diseases, such as coronary artery disease and myocardial infarction, stroke, and peripheral arterial disease. Atherosclerosis is a multifactorial disorder and its pathophysiology is highly complex. Changes in expression of multiple genes coupled with environmental and lifestyle factors initiate cascades of adverse events involving multiple types of cells (e.g. vascular endothelial cells, smooth muscle cells, and macrophages). IGF-1 is a pleiotropic factor, which is found in the circulation (endocrine IGF-1) and is also produced locally in arteries (endothelial cells and smooth muscle cells). IGF-1 exerts a variety of effects on these cell types in the context of the pathogenesis of atherosclerosis. In fact, there is an increasing body of evidence suggesting that IGF-1 has beneficial effects on the biology of atherosclerosis. This review will discuss recent findings relating to clinical investigations on the relation between IGF-1 and cardiovascular disease and basic research using animal models of atherosclerosis that have elucidated some of the mechanisms underlying atheroprotective effects of IGF-1.
1. Introduction
<Mechanisms of Atherosclerosis: Updates>
Atherosclerosis is a pathogenic condition characterized by the focal inflammatory thickening of arterial walls. It is the primary cause of cardiovascular diseases (CVDs), such as ischemic heart disease, stroke, and peripheral artery diseases. As CVDs are the leading cause of death worldwide [1], there have been significant continuing efforts to develop therapeutic strategies directly addressing atherosclerotic lesions and preventing adverse events. Nonetheless, development of new drugs has been challenging [2], and current options for medical treatment are still restricted to preventative lifestyle changes, lipid lowering therapy and control of risk factors such as hypertension and glycemic control.
Atherosclerosis is a multifactorial disease [3–5] and our understanding of its pathogenesis has advanced significantly over the past decade. By the early 2000s, major working hypotheses proposed as mechanisms of atherogenesis included the “response-to-retention hypothesis” [6], and the “oxidative modification hypothesis” [6]. These hypotheses stated that lesions are initiated when there is subendothelial retention of lipids (low-density lipoproteins) which are modified (e.g. aggregated, oxidized) to be biologically active. Modified lipids elicit subintimal infiltration of macrophages, which scavenge modified lipids to become lipid-laden macrophages (i.e. foam cells), thereby establishing an early lesion (i.e. fatty streak). Modified lipids and chemokines/cytokines from pro-inflammatory cells induce de-differentiation and migration of medial smooth muscle cells (SMCs) into the intima. The undifferentiated SMCs (“synthetic phenotype” SMCs, as opposed to differentiated “contractile phenotype” SMCs) proliferate and deposit matrix proteins leading to neointimal thickening. Under highly inflammatory and oxidative conditions, macrophages and SMCs may undergo cell death, resulting in necrotic core formation and ultimately causing plaque vulnerability.
The concept of “endothelial dysfunction” that precedes atherosclerosis development is now widely accepted [7] and further substantiated by recent findings. In addition to its classical roles in vasomotor activity, thrombosis and fibrinolysis, blood-tissue exchange, and angiogenesis, the endothelium is now recognized as a regulatory hub orchestrating vascular homeostasis [8], as a sensor and principal mediator of fluid shear stress to the arterial wall [8–10], as a regulator of proinflammatory cell recruitment and invasion [8, 11, 12], and as an integral component of mechanisms of arterial stiffness [13, 14].
New insights are also emerging about the potential roles of SMCs in atherosclerosis. The term “phenotypic switch of SMCs” used to refer to a shift of SMC phenotype from fully differentiated “contractile” state to de-differentiated “synthetic” state. However rigorous investigations in the past decade revealed that the fate of arterial SMCs under the atherogenic microenvironment is more diverse, ranging from a mesenchymal stem cell-like phenotype to a macrophage-like phenotype [15]. More than 80% of SMC-derived cells in advanced plaques do not express some or all of SMC-markers but express markers of mesenchymal stem cells [16] or even macrophages [16–18]. Vice versa, myeloid cells can express SMC-markers [19, 20]. 10–15% of α-smooth muscle actin-positive cells within an advanced plaque are derived from myeloid cells [21]. Macrophage-like cells derived from SMCs are highly pro-inflammatory, limited in phagocytic capacity, and prone to cell death [15], which may ultimately promote atheroma formation.
The significance of SMC proliferation in terms of the development of pathogenic lesions is under debate. Pathologic intimal thickening (PIT) plays an important role in the initiation and progression of atheroma formation and is distinct from non-atherosclerotic intimal thickening, which is referred to as diffuse intimal thickening (DIT) and adaptive intimal thickening (AIT, or also called eccentric intimal thickening) [22–25]. DIT has been recognized for decades [26]. DIT consists of SMCs and matrix proteins without lipid accumulation and is widespread in the arterial bed in humans. In fact, it was reported that 100 % of humans have coronary arterial DIT by 3-months of age [27, 28]. AIT is a focal thickening exceeding the thickness of DIT and frequently develops at branch-points and areas of turbulent blood flow [29], sites where atherosclerotic lesions frequently develop [30]. However there is no clear distinction between AIT and DIT in terms of their cellular or noncellular composition; they often continuously develop on a span of the arterial bed. DIT and AIT are considered to result from a physiological response to blood flow and tensile stress [22–25]; nevertheless, they may make a transition to PIT through poorly understood processes, leading to subsequent atheroma formation. Indeed, recent findings suggest that the transition of DIT/AIT to PIT involves modification of extracellular matrix proteins and deposition of non-cellular blood components in the intima [31, 32], influx of pro-inflammatory cells, and SMCs’ phenotypic switch and cell death [22–25]. It is noteworthy that most acute coronary events are related to rupture or erosion of atherosclerotic plaques that are not hemodynamically significant [33]. Thus one can speculate that early transition of AIT/DIT to PIT may contribute to development of non-obstructive but clinically significant (i.e. unstable) atherosclerotic lesions.
Macrophages play a key role in the pathophysiology of atherosclerosis, and recent findings indicate that macrophages, similarly to SMC, may undergo a phenotypic switch [15]. Thus a significant number of macrophage marker (e.g. CD68)-positive cells are of SMC origin, and vice versa, macrophages can express SMC markers (e.g. α–smooth muscle actin) [15]. These findings complicate the interpretation of previous studies relying on cell-type “specific” marker expression. A potentially beneficial function of macrophages has been proposed. In the classical “response-to-retention” hypothesis, macrophages are part of a tissue repair mechanism and remove lipids that accumulate in the intima. Macrophages also clear up dying cells within plaques via efferocytosis. There is evidence that plaque macrophages are defective in efferocytosis [34], potentially resulting in accumulation of dying/dead cells and necrotic core expansion.
We previously reviewed potential IGF-1 effects on atherosclerotic diseases [35, 36]. We now provide an update on the effects of IGF-1 on atherosclerosis after a decade of significant advances in our understanding of the pathophysiology of atherosclerosis.
<Insulin-like Growth Factor system>
Insulin-like growth factor 1 (IGF-1) is a major regulator of postnatal (prepubertal and pubertal) somatic growth, mediating many of the effects of growth hormone (GH). Circulating levels of IGF-1 reach to their highest at mid-teen years (111–647 ng/mL) and then decline with age. In healthy 40 years old adults, its reference range is approximately 50–65 % lower than its peak range (68–226 ng/mL or 9–30 nM). IGF-1 and its related peptide hormone, insulin, display similar affinities for their respective cognate receptors, but circulating IGF-1 levels are 10-fold or higher. The majority of circulating IGF-1 molecules are complexed with IGF binding proteins (IGFBPs), which hinder IGF-1 from ligating to the insulin receptor or modulate IGF-1 binding to the IGF-1 receptor (IGF1R) [37]. IGF1R is expressed in the three major cell types which are involved in the pathogenesis of atherosclerosis, namely, endothelial cells, macrophages, and SMCs. Biological effects of IGF-1 on cultured vascular endothelial cells, smooth muscle cells, and macrophages have long been described, and more recent in vivo studies have provided insights into the effects of IGF-1 on the pathogenesis of atherosclerosis.
<Growth Factors and Atherosclerosis; Permissive or Preventative?>
Traditionally, the role of growth factors in atherosclerosis has been thought to be permissive, in particular, by stimulating vascular smooth muscle cell (SMC) migration and proliferation, thereby promoting neointima formation [38, 39]. Interestingly, recent reports suggest that some growth factors have atheroprotective effects in animal models of atherosclerosis. Tang et al. [40] tested potential roles of platelet derived growth factor (PDGF)-B on cardiovascular diseases by using a murine model of atherosclerosis, apolipoprotein E-null mice (Apoe-null mice). They transplanted fetal liver cells of Pdgfb/Apoe-dual knockout mice into irradiated Apoe-null mice, therefore the recipient animals lack PDGF-B in circulating cells (such as monocytes and platelets), which are the major source of PDGF in the pathogenesis of atherosclerosis [41]. These animals demonstrated a significant change in atherosclerotic lesion composition, represented by enhanced inflammatory cell infiltration [40]. The same group demonstrated that elimination of PDGF-B in circulating cells or blockade of PDGF receptors delayed but did not inhibit smooth muscle accumulation in lesions, and more importantly, did not influence atherosclerotic burden [42]. These reports suggest a modest contribution of PDGF to atheroprogression but a major inhibitory effect on inflammatory responses and on monocyte accumulation. Transforming growth factor-β (TGF-β) promotes vascular SMC proliferation and matrix protein production (reviewed in [43]). Apoe-null mice with cardiac-specific overexpression of active TGF-β1 develop elevated cardiac and circulating TGF-β levels, less aortic root atherosclerosis, and fewer lesions in the thoracic and abdominal aortae [44]. These plaques were characterized by fewer T lymphocytes, more collagen, less lipid, and lower expression of inflammatory cytokines [44]. We found that elevation of circulating IGF-1 levels (by continuous s.c. infusion, resulted in approximately 2.3-fold elevation of total IGF-1 levels) prevented atherogenesis in Apoe-null mice [45]. These findings suggest preventive or reparative function of certain growth factors, however precise underlying mechanisms are still obscure.
2. IGF-1 and Cardiovascular Disease – Clinical Implications
IGF-1 affects vascular function and atherosclerosis in many ways, including anti-inflammatory and anti-apoptotic actions [46, 47] and stimulation of angiogenesis [48, 49]. It stimulates nitric oxide production in endothelial and vascular smooth muscle cells through activation of nitric oxide synthetase via Akt-catalyzed phosphorylation, thus also playing a role in improved cardiac contractility in exercise training [50]. There is also evidence suggesting that IGF-1 has indirect effects on the cardiovascular system by increasing Insulin sensitivity [51–54].
<Coronary Artery Disease (CAD)>
In healthy subjects a link between low levels of circulating IGF-1 and high prevalence of CAD has been suggested [55, 56]. Patients with acromegaly have a chronic excess of GH and IGF-1, which could lead to acromegalic cardiomyopathy, characterized by biventricular hypertrophy, diastolic and systolic dysfunction, and congestive heart failure[57]. Although Cansu et al. [58] suggested higher subclinical atherosclerosis and left ventricular diastolic dysfunction in a small cohort of patients with acromegaly, and Petrossians et al. [59] reported hypertension in 28.8% of 3173 patients with acromegaly in the LAS database, the incidence of baseline serious cardiovascular disease was <5% in the same patients [59]. GH deficiency impairs flow-mediated arterial dilation, and thus endothelial NO-dependent vasodilation [60] and increases cardiovascular morbidity and mortality [61–64]. GH supplementation has been reported to improve cardiovascular risk [65]. On the other hand, a small-scale study indicated a lack of evidence of premature atherosclerosis in untreated severe GH deficiency due to a GH-releasing hormone receptor mutation [66], and also a study reported a lack of evidence of elevated mortality due to cardiovascular diseases in Laron syndrome subjects [67]. Of note, a recent meta-analysis failed to confirm a significant positive trend in cardiovascular risk after short and long-term GH supplementation therapy in adult GHD patients [68]. Potentially consistent with these clinical reports, twice-daily sc injection of GH releasing peptide-2 (20 μg/injection, a dose that significantly increases GH and IGF-1 levels [69]) failed to decrease atherosclerosis in Apoe-null mice, even though it successfully elevated serum IGF-1 levels, contradicting atheroprotective effects of IGF-1 [70]. It was speculated that GH-dependent effects may blunt the effect of increased IGF-1 [70].
In possibly the largest observational study on the relation of IGF-1 to CAD in 10,600 coronary-disease free subjects in the PRIME study, Ruidavets et al. [71] found that baseline IGF-1 level was significantly lower in subjects developing an acute coronary syndrome, and that subjects in the highest quartile of IGF-1 distribution had a 55% reduction in the relative risk of developing myocardial infarction [71]. Similarly, De Leronzo et al. [72], in a small study on 36 patients, showed significantly lower IGF-1 level in patients with early-onset CAD [72].
<Stroke>
Johnson et al. [73] suggested a protective effect of IGF-1 against ischemic stroke in the observational Danish study [73]. In contrast, a case-cohort analysis in subjects >65 years old in the Cardiovascular Health Study observational study showed no effect of IGF-1 and IGF Binding Proteins (IGFBPs) on prediction of risk of cardiovascular events or stroke [74]. Saber et al. [75], using observational data from a predominantly Caucasian population of 757 subjects in the Framingham Heart Study, found the lowest incidence of ischemic stroke in patients in the highest quartile of circulating total IGF-1 (232±41.04 ng/ml), especially in diabetics and in patients in the highest waist-hip ratio quartile. They proposed that low circulating IGF-1 level could be related to increased ischemic stroke risk in diabetics and obese individuals, possibly through higher insulin resistance. The study did not find a linear relationship between IGF-1 and stroke and did not measure free IGF-1 and IGFBPs [75].
There are multiple studies on the relationship between IGF-1 and common carotid intima-media thickness (CC-IMT). In a recent study on morbidly obese individuals in a bariatric surgery program, Sirbu et al. [76] suggested that HOMA-IR (a measure of insulin resistance) and total IGF-1 z-score are associated with increased CC-IMT [76]. Similarly, Kawachi et al. [77] showed that IGF-1 levels were correlated with CC-IMT in non-obese individuals, and Splicke et al. [78] showed a positive correlation between serum IGF-1/IGFBP-3 ratio and CC-IMT [77, 78]. Ameri et al. [79] found an interesting negative association of IGF-1 with CC-IMT only in patients in the lowest 25-hydroxyvitamin D quartile in the Baltimore Longitudinal Study of Aging (BLSA) and the Microalbuminuria: A Genoa Investigation on Complications (MAGIC) study [79]. Abd El-Hafiz et al. [80], in a comparative study of metabolically healthy obese individuals with healthy controls, also suggested that IGF-1 is protective against CC-IMT in the presence of low serum vitamin D [80]. In view of the contradictory data for stroke and CC-IMT Sirbu et al. [76] have postulated that high IGF-1 could stimulate smooth muscle hyperplasia in early atherosclerosis, but promote plaque stability in advanced disease [76, 81]. Muller et al. [82] have also suggested that the variable relation of IGF-1 to stroke and CC-IMT might be explained by the lack of standardization for IGF-1 assays utilized in various studies [82].
<Mortality>
The relation of IGF-1 and long term survival/ mortality has been the subject of much discussion, and seems to be modulated by age, race and gender. Wennberg et al. [83] followed IGF-1 levels in 1618 elderly individuals in the Mayo Study of Aging. Looking primarily at bioavailable IGF-1 (ratio of IGF-1 to IGFBP-3), they reported that males have more rapid age-related decline in IGF-1 compared to females [83]. Sanders et al. [84], in a study of 945 individuals >65 years old in the Cardiovascular Health Study followed over a mean of 11.3 years, found that baseline IGF-1 level <70 ng/ml and higher IGF-1 variability were associated with higher mortality [84]. Similarly, Nillson et al. found higher mortality in hemodialysis patients with lower serum IGF-1 level, modulated by serum albumin.
Schutte et al. [85] preformed a detailed longitudinal observational analysis as part of the PURE study in a high-risk population of black South Africans. They reported that higher IGF-1 levels predicted lower mortality over 5 years after adjustment for IGFBP-3 and was significantly related to maintenance of a normotensive status. Interestingly, they did not observe any association between IGF-1 and CC-IMT, which could be attributed to the different ethnicity of this population compared to previously reported studies [85]. It is also possible that the high risk behavior in the PURE study population, with higher alcohol use, smoking and obesity, could directly lead to lower IGF-1 levels [86].
Burgers et al. [87], in a meta-analysis of 12 studies with 14,906 participants, suggested a U-shaped relationship of IGF-1 with mortality, with increased mortality in subjects with low or high IGF-1 levels compared to mid-centile reference categories [87].
<Peripheral Arterial Disease (PAD)>
There are limited studies on the association of IGF-1 with PAD. Urbaonavicience et al. [88] found higher IGF-1 and lower IGFBP-2 in obese individuals, and reported lower IGF-1 in patients with critical limb ischemia. In the same study, they did not find any improvement in prediction of cardiovascular mortality after adding IGF-1 to a conventional risk model [88]. In a cross-sectional study comparing PAD patients to healthy controls, Brevetti et al. [89] found lower levels of IGFBP-3 in the PAD group, especially in patient with an ankle/ brachial index lower than median. They also demonstrated that a high transfemoral gradient of CRP, a surrogate maker for inflammation, was independently associated with a low transfemoral gradient of IGF-1 and a high transfemoral gradient of IGFBP-3 [89].
<Association of PAPP-A with cardiovascular disease>
Pregnancy-associated plasma protein A (PAPP-A) is a metalloproteinase regulator of insulin-like growth factor bioavailability through cleavage of IGFBP-4, thus releasing bioactive IGF [90]. Hjortbjerg et al. [91], in a longitudinal study of 330 Type I diabetic patients with and without nephropathy followed for >12 years, found higher cardiovascular mortality in patients with high values of the main cleavage products NT-IGFBP-4 and CT-IGFBP-4, but not with PAPP-A [91]. There is active interest in exploring the role of stanniocalcin-2 (STC2), a potent PAPP-A inhibitor, in increasing IGF levels in patients [90, 92].
3. Atheroprotective effects of IGF-1 in animal models of atherosclerosis
<Endocrine IGF-1 and atherosclerosis>
In 2007, we reported that systemic infusion of IGF-1, which doubled circulating IGF-1 levels (665 ng/mL vs. 287 ng/mL in saline infused animals), attenuated atherosclerotic burden in Apoe-null mice [45]. Since this report, we and others have confirmed that systemic IGF-1 levels inversely correlate with atherosclerotic burden [45], consistent with atheroprotective effects of IGF-1. Sivasubramaniyam et al. [93] generated Apoe-null mice with hepatic Jak2 deficiency, which impairs the GH signaling pathway, therefore these mice have significantly lower circulating IGF-1 levels than hepatic Jak2-wild type mice [93]. These animals had significantly accelerated atherosclerosis and the causal relation between low IGF-1 and atherosclerosis was confirmed by supplementing IGF-1 by continuous infusion (at a dose that does not influence glucose tolerance or insulin sensitivity, however reverses GH levels down as low as Jak2-wild type mice [93]) or by overexpression of IGF-1 (by crossbreeding to hepatic IGF-1 overexpression mice in which serum IGF-1 levels were 2.5-fold higher than no-overexpression controls [94]) in hepatocytes of Jak2-deficient mice [93]. Svensson et al. [95] investigated diet-induced fatty streak formation in liver-specific IGF-1 inactivation mice (LI-IGF-1−/− mice), in which the serum IGF-1 level is lower than wild-type control mice by 80 % [95]. This animal model is on a C57Bl/6 background without alterations of genes related to lipid metabolism, thus it is normolipidemic and produces only early stage lesions (fatty streaks), making it a useful model to assess potential effects of endocrine IGF-1 on initiation of plaque development. Intriguingly, they observed that the deficiency of endocrine IGF-1 increased fatty streak formation only in female mice [95]. Increased fatty streak formation was associated with elevated serum cholesterol and signs of systemic inflammation, endothelial activation, and macrophage infiltration in the vascular wall, consistent with atheroprotective effects of endocrine IGF-1. It is noteworthy that the LI-IGF-1−/− mouse produces adult-onset IGF-1 deficiency (i.e. liver-specific Igf1 gene deletion induced at 3 months of age), therefore, the observed atheroprotective effects of IGF-1 are independent of any known developmental effects of IGF-1.
Elevated IGF-1 levels in the circulation not only decreased plaque burden, but also induced phenotypic changes in plaques, represented by a decrease of macrophages, attenuated pro-inflammatory cytokine expression, lowered oxidative stress, less frequent apoptosis, and increased presence of smooth muscle cells and collagen [45, 96, 97]. Vice versa, low levels of circulating IGF-1 in the 6T congenic mouse [98] and in the liver-specific IGF-1 knockout mouse [93] was associated with enhanced inflammatory phenotype and increased atherosclerotic burden. These observations suggest that IGF-1 may reduce inflammation and promote a more stable plaque phenotype, underscoring IGF-1’s therapeutic potential to prevent clinical events caused by plaque vulnerability. For a more comprehensive understanding of potential IGF-1 effects on atherosclerosis, murine atherosclerosis models which carry cell-type specific modifications of the IGF-1 system have been used and provided mechanistic insights for the vascular effects of IGF-1.
<Smooth muscle cells>
In the classical view of mechanisms of atherosclerosis, SMCs were considered to play pro-atherogenic roles. Phenotypic switching of contractile SMCs to synthetic SMCs, which migrate from the media to intima, proliferate, and deposit matrix proteins, was thought to cause plaque thickening [99, 100]. However In the past decade, it has become apparent that SMCs switch to more diverse phenotypes than merely being “synthetic” or “contractile” [15, 101]. Likewise, potential effects of growth factors on SMCs (also on SMC-derived cells and SMC-marker positive cells of other origins) need to be redefined accordingly to an updated view of SMC biology within atherosclerotic lesions. Mechanically induced neointima models (e.g. wire or balloon catheter injury-induced neointima formation) had been used to investigate potential effects of growth factors on fibroproliferative SMCs. However the biology of the neointima (neointimal cells are almost exclusively “synthetic” SMCs without intact endothelial cells covering the lesions) is grossly different from atherosclerotic lesions in humans, as the former lacks involvement of endothelial cells or pro-inflammatory cells, which are essential components in the latter. Therefore, we have avoided inclusion of reports using animal models of injury-induced intimal hyperplasia, which are now considered to represent the pathophysiology of angioplasty-induced restenosis but not atherosclerosis.
IGF-1’s effects of promoting vascular SMC migration and proliferation have been proposed as a potential mechanism of restenosis, pulmonary hypertension, and vein graft failure, using animal models of intimal hyperplasia [102–104]. However, investigations on potential effects of IGF-1 on atheroma formation had been lacking. To address the effects of autocrine/paracrine IGF-1 produced by smooth muscle cells on the pathogenesis of atherosclerosis, transgenic mice which overexpress IGF-1 in smooth muscle were created on an Apoe-null background (SMP8-Igf1 mice) [105]. When compared with Apoe-null mice, the SMP8-Igf1 mice developed a comparable plaque burden after 12 weeks on a high fat diet, suggesting that the ability of endocrine IGF-1 to reduce plaque burden [45] was mediated in large part via non-SMC target cells. However, advanced plaques in SMP8-Igf1 mice displayed features of increased plaque stability, including elevated SMC content, increased fibrous cap area, increased collagen levels, and reduced necrotic cores. Of note, the same promoter-driven IGF-1 overexpression in smooth muscle (SMP8-Igf1) substantially promoted neointima formation in an injury-induced arterial intimal hyperplasia model [106], consistent with a significant difference in pathology between arterial intimal hyperplasia and atherosclerosis. The observed consequence of IGF-1-overexpression in SMCs on atherosclerosis is consistent with the earlier studies demonstrating IGF-1 to positively support SMC differentiation [107–109] and elevate matrix protein production. We have subsequently shown that IGF-1 increases collagen synthesis by increasing LARP-6 expression [110]. In both IGF-1 infusion model (mentioned in “Endocrine IGF-1 and atherosclerosis” [45]) as well as a smooth muscle cell specific IGF-1 overexpression model, IGF-1 does not increase atherosclerotic burden. However, these studies were done at a 3-month time point of high-fat diet feeding, so potential effects of IGF-1 on early atherosclerotic lesion stage were not determined.
We also evaluated a model of SMC-specific IGF1R loss of function. For this purpose, we cross-bred IGF1R floxed mice to SM22α -CreKI mice, which have Cre recombinase gene knocked-in downstream of the promoter region of SM22α gene, resulting in IGF1R deficiency in SMCs and also in fibroblasts (FB; SM22α-CreKI/IGF1R-flox mice) [111]. IGF1R deficiency caused SMC and FB hypotrophy and decreased expression of collagen. With high-fat diet feeding, SMC-IGF1R deficient mice had increased atherosclerotic burden and inflammatory responses. Also, the IGF1R deficiency decreased plaque SMC content, markedly depleted collagen and increased indices of vulnerability in the plaques [111].
These SMC-selective IGF-1 and IGF1R gain-of-function and loss-of-function models demonstrate that one of the major effects of IGF-1 on SMC is to promote plaque stability by increasing plaque SMC content (via pro-proliferative and anti-apoptotic mechanisms) and plaque collagen matrix. It is interesting to hypothesize that IGF-1 might suppress SMC phenotype switching in favor of preventing atheroprogression, given that IGF-1 supports SMC differentiation [107–109]. Furthermore, SMC-IGF1R deficiency enhanced cell death and inflammation and enlarged necrotic cores in lesions [111]. Blackstock et al. [110] described that IGF-1 positively regulates La ribonucleoprotein domain family member 6 (LARP6), which is an essential regulator of collagen I and III synthesis and fiber assembly, in cultured aortic SMCs and in the aorta of Apoe-null mice, consistent with a novel mechanism whereby IGF-1 increases collagen fibrinogenesis and plaque stability [110].
<Macrophages>
Macrophages are in large part responsible for the development of the inflammatory milieu in plaques. Also, macrophages are major contributors to the accumulation of lipids and the degradation of extracellular matrix; consequently, all of these activities lead to a loss of the structural stability of plaques. Macrophages express IGF-1 and IGF-1R. IGF-1 production by macrophages is often described in the context of tissue regeneration, repair, and fibrosis in an inflamed tissue [112–114], where IGF-1 mediates a transition by enhancing mesenchymal/stromal cell proliferation/migration and matrix deposition. With regard to vascular inflammation, pro-atherogenic factors such as advanced glycosylation end products [115] and TNF-α [116] have been shown to increase IGF-1 synthesis in macrophages.
IGF-1 may act on surrounding cells in a paracrine manner or on macrophage themselves in an autocrine manner. Reports about potential IGF-1 effects on macrophages in the context of atherosclerosis have been conflicting. IGF-1’s potentially pro-atherogenic effects have been reported as enhancing chemotactic macrophage migration [117], stimulating tumor necrosis factor-α (TNFα) expression [118], or enhancing low-density lipoprotein (LDL) uptake and cholesterol esterification [119]. On the contrary, IGF-1 was reported to reduce lipid oxidation and foam cell formation by macrophages mediated by 12/15-lipoxygenase [120]. In fact, clinical investigations provide indirect evidence of IGF-1 exerting anti-inflammatory effects: For instance, administration of the IGF-1 and IGFBP-3 complex attenuated proinflammatory acute phase response in severely burned patients [121, 122]. Of note, some of these in vitro studies used concentrations of IGF-1 well above the physiological range, making it challenging to interpret the results. Interpretation of experiments using IGF-1 on cultured macrophages is often difficult, since experiments are often performed in a serum-containing medium, because of the high sensitivity of macrophages to serum deprivation. Due to serum-derived IGF-1, IGFBPs, insulin, and protein-degrading activity, it is difficult to estimate bioavailability of externally added IGF-1 or insulin. Careful examination of receptor usage (IGF-1R, insulin receptor, or IGF/insulin hybrid receptor) and signaling pathways may be helpful to unravel conflicting previous findings.
Recently, our group assessed a potential direct link between IGF-1 effects on macrophages and atherosclerosis [46] by creating myeloid-specific IGF1R-deficient mice on Apoe-null background (Lyz2-Cre/IGF1Rfl/fl/Apoe−/− mice). The loss of IGF-1 signaling caused an increase of atherosclerotic burden concomitant with elevated monocyte recruitment to the lesions; moreover, macrophage-IGF1R deficiency induced features of an unstable plaque phenotype [46]. Consistent with these in vivo observations, peritoneal macrophages isolated from this animal model demonstrated enhanced responses to pro-inflammatory stimuli such as interferon-gamma or lipopolysaccharide (i.e. enhanced M1 activation) as well as elevated matrix metalloproteinase production and reduced lipid efflux leading to elevated lipid accumulation [46]. Thus IGF-1 may have a fundamental role in macrophage activation and also modulate atherosclerosis by regulating MMPs expression levels and lipid metabolism.
The downstream signaling pathway from the IGF1R largely coincides with the insulin receptor signaling pathway; moreover, IGF1R and insulin receptor are structurally similar and form a heteromeric tetramer (α+β-subunits of IGF1R and α+β-subunits of insulin receptor; hybrid receptor). These hybrid receptors mediate IGF-1-dependent signaling but not insulin signaling, thus formation of hybrid receptors is inhibitory to insulin effects. Nullifying IGF1R expression could potentially enhance insulin-sensitivity via liberation of insulin receptor subunits to form holoreceptors. Our group investigated IGF1R and insulin receptor expression in mouse peritoneal macrophages and found that both receptors are expressed, however, insulin receptor is expressed predominantly, allowing insulin holoreceptor and IGF-insulin hybrid receptor expression but no IGF-1 holoreceptors [46]. Therefore, it is likely in macrophages that the hybrid receptor mediates IGF-1-dependent signaling, whereas insulin holoreceptor mediates insulin-dependent signaling. Consistently, we observed that the genetic deletion of IGF1R did not influence insulin-induced Akt phosphorylation in macrophages [46], making it unlikely that the elevated atherosclerotic burden is mediated indirectly by altered insulin signaling.
Of note, potential insulin effects on macrophages and atherosclerosis have been a subject of active investigation. In one report, myeloid-specific insulin receptor deletion in Apoe-null mice (Lyz2-Cre/Insrfl/fl/Apoe−/− mice) lowered atherosclerotic burden and this was associated with attenuated inflammatory response [123]. On the contrary, insulin receptor-deficient bone marrow transplantation into LDL receptor-null mice caused elevated plaque burden, associated with increased necrotic core size and apoptotic cell numbers [124], mediated by enhanced ER stress and apoptosis of macrophages [124]. Reasons for these divergence in findings are still obscure; it has been pointed out that the difference in the animal models (Lyz2-Cre mediated floxed-insulin receptor deletion vs. bone marrow transplantation from insulin receptor-deficient mice; Apoe-null vs. LDLR-null), or animal condition (high cholesterol diet vs. high cholesterol+ choline-supplemented diet that is more proinflammatory) may explain the difference [125, 126]. Insulin has been considered pro-inflammatory in macrophages [127, 128] and has been reported to promote foam cell formation [129], in contrast to anti-inflammatory and anti-foam cell formation effects of IGF-1 [46, 120]. Some of above papers do not evaluate receptor usage, even when relatively high dose (100 nM) of insulin had been used. Further studies are necessary to differentiate signaling mechanisms and effects of IGF-1 and insulin on macrophages, in order to elucidate their pathophysiological roles in diseases where chronic inflammation plays a vital role, such as atherosclerosis and diabetes.
<Endothelial cells>
The endothelium is the master regulator of vascular functions such as vascular permeability, vasomotor activity, and pro-or anti-thrombotic activities. The endothelium also plays a leading role in new vessel formation (i.e. angiogenesis) and in establishment of inflammation by recruiting proinflammatory cells via expression of adhesion molecules or chemoattractant factors. Any of these endothelial functions are vital to the integrity of the vasculature, and are therefore important for the pathophysiology of atherosclerosis.
Vascular endothelial cells express IGF1R and thus the endothelium is a target organ of IGF-1[130]. Potential IGF-1 effects on the endothelium have been investigated extensively (reviewed in [130, 131]); however, some of these findings have been conflicting, hence the definite roles of IGF-1 in regulation of endothelial function are still elusive. Elevated vascular permeability is an integral part of the pathogenesis of atherosclerosis and has been recently proposed to be a marker of vulnerable atherosclerotic plaques [132]. It has been reported that IGF-1 supports the endothelial blood-brain barrier function in ischemic brain, reducing permeability and proinflammatory cell infiltration [133]. In a mouse model of renal fibrosis, IGF1R overexpression reduced renal capillary permeability and proinflammatory cell infiltration, vice versa, endothelial specific deficiency of IGF1R lowered endothelial barrier function as seen by increased inflammatory cell infiltration and vascular endothelial-cadherin phosphorylation and increased the fibrosis of kidney disease [134]. On the contrary, IGF-1 was also reported to impair the blood-retinal barrier function by downregulating tight junction proteins via a GSK-3β/CREB dependent mechanism [135, 136]. It has been suggested that in the presence of hyperglycemia the IGF-I/IGF-1R axis stimulates retinal endothelial cell permeability [137]. Reasons for these apparently conflicting observations are not clear. One can speculate that different tissue-derived endothelial cells respond differently to IGF-1, or it is also possible that other factors (e.g. hormones, growth factors, or a specific metabolic condition such as high glucose) influence endothelial cells’ response to IGF-1. For instance, hyperglycemia induces the association of integrin-associated protein (IAP) with tyrosine phosphatase non-receptor type substrate-1 (SHPS-1), changing the endothelial cell response to IGF-1 and increasing permeability [138].
Impaired endothelium-dependent vasodilation precedes atherosclerosis development [8, 139, 140]. IGF-1 may promote vasodilation by upregulating eNOS activity in the endothelium and increasing nitric oxide production [141, 142]. In human subjects, low plasma IGF-1 levels are associated with impaired endothelium-dependent vasodilation [143]. In contrast, it has been also shown that IGF1R inhibits insulin-induced eNOS activity, by forming a hybrid receptor (i.e. one combination of IGF-1 alpha-beta subunits complexed with one combination of insulin alpha-beta subunit) [144]. Therefore, a reduction in IGF1R expression levels improved insulin-dependent vasorelaxation [144]; and vice versa, overexpression of IGF1R decreased nitric oxide bioavailability and insulin sensitivity in the endothelium [145]. However there is no evidence as yet that the ability of IGF-1 to stimulate eNOS plays a role in its atheroprotective effects [96]. Of note, IGF1R overexpression promoted endothelial regeneration after denudation, consistent with IGF-1’s pro-repair activity in the endothelium [145].
Intriguingly, endothelium-selective overexpression of insulin receptor blunted shear stress induced eNOS activation and elevated superoxide production by NADPH-oxidase, and these two effects combined to decrease NO bioavailability [146], leading to impaired endothelium-dependent vascular relaxation and accelerated atherosclerosis [146]. It can be speculated that the overexpression of insulin receptor could lead to increased abundance of IGF-1/insulin hybrid receptors, thereby potentially enhancing IGF-1 sensitivity in the endothelium. Therefore, the observed consequences may have been caused by enhanced IGF-1-dependent signaling activity in the endothelium. Overall, IGF-1 and insulin signaling pathways are intricately related as are their potential effects on vascular tone.
IGF-1 promotes angiogenesis and nascent vessel formation [141, 147], and also increases endothelialization of denuded artery by promoting endothelial cell proliferation and migration [145]. Promoting re-endothelialization may be beneficial to inhibit restenosis after angioplasty procedures. However, it is unclear whether it also provides benefits in atherosclerosis, since the endothelium layer stays physically intact (however functionally compromised) on atherosclerotic plaques. Angiogenesis or neovascularization in atherosclerotic lesions, which are actually promoted by hypoxia within the lesion, is considered to promote plaque progression and destabilization [148–150]. Endothelial progenitor cells (EPCs) in the circulation or in the vascular wall may contribute to endothelial repair and prevent endothelial dysfunction [151, 152]. Potential effects of IGF-1 on EPC-mediated endothelial repair are unclear. It has been reported that in healthy human subjects or in subjects with GH deficiency the GH/IGF-1 axis positively regulates circulating EPC levels [153–155]. Consistent with these observations in human subjects, IGF-1 administration elevated circulating EPC levels in animal models [45, 153, 156]. More recent reports [157, 158] investigated acromegalic subjects, and interestingly, these two reports found contradictory results of increased [157] or reduced [158] levels of circulating EPCs compared to control subjects. Reasons for the discrepancy are unclear, however an interesting notion from these studies is that there seems to be an inverted U-shaped relationship between GH/IGF-1 and circulating EPC levels (i.e. these seems to be an optimal range of GH/IGF-1 for maintaining circulating EPC levels). Future investigations to explore mechanisms whereby IGF-1 can positively/negatively regulate EPC availability in vivo would be necessary to comprehend IGF-1’s effects on endothelial repair.
There is evidence suggesting potential anti-oxidant effects of IGF-1 in vascular endothelial cells. IGF-1 promoted Nrf2-dependent antioxidant responses in cultured endothelial cells and low circulating IGF-1 levels impaired the Nrf2-dependent antioxidant response [159]. IGF-1 has been also shown to positively regulate expression levels of GPX1, a major anti-oxidant enzyme, and attenuate oxidative stress-induced premature senescence of endothelial cells [160]. Oxidative stress and premature senescence of endothelial cells is considered integral to the mechanisms of endothelial dysfunction, and thus causally related to atherosclerosis [161, 162]. Therefore, potential anti-oxidant effects of IGF-1 in the endothelium may contribute to the atheroprotective activity of IGF-1. Further studies are needed to investigate a direct link between the potential antioxidant effects of IGF-1 and reduction of atherosclerotic burden.
4. Modifiers and effectors of IGF-1 on the vasculature
Pregnancy-associated plasma protein A (PAPP-A) is a metalloproteinase and its known substrates are IGFBP-4, IGFBP-2, and IGFBP-5 [163, 164]. Circulating PAPP-A has been recognized for its positive association with prevalence of cardiovascular diseases [165–168]. There has been debate whether elevated PAPP-A levels are related to cardiovascular diseases [169, 170], and recent findings in animal models suggest a causal role of PAPP-A in vascular complications, such as injury-induced stenosis [171] and atherosclerosis [172–174], and also in metabolic dysfunction induced by high-fat/high-sucrose diet [175]. SMC-targeted overexpression of mutated PAPP-A, which is defective in IGFBP-4 proteolysis (Asp1499 to Ala) or both IGFBP-4 and −5 proteolysis (Glu483 to Ala), did not promote lesion formation, whilst an overexpression of wild-type (i.e. proteolysis-active) PAPP-A promoted lesion development, suggesting that proteolytic activity is essential for PAPP-A to promote atherosclerosis [176]. Since IGFBP-4 is considered inhibitory to IGF-1 action, proteolysis of IGFBP-4 should increase the bioavailability of IGF-1 to adjacent cells. In accordance with the notion, SMC-targeted overexpression of PAPP-A increased IGFBP-5 mRNA expression (known to be positively regulated by IGF-1) in aorta [173]. Therefore, it has been speculated that PAPP-A promotes atherosclerosis by liberating IGF-1 from IGFBP-4, thereby enhancing IGF-1 bioavailability [173, 176]. This is in contradiction to other reports showing atheroprotective effects of IGF-1 [45, 93, 95–98, 105]. PAPP-A’s IGF-independent mechanisms of action remain a possibility. Of note, IGFBP-4 is known to have potent IGF-independent anti-angiogenic and anti-tumorigenic effects [177] and these effects are associated with anti-cathepsin B activity [178]. Pharmacological and gene therapy approaches have been attempted to test PAPP-A as a therapeutic target in animal models of atherosclerosis. Targeting of PAPP-A with a monoclonal antibody resulted in a 70% reduction in atherosclerotic burden in Apoe-null mice [179]. Stanniocalcin-2 is a protein that binds and inhibits PAPP-A, and AAV8-mediated overexpression of stanniocalcin-2 in liver reduced atherosclerosis in Apoe-null mice [180]. There reports suggest that systemic inhibition of PAPP-A may be a promising approach for treating atherosclerosis.
Diabetic status is a significant risk factor for atherosclerosis, and it has been shown that in vascular smooth muscle cells hyperglycemia diverts the IGF-1 signaling pathway from its canonical IRS-1-dependent phosphorylation cascades to a SHPS-1 mediated pathway, leading to enhanced proliferation, migration, and dedifferentiation [181, 182]. Detailed mechanisms have been described indicating that the shift of IGF-1/IGF-1R signaling pathway is mediated via SHPS-1, which requires ligation of integrin αVβ3 with its ligand such as thrombospondin, vitronectin and osteopontin [183]. SMC expression levels of these ligands are elevated under hyperglycemic condition, consistent with hyperglycemia induced shift of IGF-1 signaling. In fact, a monoclonal antibody against αVβ3 integrin inhibited development of atherosclerotic lesions in diabetic pigs [184]. It has been also shown that hyperglycemia downregulates IRS-1, which should also contribute to the shift to the SHPS-1-dependent signaling pathway [182]. Moreover, hyperglycemia enhances smooth muscle cells’ sensitivity to IGF-1 via p62/PKCζ-dependent NADPH-oxidase 4 upregulation [185], further promoting smooth muscle cell proliferation/migration. In light of the strikingly diverse phenotypes of SMC-derived cells within atherosclerotic plaques, it would be valuable to investigate potential consequences of the diversion of IGF-1R signaling pathways on phenotypic switching and pathologic intimal thickening.
Several miRNAs have been reported to regulate the IGF-1 system in cardiac tissue [186–189] or in arteries [190, 191]. miR-133a increased IGF1R mRNA half-life and IGF1R expression levels in SMCs, thereby promoting proliferation [190]. miR-490–3p is expressed in aortic SMCs and negatively regulates PAPP-A [191]. Of note, miR-490–3p was found to be downregulated in atherosclerotic plaques with a concomitant increase in PAPP-A levels [191], potentially enhancing IGF’s effects.
Extracellular vesicles (EVs) are cell-derived spherical structures of various origins, including exosomes and microvesicles [192], which can transport proteins, mRNAs or non-coding RNAs. It has been suggested that EVs can positively regulate the IGF-1 system, leading to beneficial outcomes, such as enhancing angiogenesis in ischemic skeletal muscle [193] and protecting myocardium from ischemic damage[194, 195]. However, EVs could also negatively regulate the IGF-1 system, thereby inhibiting angiogenesis in diabetic heart [196] or potentially promoting apoptosis of endothelial cells [197]. IGF-1 may also regulate release of EVs. For instance, IGF-1 has been reported to facilitate release of EVs by cardiomyocytes and exert cardioprotection [198]. The potential role of EVs in modulating the IGF-1 system in atherosclerosis is largely unexplored.
5. Future perspectives
There is now a solid body of evidence testing IGF-1’s effects in a cell-type specific manner using murine models of atherosclerosis. Thus far, outcomes of these investigations point to atheroprotective effects of IGF-1, and in particular the ability of IGF-1 to promote features of a more stable atherosclerotic plaque. Consistent with our observations, several clinical trials established a strong association between low IGF-1 (or high IGFBP levels) and increased risk of CVD. Yet, it should be noted that murine models of atherosclerosis have notable limitations [199], and outcomes in murine models cannot be directly inferred to the pathology of human disease. For instance rodents and humans have significant differences in mechanisms of lipid metabolism and in their immune systems, which play essential roles in the pathology of atherosclerosis [199]. Atherosclerotic plaques in mice rarely develop into atherothrombotic plaques [199], whereas in humans plaque erosion or rupture commonly lead to acute ischemic events. Moreover, rodents do not seem to develop diffuse/adoptive intimal thickening, however large mammals such as pigs and horses are similar to humans [200–202]. Although there are no conventional animal models that develop spontaneous atherothrombotic events in a manner similar to human pathology, use of large animal models such as swine may in the future prove to be very informative [2, 199].
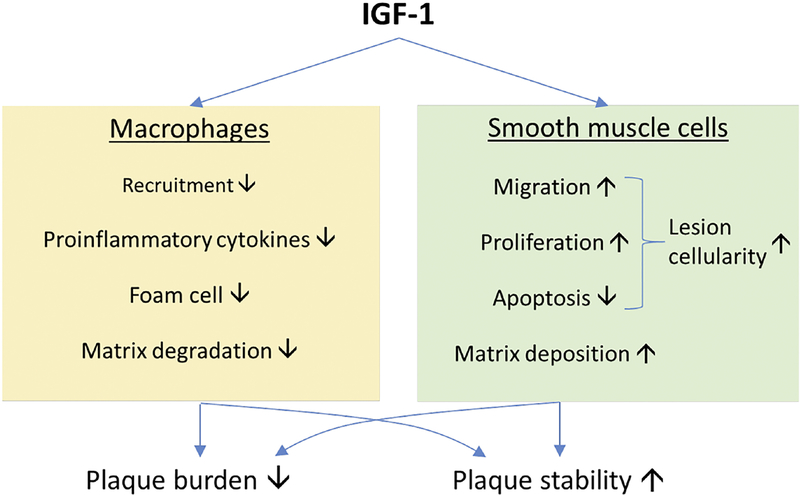
Figure. Cell-specific mechanisms underlying atheroprotective effect of IGF-1.
Cumulative reports of in vitro and in vivo investigations suggest that IGF-1 is suppressive to the recruitment of monocytes/macrophages to atherosclerotic plaques, production of proinflammatory cytokines, conversion of macrophages into lipid-laden foam cells, and extracellular matrix degradation. On the other hand, IGF-1 promotes smooth muscle cell (SMC) migration, proliferation and SMC-dependent matrix deposition. These cell-specific mechanisms may contribute to IGF-1-induced reduction in plaque burden and increase in plaque stability.
Highlights.
Low circulating IGF-1 levels have been associated with cardiovascular diseases.
Cell-type specific alterations of the IGF-1 system in animal models are consistent with atheroprotective effects of IGF-1.
Evidence suggests beneficial roles of IGF-1 in the pathology of atherosclerosis.
Acknowledgements
This work was supported by a grant from the National Institute of Health 5 -R01-HL070241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
We have no conflict of interest to declare.
Reference
- [1].G B D C o D Collaborators, Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet, 390 (2017) 1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vatner SF, Why So Few New Cardiovascular Drugs Translate to the Clinics, Circ Res, 119 (2016) 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Badimon JJ, Fuster V, Chesebro JH, Badimon L, Coronary atherosclerosis. A multifactorial disease, Circulation, 87 (1993) II3–16. [PubMed] [Google Scholar]
- [4].Poulter N, Coronary heart disease is a multifactorial disease, Am J Hypertens, 12 (1999) 92S–95S. [DOI] [PubMed] [Google Scholar]
- [5].Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS, Pathogenesis of atherosclerosis: A multifactorial process, Exp Clin Cardiol, 7 (2002) 40–53. [PMC free article] [PubMed] [Google Scholar]
- [6].Stocker R, Keaney JF Jr, Role of oxidative modifications in atherosclerosis, Physiol Rev, 84 (2004) 1381–1478. [DOI] [PubMed] [Google Scholar]
- [7].Verma S, Anderson TJ, Fundamentals of endothelial function for the clinical cardiologist, Circulation, 105 (2002) 546–549. [DOI] [PubMed] [Google Scholar]
- [8].Gimbrone MA Jr, Garcia-Cardena G, Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis, Circ Res, 118 (2016) 620–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA, Endothelial fluid shear stress sensing in vascular health and disease, J Clin Invest, 126 (2016) 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Givens C, Tzima E, Endothelial Mechanosignaling: Does One Sensor Fit All?, Antioxid Redox Signal, 25 (2016) 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].van Hinsbergh VW, Endothelium--role in regulation of coagulation and inflammation, Semin Immunopathol, 34 (2012) 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Muller WA, How endothelial cells regulate transmigration of leukocytes in the inflammatory response, Am J Pathol, 184 (2014) 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR, The role of tissue Renin-Angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness, Front Endocrinol (Lausanne), 4 (2013) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J, Endothelial dysfunction, arterial stiffness, and heart failure, J Am Coll Cardiol, 60 (2012) 1455–1469. [DOI] [PubMed] [Google Scholar]
- [15].Bennett MR, Sinha S, Owens GK, Vascular Smooth Muscle Cells in Atherosclerosis, Circ Res, 118 (2016) 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK, KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis, Nat Med, 21 (2015) 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R, Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis, Circ Res, 115 (2014) 662–667. [DOI] [PubMed] [Google Scholar]
- [18].Gomez D, Shankman LS, Nguyen AT, Owens GK, Detection of histone modifications at specific gene loci in single cells in histological sections, Nat Methods, 10 (2013) 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R, Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis, Nat Med, 8 (2002) 403–409. [DOI] [PubMed] [Google Scholar]
- [20].Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, Russell SJ, Litzow MR, Edwards WD, Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation, Proc Natl Acad Sci U S A, 100 (2003) 4754–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R, Bone marrow-derived cells contribute to vascular inflammation but do not differentiate into smooth muscle cell lineages, Circulation, 122 (2010) 2048–2057. [DOI] [PubMed] [Google Scholar]
- [22].Kolodgie FD, Burke AP, Nakazawa G, Virmani R, Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease?, Arterioscler Thromb Vasc Biol, 27 (2007) 986–989. [DOI] [PubMed] [Google Scholar]
- [23].Johnson JL, Emerging regulators of vascular smooth muscle cell function in the development and progression of atherosclerosis, Cardiovasc Res, 103 (2014) 452–460. [DOI] [PubMed] [Google Scholar]
- [24].Nakagawa K, Nakashima Y, Pathologic intimal thickening in human atherosclerosis is formed by extracellular accumulation of plasma-derived lipids and dispersion of intimal smooth muscle cells, Atherosclerosis, 274 (2018) 235–242. [DOI] [PubMed] [Google Scholar]
- [25].Sakamoto A, Torii S, Jinnouchi H, Finn AV, Virmani R, Kolodgie FD, Pathologic intimal thickening: Are we any closer to understand early transitional plaques that lead to symptomatic disease?, Atherosclerosis, 274 (2018) 227–229. [DOI] [PubMed] [Google Scholar]
- [26].Wilens SL, The nature of diffuse intimal thickening of arteries, Am J Pathol, 27 (1951) 825–839. [PMC free article] [PubMed] [Google Scholar]
- [27].Ikari Y, McManus BM, Kenyon J, Schwartz SM, Neonatal intima formation in the human coronary artery, Arterioscler Thromb Vasc Biol, 19 (1999) 2036–2040. [DOI] [PubMed] [Google Scholar]
- [28].Movat HZ, More RH, Haust MD, The diffuse intimal thickening of the human aorta with aging, Am J Pathol, 34 (1958) 1023–1031. [PMC free article] [PubMed] [Google Scholar]
- [29].Velican C, Velican D, Study of coronary intimal thickening, Atherosclerosis, 56 (1985) 331–344. [DOI] [PubMed] [Google Scholar]
- [30].Doran AC, Meller N, McNamara CA, Role of smooth muscle cells in the initiation and early progression of atherosclerosis, Arterioscler Thromb Vasc Biol, 28 (2008) 812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Otsuka F, Kramer MC, Woudstra P, Yahagi K, Ladich E, Finn AV, de Winter RJ, Kolodgie FD, Wight TN, Davis HR, Joner M, Virmani R, Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study, Atherosclerosis, 241 (2015) 772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ, Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation, J Biol Chem, 268 (1993) 20419–20432. [PubMed] [Google Scholar]
- [33].Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, Borrico S, Gorlin R, Fuster V, Angiographic progression of coronary artery disease and the development of myocardial infarction, J Am Coll Cardiol, 12 (1988) 56–62. [DOI] [PubMed] [Google Scholar]
- [34].Tabas I, Bornfeldt KE, Macrophage Phenotype and Function in Different Stages of Atherosclerosis, Circ Res, 118 (2016) 653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P, IGF-1, oxidative stress and atheroprotection, Trends Endocrinol Metab, 21 (2010) 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P, Aging, atherosclerosis, and IGF-1, J Gerontol A Biol Sci Med Sci, 67 (2012) 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Allard JB, Duan C, IGF-Binding Proteins: Why Do They Exist and Why Are There So Many?, Front Endocrinol (Lausanne), 9 (2018) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nilsson J, Growth factors and the pathogenesis of atherosclerosis, Atherosclerosis, 62 (1986) 185–199. [DOI] [PubMed] [Google Scholar]
- [39].Ross R, The pathogenesis of atherosclerosis: a perspective for the 1990s, Nature, 362 (1993) 801–809. [DOI] [PubMed] [Google Scholar]
- [40].Tang J, Kozaki K, Farr AG, Martin PJ, Lindahl P, Betsholtz C, Raines EW, The absence of platelet-derived growth factor-B in circulating cells promotes immune and inflammatory responses in atherosclerosis-prone ApoE−/− mice, Am J Pathol, 167 (2005) 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H, Localization of PDGF-B protein in macrophages in all phases of atherogenesis, Science, 248 (1990) 1009–1012. [DOI] [PubMed] [Google Scholar]
- [42].Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW, Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice, Am J Pathol, 161 (2002) 1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Raines EW, Ferri N, Thematic review series: The immune system and atherogenesis. Cytokines affecting endothelial and smooth muscle cells in vascular disease, J Lipid Res, 46 (2005) 1081–1092. [DOI] [PubMed] [Google Scholar]
- [44].Frutkin AD, Otsuka G, Stempien-Otero A, Sesti C, Du L, Jaffe M, Dichek HL, Pennington CJ, Edwards DR, Nieves-Cintron M, Minter D, Preusch M, Hu JH, Marie JC, Dichek DA, TGF-[beta]1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice, Arterioscler Thromb Vasc Biol, 29 (2009) 1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P, IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in Apo E-deficient mice, Arterioscler Thromb Vasc Biol, 27 (2007) 2684–2690. [DOI] [PubMed] [Google Scholar]
- [46].Higashi Y, Sukhanov S, Shai SY, Danchuk S, Tang R, Snarski P, Li Z, Lobelle-Rich P, Wang M, Wang D, Yu H, Korthuis R, Delafontaine P, Insulin-Like Growth Factor-1 Receptor Deficiency in Macrophages Accelerates Atherosclerosis and Induces an Unstable Plaque Phenotype in Apolipoprotein E-Deficient Mice, Circulation, 133 (2016) 2263–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li Y, Higashi Y, Itabe H, Song YH, Du J, Delafontaine P, Insulin-like growth factor-1 receptor activation inhibits oxidized LDL-induced cytochrome C release and apoptosis via the phosphatidylinositol 3 kinase/Akt signaling pathway, Arterioscler Thromb Vasc Biol, 23 (2003) 2178–2184. [DOI] [PubMed] [Google Scholar]
- [48].Hutter R, Sauter BV, Reis ED, Roque M, Vorchheimer D, Carrick FE, Fallon JT, Fuster V, Badimon JJ, Decreased reendothelialization and increased neointima formation with endostatin overexpression in a mouse model of arterial injury, Circulation, 107 (2003) 1658–1663. [DOI] [PubMed] [Google Scholar]
- [49].Nakao-Hayashi J, Ito H, Kanayasu T, Morita I, Murota S, Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells, Atherosclerosis, 92 (1992) 141–149. [DOI] [PubMed] [Google Scholar]
- [50].Burgos JI, Yeves AM, Barrena JP, Portiansky EL, Vila-Petroff MG, Ennis IL, Nitric oxide and CaMKII: Critical steps in the cardiac contractile response To IGF-1 and swim training, J Mol Cell Cardiol, 112 (2017) 16–26. [DOI] [PubMed] [Google Scholar]
- [51].Russell-Jones DL, Bates AT, Umpleby AM, Hennessy TR, Bowes SB, Hopkins KD, Jackson N, Kelly J, Shojaee-Moradie F, Jones RH, et al. , A comparison of the effects of IGF-I and insulin on glucose metabolism, fat metabolism and the cardiovascular system in normal human volunteers, Eur J Clin Invest, 25 (1995) 403–411. [DOI] [PubMed] [Google Scholar]
- [52].Sesti G, Sciacqua A, Cardellini M, Marini MA, Maio R, Vatrano M, Succurro E, Lauro R, Federici M, Perticone F, Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance, Diabetes Care, 28 (2005) 120–125. [DOI] [PubMed] [Google Scholar]
- [53].Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, Le Roith D, Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity, Diabetes, 50 (2001) 1110–1118. [DOI] [PubMed] [Google Scholar]
- [54].Sjogren K, Wallenius K, Liu JL, Bohlooly YM, Pacini G, Svensson L, Tornell J, Isaksson OG, Ahren B, Jansson JO, Ohlsson C, Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism, Diabetes, 50 (2001) 1539–1545. [DOI] [PubMed] [Google Scholar]
- [55].Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T, Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study, Circulation, 106 (2002) 939–944. [DOI] [PubMed] [Google Scholar]
- [56].Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D, The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study, J Clin Endocrinol Metab, 89 (2004) 114–120. [DOI] [PubMed] [Google Scholar]
- [57].Sharma MD, Nguyen AV, Brown S, Robbins RJ, Cardiovascular Disease in Acromegaly, Methodist Debakey Cardiovasc J, 13 (2017) 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cansu GB, Yilmaz N, Yanikoglu A, Ozdem S, Yildirim AB, Suleymanlar G, Altunbas HA, Assessment of Diastolic Dysfunction, Arterial Stiffness, and Carotid Intima-Media Thickness in Patients with Acromegaly, Endocr Pract, 23 (2017) 536–545. [DOI] [PubMed] [Google Scholar]
- [59].Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun-Fathallah M, Auriemma R, Diallo AM, Hulting AL, Ferone D, Hana V Jr, Filipponi S, Sievers C, Nogueira C, Fajardo-Montanana C, Carvalho D, Hana V, Stalla GK, Jaffrain-Rea ML, Delemer B, Colao A, Brue T, Neggers S, Zacharieva S, Chanson P, Beckers A, Acromegaly at diagnosis in 3173 patients from the Liege Acromegaly Survey (LAS) Database, Endocr Relat Cancer, 24 (2017) 505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elhadd TA, Abdu TA, Oxtoby J, Kennedy G, McLaren M, Neary R, Belch JJ, Clayton RN, Biochemical and biophysical markers of endothelial dysfunction in adults with hypopituitarism and severe GH deficiency, J Clin Endocrinol Metab, 86 (2001) 4223–4232. [DOI] [PubMed] [Google Scholar]
- [61].Colao A, di Somma C, Pivonello R, Cuocolo A, Spinelli L, Bonaduce D, Salvatore M, Lombardi G, The cardiovascular risk of adult GH deficiency (GHD) improved after GH replacement and worsened in untreated GHD: a 12-month prospective study, J Clin Endocrinol Metab, 87 (2002) 1088–1093. [DOI] [PubMed] [Google Scholar]
- [62].Rosen T, Eden S, Larson G, Wilhelmsen L, Bengtsson BA, Cardiovascular risk factors in adult patients with growth hormone deficiency, Acta Endocrinol (Copenh), 129 (1993) 195–200. [DOI] [PubMed] [Google Scholar]
- [63].Bulow B, Hagmar L, Mikoczy Z, Nordstrom CH, Erfurth EM, Increased cerebrovascular mortality in patients with hypopituitarism, Clin Endocrinol (Oxf), 46 (1997) 75–81. [DOI] [PubMed] [Google Scholar]
- [64].Bates AS, Van’t Hoff W, Jones PJ, Clayton RN, The effect of hypopituitarism on life expectancy, J Clin Endocrinol Metab, 81 (1996) 1169–1172. [DOI] [PubMed] [Google Scholar]
- [65].Burger AG, Monson JP, Colao AM, Klibanski A, Cardiovascular risk in patients with growth hormone deficiency: effects of growth hormone substitution, Endocr Pract, 12 (2006) 682–689. [DOI] [PubMed] [Google Scholar]
- [66].Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, Ximenes Filho R, de Oliveira Britto AV, Oliveira Souza AH, Prado CM, Pereira Oliveira CR, Pereira RM, Ribeiro Vicente Tde A, Farias CT, Aguiar-Oliveira MH, Salvatori R, Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone (GH) deficiency due to a GH-releasing hormone receptor mutation, J Clin Endocrinol Metab, 91 (2006) 2093–2099. [DOI] [PubMed] [Google Scholar]
- [67].Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD, Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans, Sci Transl Med, 3 (2011) 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Giagulli VA, Castellana M, Perrone R, Guastamacchia E, Iacoviello M, Triggiani V, GH Supplementation Effects on Cardiovascular Risk in GH Deficient Adult Patients: A Systematic Review and Meta-analysis, Endocr Metab Immune Disord Drug Targets, 17 (2017) 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alba M, Fintini D, Bowers CY, Parlow AF, Salvatori R, Effects of long-term treatment with growth hormone-releasing peptide-2 in the GHRH knockout mouse, Am J Physiol Endocrinol Metab, 289 (2005) E762–767. [DOI] [PubMed] [Google Scholar]
- [70].Titterington JS, Sukhanov S, Higashi Y, Vaughn C, Bowers C, Delafontaine P, Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in ApoE−/− mice but does not reduce atherosclerosis, Endocrinology, 150 (2009) 5478–5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ruidavets JB, Luc G, Machez E, Genoux AL, Kee F, Arveiler D, Morange P, Woodside JV, Amouyel P, Evans A, Ducimetiere P, Bingham A, Ferrieres J, Perret B, Effects of insulin-like growth factor 1 in preventing acute coronary syndromes: the PRIME study, Atherosclerosis, 218 (2011) 464–469. [DOI] [PubMed] [Google Scholar]
- [72].De Lorenzo A, Moreira AS, Souza EG, Oliveira GM, Insulin-like growth factor-1 in early-onset coronary artery disease: Insights into the pathophysiology of atherosclerosis, Int J Cardiol, 202 (2016) 1–2. [DOI] [PubMed] [Google Scholar]
- [73].Johnsen SP, Hundborg HH, Sorensen HT, Orskov H, Tjonneland A, Overvad K, Jorgensen JO, Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke, J Clin Endocrinol Metab, 90 (2005) 5937–5941. [DOI] [PubMed] [Google Scholar]
- [74].Kaplan RC, McGinn AP, Pollak MN, Kuller LH, Strickler HD, Rohan TE, Cappola AR, Xue X, Psaty BM, Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFBP-1), and IGFBP-3 levels with incident coronary events and ischemic stroke, J Clin Endocrinol Metab, 92 (2007) 1319–1325. [DOI] [PubMed] [Google Scholar]
- [75].Saber H, Himali JJ, Beiser AS, Shoamanesh A, Pikula A, Roubenoff R, Romero JR, Kase CS, Vasan RS, Seshadri S, Serum Insulin-Like Growth Factor 1 and the Risk of Ischemic Stroke: The Framingham Study, Stroke, 48 (2017) 1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sirbu A, Nicolae H, Martin S, Barbu C, Copaescu C, Florea S, Panea C, Fica S, IGF-1 and Insulin Resistance Are Major Determinants of Common Carotid Artery Thickness in Morbidly Obese Young Patients, Angiology, 67 (2016) 259–265. [DOI] [PubMed] [Google Scholar]
- [77].Kawachi S, Takeda N, Sasaki A, Kokubo Y, Takami K, Sarui H, Hayashi M, Yamakita N, Yasuda K, Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis, Arterioscler Thromb Vasc Biol, 25 (2005) 617–621. [DOI] [PubMed] [Google Scholar]
- [78].Spilcke-Liss E, Friedrich N, Dorr M, Schminke U, Volzke H, Brabant G, Nauck M, Wallaschofski H, Serum insulin-like growth factor I and its binding protein 3 in their relation to intima-media thickness: results of the study of health in Pomerania (SHIP), Clin Endocrinol (Oxf), 75 (2011) 70–75. [DOI] [PubMed] [Google Scholar]
- [79].Ameri P, Canepa M, Fabbi P, Leoncini G, Milaneschi Y, Mussap M, AlGhatrif M, Balbi M, Viazzi F, Murialdo G, Pontremoli R, Brunelli C, Ferrucci L, Vitamin D modulates the association of circulating insulin-like growth factor-1 with carotid artery intima-media thickness, Atherosclerosis, 236 (2014) 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].El-Hafez H Abd, Elrakhawy MM, El-Baiomy AA, El-Eshmawy MM, Carotid Intima Media Thickness Is Independently Associated with Male Gender, Middle Age, and IGF-1 in Metabolically Healthy Obese Individuals, ISRN Obes, 2014 (2014) 545804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang J, Razuvaev A, Folkersen L, Hedin E, Roy J, Brismar K, Hedin U, The expression of IGFs and IGF binding proteins in human carotid atherosclerosis, and the possible role of IGF binding protein-1 in the regulation of smooth muscle cell proliferation, Atherosclerosis, 220 (2012) 102–109. [DOI] [PubMed] [Google Scholar]
- [82].Muller C, Wallaschofski H, Brabant G, Wahnschaffe U, Samietz S, Nauck M, Friedrich N, The association between IGF-I/IGFBP-3 and subclinical end points: epidemiology faces the limits, J Clin Endocrinol Metab, 99 (2014) 2804–2812. [DOI] [PubMed] [Google Scholar]
- [83].Wennberg AMV, Hagen CE, Petersen RC, Mielke MM, Trajectories of plasma IGF-1, IGFBP-3, and their ratio in the Mayo Clinic Study of Aging, Exp Gerontol, 106 (2018) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Sanders JL, Guo W, O’Meara ES, Kaplan RC, Pollak MN, Bartz TM, Newman AB, Fried LP, Cappola AR, Trajectories of IGF-I Predict Mortality in Older Adults: The Cardiovascular Health Study, J Gerontol A Biol Sci Med Sci, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schutte AE, Conti E, Mels CM, Smith W, Kruger R, Botha S, Gnessi L, Volpe M, Huisman HW, Attenuated IGF-1 predicts all-cause and cardiovascular mortality in a Black population: A five-year prospective study, Eur J Prev Cardiol, 23 (2016) 1690–1699. [DOI] [PubMed] [Google Scholar]
- [86].Schutte AE, Huisman HW, van Rooyen JM, Malan L, Malan NT, Fourie CM, Louw R, van der Westhuizen FH, Schutte R, A significant decline in IGF-I may predispose young Africans to subsequent cardiometabolic vulnerability, J Clin Endocrinol Metab, 95 (2010) 2503–2507. [DOI] [PubMed] [Google Scholar]
- [87].Burgers AM, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, Dekkers OM, Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality, J Clin Endocrinol Metab, 96 (2011) 2912–2920. [DOI] [PubMed] [Google Scholar]
- [88].Urbonaviciene G, Frystyk J, Urbonavicius S, Lindholt JS, IGF-I and IGFBP2 in peripheral artery disease: results of a prospective study, Scand Cardiovasc J, 48 (2014) 99–105. [DOI] [PubMed] [Google Scholar]
- [89].Brevetti G, Colao A, Schiano V, Pivonello R, Laurenzano E, Di Somma C, Lombardi G, Chiariello M, IGF system and peripheral arterial disease: relationship with disease severity and inflammatory status of the affected limb, Clin Endocrinol (Oxf), 69 (2008) 894–900. [DOI] [PubMed] [Google Scholar]
- [90].Oxvig C, The role of PAPP-A in the IGF system: location, location, location, J Cell Commun Signal, 9 (2015) 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hjortebjerg R, Tarnow L, Jorsal A, Parving HH, Rossing P, Bjerre M, Frystyk J, IGFBP-4 Fragments as Markers of Cardiovascular Mortality in Type 1 Diabetes Patients With and Without Nephropathy, J Clin Endocrinol Metab, 100 (2015) 3032–3040. [DOI] [PubMed] [Google Scholar]
- [92].Fujimoto M, Hwa V, Dauber A, Novel Modulators of the Growth Hormone - Insulin-Like Growth Factor Axis: Pregnancy-Associated Plasma Protein-A2 and Stanniocalcin-2, J Clin Res Pediatr Endocrinol, 9 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sivasubramaniyam T, Schroer SA, Li A, Luk CT, Shi SY, Besla R, Dodington DW, Metherel AH, Kitson AP, Brunt JJ, Lopes J, Wagner KU, Bazinet RP, Bendeck MP, Robbins CS, Woo M, Hepatic JAK2 protects against atherosclerosis through circulating IGF-1, JCI Insight, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wu Y, Sun H, Yakar S, LeRoith D, Elevated levels of insulin-like growth factor (IGF)-I in serum rescue the severe growth retardation of IGF-I null mice, Endocrinology, 150 (2009) 4395–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Svensson J, Sjogren K, Levin M, Boren J, Tivesten A, Ohlsson C, Increased diet-induced fatty streak formation in female mice with deficiency of liver-derived insulin-like growth factor-I, Endocrine, 52 (2016) 550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sukhanov S, Higashi Y, Shai SY, Blackstock C, Galvez S, Vaughn C, Titterington J, Delafontaine P, Differential requirement for nitric oxide in IGF-1-induced anti-apoptotic, anti-oxidant and anti-atherosclerotic effects, FEBS Lett, 585 (2011) 3065–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].von der Thusen JH, Borensztajn KS, Moimas S, van Heiningen S, Teeling P, J van Berkel T, Biessen EA, IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype, Am J Pathol, 178 (2011) 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P, Low circulating insulin-like growth factor I increases atherosclerosis in Apo E-deficient mice, Am J Physiol Heart Circ Physiol, 300 (2011) H1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ross R, Glomset JA, Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis, Science, 180 (1973) 1332–1339. [DOI] [PubMed] [Google Scholar]
- [100].Schwartz SM, Campbell GR, Campbell JH, Replication of smooth muscle cells in vascular disease, Circ Res, 58 (1986) 427–444. [DOI] [PubMed] [Google Scholar]
- [101].Shanahan CM, Weissberg PL, Smooth muscle cell phenotypes in atherosclerotic lesions, Curr Opin Lipidol, 10 (1999) 507–513. [DOI] [PubMed] [Google Scholar]
- [102].Beneit N, Fernandez-Garcia CE, Martin-Ventura JL, Perdomo L, Escribano O, Michel JB, Garcia-Gomez G, Fernandez S, Diaz-Castroverde S, Egido J, Gomez-Hernandez A, Benito M, Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis, Cardiovasc Diabetol, 15 (2016) 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sun M, Ramchandran R, Chen J, Yang Q, Raj JU, Smooth Muscle Insulin-Like Growth Factor-1 Mediates Hypoxia-Induced Pulmonary Hypertension in Neonatal Mice, Am J Respir Cell Mol Biol, 55 (2016) 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Jia G, Mitra AK, Gangahar DM, Agrawal DK, Insulin-like growth factor-1 induces phosphorylation of PI3K-Akt/PKB to potentiate proliferation of smooth muscle cells in human saphenous vein, Exp Mol Pathol, 89 (2010) 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Shai SY, Sukhanov S, Higashi Y, Vaughn C, Kelly J, Delafontaine P, Smooth muscle cell -specific insulin-like growth factor-1 overexpression in Apoe−/− mice does not alter atherosclerotic plaque burden but increases features of plaque stability, Arterioscler Thromb Vasc Biol, 30 (2010) 1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA, Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury, Endocrinology, 142 (2001) 3598–3606. [DOI] [PubMed] [Google Scholar]
- [107].Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, Brown DJ, Chiu HY, Shyu M, Drapeau BL, Wagner RJ, Powell RJ, Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling, J Biol Chem, 282 (2007) 36112–36120. [DOI] [PubMed] [Google Scholar]
- [108].Hayashi K, Saga H, Chimori Y, Kimura K, Yamanaka Y, Sobue K, Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase, J Biol Chem, 273 (1998) 28860–28867. [DOI] [PubMed] [Google Scholar]
- [109].Liu ZP, Wang Z, Yanagisawa H, Olson EN, Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin, Dev Cell, 9 (2005) 261–270. [DOI] [PubMed] [Google Scholar]
- [110].Blackstock CD, Higashi Y, Sukhanov S, Shai SY, Stefanovic B, Tabony AM, Yoshida T, Delafontaine P, Insulin-like growth factor-1 increases synthesis of collagen type I via induction of the mRNA-binding protein LARP6 expression and binding to the 5’ stem-loop of COL1a1 and COL1a2 mRNA, J Biol Chem, 289 (2014) 7264–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sukhanov s, Higashi y, Shai s Y, Snarski p, Danchuk s, D’ambra v, Tabony m, Woods t C, Hou x, Li z, Ozoe a, Bysani c, Takahashi s I, Delafontaine p, SM22α (Smooth Muscle Protein 22-α) Promoter-Driven IGF1R (Insulin-Like Growth Factor 1 Receptor) Deficiency Promotes Atherosclerosis, Arteriosclerosis, Thrombosis, and Vascular Biology, In press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tonkin J, Temmerman L, Sampson RD, Gallego-Colon E, Barberi L, Bilbao D, Schneider MD, Musaro A, Rosenthal N, Monocyte/Macrophage-derived IGF-1 Orchestrates Murine Skeletal Muscle Regeneration and Modulates Autocrine Polarization, Mol Ther, 23 (2015) 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Tidball JG, Welc SS, Macrophage-Derived IGF-1 Is a Potent Coordinator of Myogenesis and Inflammation in Regenerating Muscle, Mol Ther, 23 (2015) 1134–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Aston C, Jagirdar J, Lee TC, Hur T, Hintz RL, Rom WN, Enhanced insulin-like growth factor molecules in idiopathic pulmonary fibrosis, Am J Respir Crit Care Med, 151 (1995) 1597–1603. [DOI] [PubMed] [Google Scholar]
- [115].Kirstein M, Aston C, Hintz R, Vlassara H, Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins, J Clin Invest, 90 (1992) 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fournier T, Riches DW, Winston BW, Rose DM, Young SK, Noble PW, Lake FR, Henson PM, Divergence in macrophage insulin-like growth factor-I (IGF-I) synthesis induced by TNF-alpha and prostaglandin E2, J Immunol, 155 (1995) 2123–2133. [PubMed] [Google Scholar]
- [117].Furundzija V, Fritzsche J, Kaufmann J, Meyborg H, Fleck E, Kappert K, Stawowy P, IGF-1 increases macrophage motility via PKC/p38-dependent alphavbeta3-integrin inside-out signaling, Biochem Biophys Res Commun, 394 (2010) 786–791. [DOI] [PubMed] [Google Scholar]
- [118].Renier G, Clement I, Desfaits AC, Lambert A, Direct stimulatory effect of insulin-like growth factor-I on monocyte and macrophage tumor necrosis factor-alpha production, Endocrinology, 137 (1996) 4611–4618. [DOI] [PubMed] [Google Scholar]
- [119].Hochberg Z, Hertz P, Maor G, Oiknine J, Aviram M, Growth hormone and insulin-like growth factor-I increase macrophage uptake and degradation of low density lipoprotein, Endocrinology, 131 (1992) 430–435. [DOI] [PubMed] [Google Scholar]
- [120].Sukhanov S, Snarski P, Vaughn C, Lobelle-Rich P, Kim C, Higashi Y, Shai SY, Delafontaine P, Insulin-like growth factor I reduces lipid oxidation and foam cell formation via downregulation of 12/15-lipoxygenase, Atherosclerosis, 238 (2015) 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jeschke MG, Barrow RE, Herndon DN, Insulinlike growth factor I plus insulinlike growth factor binding protein 3 attenuates the proinflammatory acute phase response in severely burned children, Ann Surg, 231 (2000) 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Spies M, Wolf SE, Barrow RE, Jeschke MG, Herndon DN, Modulation of types I and II acute phase reactants with insulin-like growth factor-1/binding protein-3 complex in severely burned children, Crit Care Med, 30 (2002) 83–88. [DOI] [PubMed] [Google Scholar]
- [123].Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC, Myeloid lineage cell-restricted insulin resistance protects apolipoprotein E-deficient mice against atherosclerosis, Cell Metab, 3 (2006) 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR, Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions, Cell Metab, 3 (2006) 257–266. [DOI] [PubMed] [Google Scholar]
- [125].Bornfeldt KE, Tabas I, Insulin resistance, hyperglycemia, and atherosclerosis, Cell Metab, 14 (2011) 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Mauer J, Chaurasia B, Plum L, Quast T, Hampel B, Bluher M, Kolanus W, Kahn CR, Bruning JC, Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance, PLoS Genet, 6 (2010) e1000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Iida KT, Shimano H, Kawakami Y, Sone H, Toyoshima H, Suzuki S, Asano T, Okuda Y, Yamada N, Insulin up-regulates tumor necrosis factor-alpha production in macrophages through an extracellular-regulated kinase-dependent pathway, J Biol Chem, 276 (2001) 32531–32537. [DOI] [PubMed] [Google Scholar]
- [128].Manowsky J, Camargo RG, Kipp AP, Henkel J, Puschel GP, Insulin-induced cytokine production in macrophages causes insulin resistance in hepatocytes, Am J Physiol Endocrinol Metab, 310 (2016) E938–946. [DOI] [PubMed] [Google Scholar]
- [129].Park YM, S RK, J AM, Silverstein RL, Insulin promotes macrophage foam cell formation: potential implications in diabetes-related atherosclerosis, Lab Invest, 92 (2012) 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bach LA, Endothelial cells and the IGF system, J Mol Endocrinol, 54 (2015) R1–13. [DOI] [PubMed] [Google Scholar]
- [131].Higashi Y, Quevedo HC, Tiwari S, Sukhanov S, Shai SY, Anwar A, Delafontaine P, Interaction between insulin-like growth factor-1 and atherosclerosis and vascular aging, Front Horm Res, 43 (2014) 107–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Phinikaridou A, Andia ME, Lavin B, Smith A, Saha P, Botnar RM, Increased Vascular Permeability Measured With an Albumin-Binding Magnetic Resonance Contrast Agent Is a Surrogate Marker of Rupture-Prone Atherosclerotic Plaque, Circ Cardiovasc Imaging, 9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Bake S, Okoreeh AK, Alaniz RC, Sohrabji F, Insulin-Like Growth Factor (IGF)-I Modulates Endothelial Blood-Brain Barrier Function in Ischemic Middle-Aged Female Rats, Endocrinology, 157 (2016) 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liang M, Woodard LE, Liang A, Luo J, Wilson MH, Mitch WE, Cheng J, Protective role of insulin-like growth factor-1 receptor in endothelial cells against unilateral ureteral obstruction-induced renal fibrosis, Am J Pathol, 185 (2015) 1234–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Haurigot V, Villacampa P, Ribera A, Llombart C, Bosch A, Nacher V, Ramos D, Ayuso E, Segovia JC, Bueren JA, Ruberte J, Bosch F, Increased intraocular insulin-like growth factor-I triggers blood-retinal barrier breakdown, J Biol Chem, 284 (2009) 22961–22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Devi TS, Singh LP, Hosoya K, Terasaki T, GSK-3beta/CREB axis mediates IGF-1-induced ECM/adhesion molecule expression, cell cycle progression and monolayer permeability in retinal capillary endothelial cells: Implications for diabetic retinopathy, Biochim Biophys Acta, 1812 (2011) 1080–1088. [DOI] [PubMed] [Google Scholar]
- [137].Spoerri PE, Afzal A, Li Calzi S, Shaw LC, Cai J, Pan H, Boulton M, Grant MB, Effects of VEGFR-1, VEGFR-2, and IGF-IR hammerhead ribozymes on glucose-mediated tight junction expression in cultured human retinal endothelial cells, Mol Vis, 12 (2006) 32–42. [PubMed] [Google Scholar]
- [138].Maile LA, Gollahon K, Wai C, Byfield G, Hartnett ME, Clemmons D, Disruption of the association of integrin-associated protein (IAP) with tyrosine phosphatase non-receptor type substrate-1 (SHPS)-1 inhibits pathophysiological changes in retinal endothelial function in a rat model of diabetes, Diabetologia, 55 (2012) 835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Davignon J, Ganz P, Role of endothelial dysfunction in atherosclerosis, Circulation, 109 (2004) III27–32. [DOI] [PubMed] [Google Scholar]
- [140].Zeiher AM, Drexler H, Wollschlager H, Just H, Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis, Circulation, 84 (1991) 1984–1992. [DOI] [PubMed] [Google Scholar]
- [141].Cho YL, Hur SM, Kim JY, Kim JH, Lee DK, Choe J, Won MH, Ha KS, Jeoung D, Han S, Ryoo S, Lee H, Min JK, Kwon YG, Kim DH, Kim YM, Specific activation of insulin-like growth factor-1 receptor by ginsenoside Rg5 promotes angiogenesis and vasorelaxation, J Biol Chem, 290 (2015) 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Tsukahara H, Gordienko DV, Tonshoff B, Gelato MC, Goligorsky MS, Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells, Kidney Int, 45 (1994) 598–604. [DOI] [PubMed] [Google Scholar]
- [143].Perticone F, Sciacqua A, Perticone M, Laino I, Miceli S, Care I, Galiano Leone G, Andreozzi F, Maio R, Sesti G, Low-plasma insulin-like growth factor-I levels are associated with impaired endothelium-dependent vasodilatation in a cohort of untreated, hypertensive Caucasian subjects, J Clin Endocrinol Metab, 93 (2008) 2806–2810. [DOI] [PubMed] [Google Scholar]
- [144].Abbas A, Imrie H, Viswambharan H, Sukumar P, Rajwani A, Cubbon RM, Gage M, Smith J, Galloway S, Yuldeshava N, Kahn M, Xuan S, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT, The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium, Diabetes, 60 (2011) 2169–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Imrie H, Viswambharan H, Sukumar P, Abbas A, Cubbon RM, Yuldasheva N, Gage M, Smith J, Galloway S, Skromna A, Rashid ST, Futers TS, Xuan S, Gatenby VK, Grant PJ, Channon KM, Beech DJ, Wheatcroft SB, Kearney MT, Novel role of the IGF-1 receptor in endothelial function and repair: studies in endothelium-targeted IGF-1 receptor transgenic mice, Diabetes, 61 (2012) 2359–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Viswambharan H, Yuldasheva NY, Sengupta A, Imrie H, Gage MC, Haywood N, Walker AM, Skromna A, Makova N, Galloway S, Shah P, Sukumar P, Porter KE, Grant PJ, Shah AM, Santos CX, Li J, Beech DJ, Wheatcroft SB, Cubbon RM, Kearney MT, Selective Enhancement of Insulin Sensitivity in the Endothelium In Vivo Reveals a Novel Proatherosclerotic Signaling Loop, Circ Res, 120 (2017) 784–798. [DOI] [PubMed] [Google Scholar]
- [147].Jacobo SM, Kazlauskas A, Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels, J Biol Chem, 290 (2015) 6349–6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, Cleutjens JP, van den Akker LH, Corvol P, Wouters BG, Daemen MJ, Bijnens AP, Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis, J Am Coll Cardiol, 51 (2008) 1258–1265. [DOI] [PubMed] [Google Scholar]
- [149].Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J, Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage, Arterioscler Thromb Vasc Biol, 25 (2005) 2054–2061. [DOI] [PubMed] [Google Scholar]
- [150].Camare C, Pucelle M, Negre-Salvayre A, Salvayre R, Angiogenesis in the atherosclerotic plaque, Redox Biol, 12 (2017) 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM, Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells, Circulation, 105 (2002) 3017–3024. [DOI] [PubMed] [Google Scholar]
- [152].Foteinos G, Hu Y, Xiao Q, Metzler B, Xu Q, Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice, Circulation, 117 (2008) 1856–1863. [DOI] [PubMed] [Google Scholar]
- [153].Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J, Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1, Circ Res, 100 (2007) 434–443. [DOI] [PubMed] [Google Scholar]
- [154].Thum T, Fleissner F, Klink I, Tsikas D, Jakob M, Bauersachs J, Stichtenoth DO, Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I, J Clin Endocrinol Metab, 92 (2007) 4172–4179. [DOI] [PubMed] [Google Scholar]
- [155].van der Klaauw AA, Pereira AM, Rabelink TJ, Corssmit EP, Zonneveld AJ, Pijl H, de Boer HC, Smit JW, Romijn JA, de Koning EJ, Recombinant human GH replacement increases CD34+ cells and improves endothelial function in adults with GH deficiency, Eur J Endocrinol, 159 (2008) 105–111. [DOI] [PubMed] [Google Scholar]
- [156].Cittadini A, Monti MG, Castiello MC, D’Arco E, Galasso G, Sorriento D, Saldamarco L, De Paulis A, Napoli R, Iaccarino G, Sacca L, Insulin-like growth factor-1 protects from vascular stenosis and accelerates re-endothelialization in a rat model of carotid artery injury, J Thromb Haemost, 7 (2009) 1920–1928. [DOI] [PubMed] [Google Scholar]
- [157].Bellastella G, Maiorino MI, Pivonello R, Grasso LF, Galdiero M, Sinisi AA, Colao A, Giugliano D, Esposito K, Circulating endothelial progenitor cells in acromegaly, J Endocrinol Invest, 36 (2013) 825–830. [DOI] [PubMed] [Google Scholar]
- [158].Fadini GP, Dassie F, Albiero M, Boscaro E, Albano I, Martini C, de Kreutzenberg SV, Agostini C, Avogaro A, Vettor R, Maffei P, Endothelial progenitor cells are reduced in acromegalic patients and can be restored by treatment with somatostatin analogs, J Clin Endocrinol Metab, 99 (2014) E2549–2556. [DOI] [PubMed] [Google Scholar]
- [159].Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A, Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging, J Gerontol A Biol Sci Med Sci, 67 (2012) 313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Higashi Y, Pandey A, Goodwin B, Delafontaine P, Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1, Biochim Biophys Acta, 1832 (2013) 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Munzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC, Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series, J Am Coll Cardiol, 70 (2017) 212–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Sack MN, Fyhrquist FY, Saijonmaa OJ, Fuster V, Kovacic JC, Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series, J Am Coll Cardiol, 70 (2017) 196–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mazerbourg S, Monget P, Insulin-Like Growth Factor Binding Proteins and IGFBP Proteases: A Dynamic System Regulating the Ovarian Folliculogenesis, Front Endocrinol (Lausanne), 9 (2018) 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Conover CA, Key questions and answers about pregnancy-associated plasma protein-A, Trends Endocrinol Metab, 23 (2012) 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR Jr, Virmani R, Oxvig C, Schwartz RS, Pregnancy-associated plasma protein A as a marker of acute coronary syndromes, N Engl J Med, 345 (2001) 1022–1029. [DOI] [PubMed] [Google Scholar]
- [166].Beaudeux JL, Burc L, Imbert-Bismut F, Giral P, Bernard M, Bruckert E, Chapman MJ, Serum plasma pregnancy-associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk, Arterioscler Thromb Vasc Biol, 23 (2003) e7–10. [DOI] [PubMed] [Google Scholar]
- [167].Lund J, Qin QP, Ilva T, Pettersson K, Voipio-Pulkki LM, Porela P, Pulkki K, Circulating pregnancy-associated plasma protein a predicts outcome in patients with acute coronary syndrome but no troponin I elevation, Circulation, 108 (2003) 1924–1926. [DOI] [PubMed] [Google Scholar]
- [168].Bonaca MP, Scirica BM, Sabatine MS, Jarolim P, Murphy SA, Chamberlin JS, Rhodes DW, Southwick PC, Braunwald E, Morrow DA, Prospective evaluation of pregnancy-associated plasma protein-a and outcomes in patients with acute coronary syndromes, J Am Coll Cardiol, 60 (2012) 332–338. [DOI] [PubMed] [Google Scholar]
- [169].Conti E, Volpe M, Carrozza C, Marzo F, Zuppi C, Crea F, Andreotti F, Pregnancy-associated plasma protein-A and acute coronary syndromes: cause or consequence?, J Am Coll Cardiol, 46 (2005) 1583–1584; author reply 1584–1585. [DOI] [PubMed] [Google Scholar]
- [170].Iversen K, Teisner A, Dalager S, Olsen KE, Floridon C, Teisner B, Pregnancy associated plasma protein-A (PAPP-A) is not a marker of the vulnerable atherosclerotic plaque, Clin Biochem, 44 (2011) 312–318. [DOI] [PubMed] [Google Scholar]
- [171].Conover CA, Bale LK, Powell DR, Inducible knock out of pregnancy-associated plasma protein-a gene expression in the adult mouse: effect on vascular injury response, Endocrinology, 154 (2013) 2734–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Harrington SC, Simari RD, Conover CA, Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet, Circ Res, 100 (2007) 1696–1702. [DOI] [PubMed] [Google Scholar]
- [173].Conover CA, Mason MA, Bale LK, Harrington SC, Nyegaard M, Oxvig C, Overgaard MT, Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development, Am J Physiol Heart Circ Physiol, 299 (2010) H284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Bale LK, Chakraborty S, Conover CA, Inducible reduction in pregnancy-associated plasma protein-A gene expression inhibits established atherosclerotic plaque progression in mice, Endocrinology, 155 (2014) 1184–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hill CM, Arum O, Boparai RK, Wang F, Fang Y, Sun LY, Masternak MM, Bartke A, Female PAPP-A knockout mice are resistant to metabolic dysfunction induced by high-fat/high-sucrose feeding at middle age, Age (Dordr), 37 (2015) 9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Boldt HB, Bale LK, Resch ZT, Oxvig C, Overgaard MT, Conover CA, Effects of mutated pregnancy-associated plasma protein-a on atherosclerotic lesion development in mice, Endocrinology, 154 (2013) 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mohan S, Baylink DJ, IGF-binding proteins are multifunctional and act via IGF-dependent and - independent mechanisms, J Endocrinol, 175 (2002) 19–31. [DOI] [PubMed] [Google Scholar]
- [178].Moreno MJ, Ball M, Rukhlova M, Slinn J, L’Abbe D, Iqbal U, Monette R, Hagedorn M, O’Connor-McCourt MD, Durocher Y, Stanimirovic DB, IGFBP-4 anti-angiogenic and anti-tumorigenic effects are associated with anti-cathepsin B activity, Neoplasia, 15 (2013) 554–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Conover CA, Bale LK, Oxvig C, Targeted Inhibition of Pregnancy-Associated Plasma Protein-A Activity Reduces Atherosclerotic Plaque Burden in Mice, J Cardiovasc Transl Res, 9 (2016) 77–79. [DOI] [PubMed] [Google Scholar]
- [180].Steffensen LB, Conover CA, Bjorklund MM, Ledet T, Bentzon JF, Oxvig C, Stanniocalcin-2 overexpression reduces atherosclerosis in hypercholesterolemic mice, Atherosclerosis, 248 (2016) 36–43. [DOI] [PubMed] [Google Scholar]
- [181].Radhakrishnan Y, Shen X, Maile LA, Xi G, Clemmons DR, IGF-I stimulates cooperative interaction between the IGF-I receptor and CSK homologous kinase that regulates SHPS-1 phosphorylation in vascular smooth muscle cells, Mol Endocrinol, 25 (2011) 1636–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Xi G, Wai C, White MF, Clemmons DR, Down-regulation of Insulin Receptor Substrate 1 during Hyperglycemia Induces Vascular Smooth Muscle Cell Dedifferentiation, J Biol Chem, 292 (2017) 2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Clemmons DR, Maile LA, Ling Y, Yarber J, Busby WH, Role of the integrin alphaVbeta3 in mediating increased smooth muscle cell responsiveness to IGF-I in response to hyperglycemic stress, Growth Horm IGF Res, 17 (2007) 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Maile LA, Busby WH, Nichols TC, Bellinger DA, Merricks EP, Rowland M, Veluvolu U, Clemmons DR, A monoclonal antibody against alphaVbeta3 integrin inhibits development of atherosclerotic lesions in diabetic pigs, Sci Transl Med, 2 (2010) 18ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Xi G, Shen X, Wai C, Vilas CK, Clemmons DR, Hyperglycemia stimulates p62/PKCzeta interaction, which mediates NF-kappaB activation, increased Nox4 expression, and inflammatory cytokine activation in vascular smooth muscle, FASEB J, 29 (2015) 4772–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Song CL, Liu B, Diao HY, Shi YF, Zhang JC, Li YX, Liu N, Yu YP, Wang G, Wang JP, Li Q, Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1, Oncotarget, 7 (2016) 39740–39757. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [187].Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD, Regulation of progenitor cell proliferation and granulocyte function by microRNA-223, Nature, 451 (2008) 1125–1129. [DOI] [PubMed] [Google Scholar]
- [188].Shi L, Kojonazarov B, Elgheznawy A, Popp R, Dahal BK, Bohm M, Pullamsetti SS, Ghofrani HA, Godecke A, Jungmann A, Katus HA, Muller OJ, Schermuly RT, Fisslthaler B, Seeger W, I Fleming, miR-223-IGF-IR signalling in hypoxia- and load-induced right-ventricular failure: a novel therapeutic approach, Cardiovasc Res, 111 (2016) 184–193. [DOI] [PubMed] [Google Scholar]
- [189].Abonnenc M, Nabeebaccus AA, Mayr U, Barallobre-Barreiro J, Dong X, Cuello F, Sur S, Drozdov I, Langley SR, Lu R, Stathopoulou K, Didangelos A, Yin X, Zimmermann WH, Shah AM, Zampetaki A, Mayr M, Extracellular matrix secretion by cardiac fibroblasts: role of microRNA-29b and microRNA-30c, Circ Res, 113 (2013) 1138–1147. [DOI] [PubMed] [Google Scholar]
- [190].Gao S, Wassler M, Zhang L, Li Y, Wang J, Zhang Y, Shelat H, Williams J, Geng YJ, MicroRNA-133a regulates insulin-like growth factor-1 receptor expression and vascular smooth muscle cell proliferation in murine atherosclerosis, Atherosclerosis, 232 (2014) 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Sun Y, Chen D, Cao L, Zhang R, Zhou J, Chen H, Li Y, Li M, Cao J, Wang Z, MiR-490–3p modulates the proliferation of vascular smooth muscle cells induced by ox-LDL through targeting PAPP-A, Cardiovasc Res, 100 (2013) 272–279. [DOI] [PubMed] [Google Scholar]
- [192].van Niel G, D’Angelo G, Raposo G, Shedding light on the cell biology of extracellular vesicles, Nat Rev Mol Cell Biol, 19 (2018) 213–228. [DOI] [PubMed] [Google Scholar]
- [193].Cavallari C, Ranghino A, Tapparo M, Cedrino M, Figliolini F, Grange C, Giannachi V, Garneri P, Deregibus MC, Collino F, Rispoli P, Camussi G, Brizzi MF, Serum-derived extracellular vesicles (EVs) impact on vascular remodeling and prevent muscle damage in acute hind limb ischemia, Sci Rep, 7 (2017) 8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H, Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction, Int J Cardiol, 178 (2015) 239–246. [DOI] [PubMed] [Google Scholar]
- [195].Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, Bolis S, Altomare C, Matteucci M, Di Silvestre D, Brambilla F, Fertig TE, Torre T, Demertzis S, Mauri P, Moccetti T, Vassalli G, Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A, Cardiovasc Res, 114 (2018) 992–1005. [DOI] [PubMed] [Google Scholar]
- [196].Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC, Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells, J Mol Cell Cardiol, 74 (2014) 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K, Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor, J Immunol, 192 (2014) 437–446. [DOI] [PubMed] [Google Scholar]
- [198].Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J, Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury, Basic Res Cardiol, 112 (2017) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Daugherty A, Tall AR, Daemen M, Falk E, Fisher EA, Garcia-Cardena G, Lusis AJ, Owens AP 3rd Rosenfeld ME, Virmani R, T American Heart Association Council on Arteriosclerosis, Vascular B, S Council on Basic Cardiovascular, Recommendation on Design, Execution, and Reporting of Animal Atherosclerosis Studies: A Scientific Statement From the American Heart Association, Circ Res, 121 (2017) e53–e79. [DOI] [PubMed] [Google Scholar]
- [200].French JE, Atherosclerosis in relation to the structure and function of the arterial intima, with special reference to th endothelium, Int Rev Exp Pathol, 5 (1966) 253–353. [PubMed] [Google Scholar]
- [201].Stout LC, Pathogenesis of diffuse intimal thickening (DIT) in aortas and coronary arteries of 2 1/2-year-old miniature pigs, Exp Mol Pathol, 37 (1982) 427–432. [DOI] [PubMed] [Google Scholar]
- [202].Schwartz SM, deBlois D, O’Brien ER, The intima. Soil for atherosclerosis and restenosis, Circ Res, 77 (1995) 445–465. [DOI] [PubMed] [Google Scholar]