Abstract
Configurationally stable, atropisomeric motifs are an important structural element in a number of molecules, including chiral ligands, catalysts, and molecular devices. Thus, understanding features that stabilize chiral axes is of fundamental interest throughout the chemical sciences. The following details the high rotational barriers about the Ar–C(O) bond of tropone amides, which significantly exceed those of analogous benzamides. These studies are supported by both experimental and computational rotational barrier measurements. We also report the resolution of an axially chiral α-hydroxytropolone amide into its individual atropisomers, and demonstrate its configurational stability at physiological pH and temperatures over 24 hours.
Graphical Absract:
Atropisomerism, a form of chirality arising from restricted rotation about an asymmetric axis, plays an important role in a number of functional molecules1 including chiral biaryl ligands and catalysts (e.g., 1,2 Figure 1A),3 as well as unidirectional molecular devices and switches.4 Single atropisomer therapeutics also exist, although they often have their roots in natural products (e.g., 2,5 Figure 1A).6 However, given the critical importance of chirality in drug development, they are also becoming increasingly prevalent in de novo drug design (e.g., 3,7 Figure 1A).8 Compared to 5- and 6-membered aromatic rings commonly found in drugs, troponoids should have an increased likelihood of being atropisomeric due to their decreased external bond angles (Figure 1B). However, other features such as tropylium characteristics,9 ring puckering,10 and decreased aromaticity11 could influence the rotational barriers, as well. Only a few troponoids are known to exhibit atropisomerism, including colchicine (2, Figure 1A, ΔG‡298 K = 22 kcal/mol)5 and bistropone homodimer 4 (Figure 1C, ΔG‡298 K = 20.7 kcal/mol),12 both of which have relatively low rotational barriers. Given the growing interest in troponoid drug development,13 as well as the importance of atropisomerism throughout the chemical sciences, understanding how this motif influences atropisomerism compared to benzenoids would be helpful in designing new, functional atropisomeric molecules.
Figure 1. Atropisomerism and troponoids.
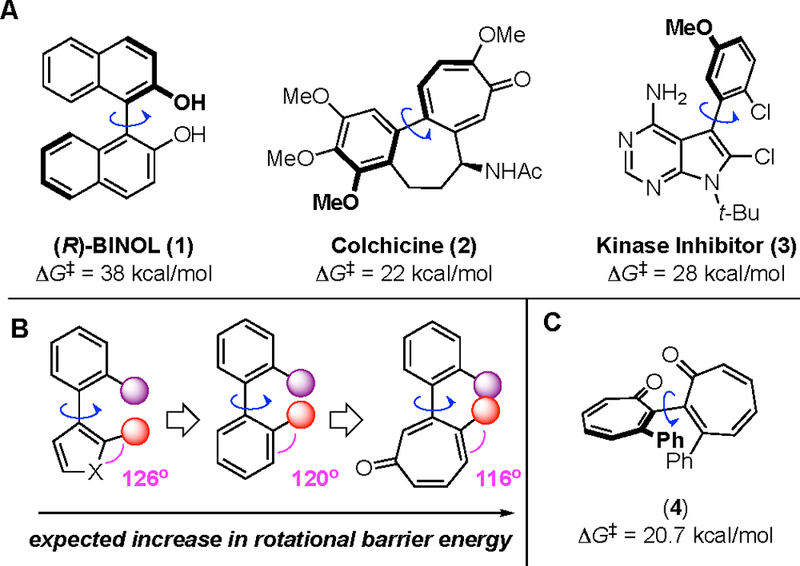
(A) Examples of valuable molecules exhibiting atropisomerism. (B) External bond angle differences between 5, 6, and 7-membered aromatic rings, and their influence on proximity of ortho-substituents. (C) Bistropone homodimer 4 with an experimental rotational barrier measurement.
Our investigation began with DFT computations on a series of axially chiral tropolones and analogous benzenoids (Table 1).14 Troponoid substrates consistently exhibited higher rotational barriers than those of the corresponding benzenoids, with rotational energy barrier increases of up to 4.3 kcal/mol (Entry 4), and increases in half-lives to enantiomerization at room temperature of up to four orders of magnitude.
Table 1. Computed rotational energy barriers of related troponoids and benzenoids.
Rotational barriers were computed at the M06–2X/6–311++G(2d,3p)//B3LYP/6–31+G(d,p) level of theory at 298.15 K and 1 atm using the Gaussian 09 suite.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Benzenoids | Troponoids | |||||||
| Entry | R1 | R2 | No. |
t½ (s) |
ΔG‡ (kcal/mol) |
No. |
t½ (s) |
ΔG‡ (kcal/mol) |
| 1 | Ph | Me | 5a | 6 × 10–11 | 3.7 | 6a | 1 × 10–8 | 6.9 |
| 2 | N(CH2)5 | H | 5b | 6 × 10–6 | 10.5 | 6b | 1 × 10–4 | 12.3 |
| 3 | N(CH2)5 | Me | 5c | 4 × 10–4 | 13.0 | 6c | 0.3 | 16.9 |
| 4 | N(i-Pr)2 | Me | 5d | 5 × 10–4 | 13.2 | 6d | 0.7 | 17.5 |
To confirm these energy barriers experimentally, we turned to α-hydroxytropolone 7 and benzamide 8 (Figure 2A, B), which were readily accessible from the corresponding carboxylic acids.15,16 1H NMR spectra of these molecules have diagnostic diastereotopic signals and observable E and Z amide isomers useful for probing the configurational stability of the Ar–C(O) and N–C(O) axes, respectively, through variable temperature NMR experiments (Figure 2A, B). Prior studies on hindered benzamides have established that, in addition to isolated Ar–C(O) and N–C(O) rotations, isomerization can also proceed through an often energetically intermediate, concerted Ar–C(O)/N–C(O) process (Figure 2C).17 Consistent with this precedent, computational modeling on both 7 and 8 revealed that Ar–C(O) rotational barriers were lower in energy than N–C(O) rotation, and the concerted Ar–C(O)/N–C(O) rotation was the lowest energy pathway for effective N–C(O) amide isomerization (Figure 2C). These results were also validated by variable temperature NMR experiments. Heating a solution of 7 in DMSO-d6 led to coalescence of only the diastereotopic signals, which allowed us to measure the individual rotational energy barriers of the Ar–C(O) bonds of both E and Z isomers (ΔG‡E = 16.7 kcal/mol, ΔG‡Z =16.5 kcal/mol). Conversely, cooling a sample of 8 revealed proton diastereotopicity, and allowed an experimental Ar–C(O) rotational barrier measurement (ΔG‡E = 12.2 kcal/mol, ΔG‡Z = 12.1 kcal/mol, Figure 2B). Finally, while no coalescence of the amide rotamers of 7 was observed at higher temperatures, heating a solution of 8 in DMSO-d6 to 90 °C led to complete coalescence of its amide rotamers (Figure 2B). The increased stability of the N–C(O) axis of 7 relative to 8 demonstrates the ancillary rigidity provided by the tropone.
Figure 2.
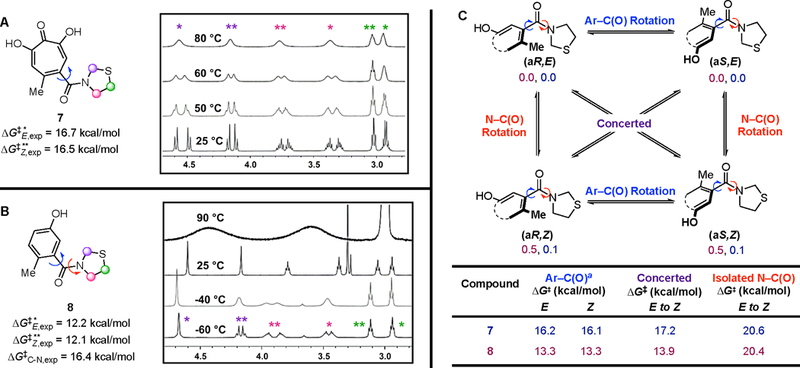
Variable temperature 1H-NMR spectra of (A) troponoid, 7 (DMSO-d6), and (B) benzamide, 8 (CD2Cl2 and DMSO-d6) on an Agilent 500 MHz NMR spectrometer. The peaks assigned to the protons of the Z and E amide conformers are denoted by (*) and (**), respectively. Peak intensities normalized for clarity. (C) Proposed pathways to enantiomerization (Ar–C(O) rotation) and amide isomerization (N–C(O) rotation) for differentially substituted thiazolidine systems with energy values (kcal/mol) indicated for ground states, as well as computed rotational barriers denoted in the subsequent table (calculated at the M06–2X-D3/6–311++G(2d,3p) level of theory with using the Gaussian 09 suite). a Obtained from considering both isolated Ar-C(O) and concerted pathways.
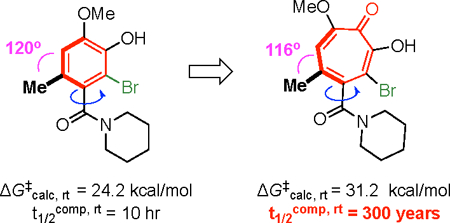
In order to observe these rotational barrier differences in more configurationally stable systems, we modeled brominated variants of the compounds shown in Table 1 (Figure 3A).7,14,18 Rotational barriers increased by upwards of 11 kcal/mol for the benzenoids and by 14 kcal/mol for the troponoids. A corollary of this effect is observed in the half-life to enantiomerization of 10c, which was computed to take place over centuries at room temperature (t½, 298 K = ~300 years), relative to hours for benzenoid 9c (t½, 298 K = ~10 hours). Based on the classification system put forward by LaPlante and co-workers, these two molecules are considered to be class 3 (ΔG‡ > 28 kcal/mol) and class 2 (ΔG‡ ≈ 20–28 kcal/mol) atropisomers, respectively (Figure 3B).19 Class 2 molecules fall into a category where the molecules are atropisomeric, but the rotational barriers might not be high enough to develop them as single atropisomers. Class 3 molecules are those with rotational barriers sufficiently high to confidently develop as single-atropisomer drugs.
Figure 3.
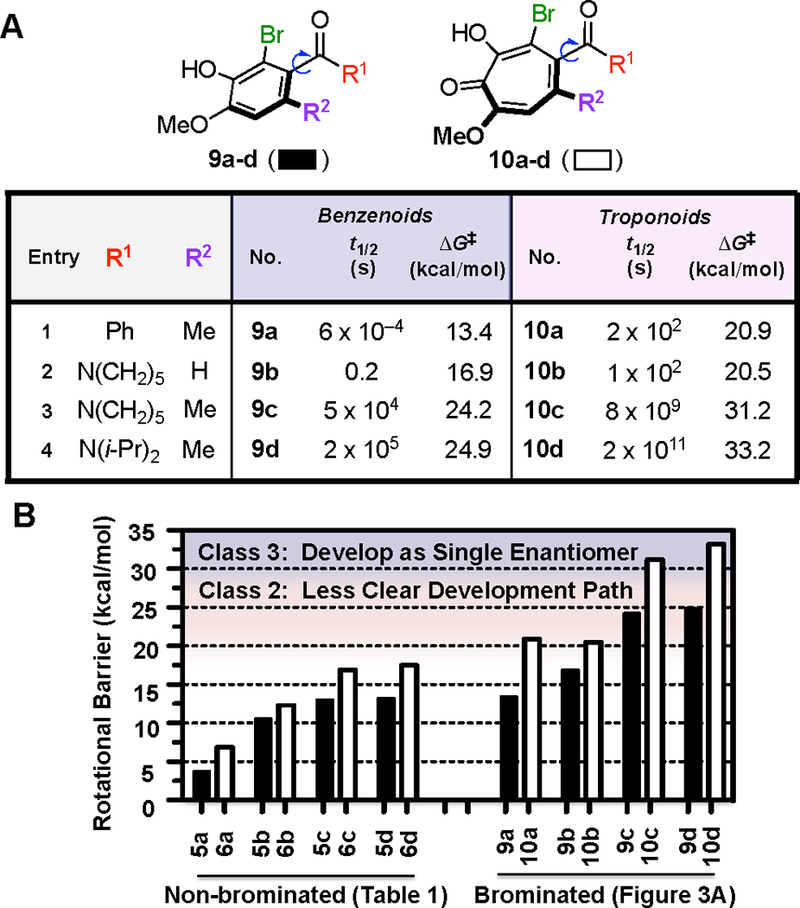
(A) Computed rotational energy barriers of brominated troponoids and benzenoids, with (B) comparison to non-brominated molecules. Class 2 = intermediate barrier and Class 3 = stable atropisomerism. Rotational barriers were computed at the M06–2X/6–311++G(2d,3p)//B3LYP/6–31+G(d,p) level of theory at 298.15 K and 1 atm using the Gaussian 09 suite.
To obtain the quantities of enantioenriched 10c necessary to confirm these high energy barriers experimentally, we turned our attention to a peptide-catalyzed dynamic kinetic atroposelective halogenation14,18,20 that had been established on structurally analogous benzamides (i.e. 11 vs. 6c, Figure 4A/B).20a Employing slightly modified conditions to those used previously on 11, we were able to obtain 10c in 75:25 er.21 We next monitored enantioerosion of a solution of enantioenriched 10c (93:7 er) in triglyme over 60 minutes at 145 °C, and obtained an experimental energy barrier of 30.1 kcal/mol.16 A discrepancy between this value and that computed using DFT at the same temperature (ΔG‡418 K = 32.7 kcal/mol) may be due to the triglyme solvent.
Figure 4.
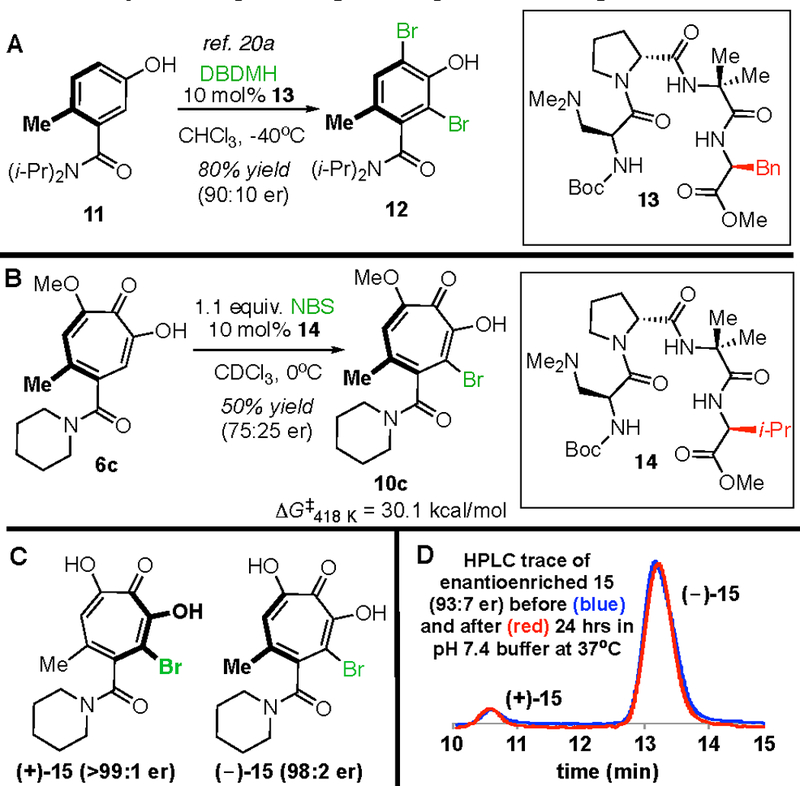
(A) Atroposelective halogenation of close troponoid structural homolog 11 using peptide 13. (B) Peptide 14 was found to deliver enantioenriched 10c. (C) a-Hydroxytropolone atropisomers of 15. (D) Enantiomerically enriched (–)-15 before (blue) and after (red) incubation at 37 °C in phosphate buffer (pH = 7.4) as monitored by analytical HPLC (CHIRALPAK® IC, 250 mm, i.d. 4.6 mm, 30% acetonitrile in water with 0.1% TFA, 1.5 mL/min). Peak intensities normalized for clarity.
Methoxytropolones, such as 10c, are known precursors to α-hydroxytropolones,22 which are promising dinuclear metalloenzyme-inhibiting fragments we have been leveraging in drug-development pursuits for a number of different diseases.23 The high energy barriers we identified for 10c suggested that structurally analogous α-hydroxytropolones could be studied as single atropisomers. α-Hydroxytropolone 15 was thus synthesized through demethylation of 10c and resolved into individual enantiomers with a preparatory scale chiral stationary phase column.16 To test stability under physiological conditions, an enantiomerically enriched sample consisting of 93% (−)-15 and 7% (+)-15 was dissolved in phosphate buffer (pH = 7.4) and heated to 37 °C for 24 hours; no change was observed in the enantiomeric ratio over this time period (Figure 4E). This result is important for tropolone development as single atropisomers, since ionization states can change rotational barriers24 and tropolones are likely to exist in an anionic state at physiological pH.25
In conclusion, computational and experimental rotational barrier measurements demonstrate that tropone-amide chiral axes are substantially higher than those of analogous benzamides. We also synthesized and resolved a configurationally stable, axially chiral α-hydroxytropolone amide, and we found the enantiomers to be stable at physiological temperature and pH for over 24 hrs. Given the importance of configurationally stable atropisomeric molecules throughout the chemical sciences, these studies suggest that tropones could find a valuable role in atropisomeric molecule design.
Supplementary Material
ACKNOWLEDGMENT
This work is supported by the National Institute of General Medicinal Sciences of the United States National Institutes of Health in the form of Grants to RPM (SC1GM111158) and SJM (R37GM068649). DRH was supported by the CUNY Mina Rees Dissertation Fellowship. AJM was supported by the NSF Graduate Research Fellowship Program. EAS acknowledges the NIH Molecular Biophysical Predoctoral Training Grant (T32 GM008283) and the NSF Graduate Research Fellowship Program for research support. GS is grateful to the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship (STO 1175/1–1). We also want to thank Dr. Sean Colvin (Yale University) and John Stasiak (Brooklyn College) for technical assistance.
Footnotes
Supporting Information
Supporting information including detailed experimental procedures and 1H and 13C NMR spectra. These materials are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) For lead reviews, see: Kumarasamy E; Raghunathan R; Sibi MP; Sivaguru, J. Chem. Rev 2015, 115, 11239–300. [DOI] [PubMed] [Google Scholar]; (b) Gluntz PW Biorg. Med. Chem. Lett 2018, 28, 53–60. [DOI] [PubMed] [Google Scholar]
- (2).Meca L; Řeha D; Havlas ZJ Org. Chem 2003, 68, 5677– 80. [DOI] [PubMed] [Google Scholar]
- (3).(a) Tang W; Zhang X Chem. Rev 2003, 103, 3029–3069. [DOI] [PubMed] [Google Scholar]; (b) Cardo-so FSP; Abboud KA; Aponick AJ Am. Chem. Soc 2013, 135, 14548–51. [DOI] [PubMed] [Google Scholar]; (c) Fernández E; Guiry PJ; Connole KP; Brown JM J. Org. Chem 2014, 79, 5391–400. [DOI] [PubMed] [Google Scholar]
- (4).(a) For lead examples, see: Kelly TR; De Silva H; Silva RA Nature 1999, 401, 150–152. [DOI] [PubMed] [Google Scholar]; (b) Collins BSK; Kistemaker JCM; Otten E; Feringa BL Nat. Chem 2016, 8, 860–866. [Google Scholar]; (c) Feringa BL; Koumura N; Zijlstra RWJ; Van Delden RA; Harada N Nature, 1999, 401, 152–155. [DOI] [PubMed] [Google Scholar]
- (5).(a) Hartung E Ann Rhem. Dis 1954, 13, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brossi A; Yeh HJ; Chrzanowska M; Wolff J; Hamel E; Lin CM; Quin F; Suffness M; Silverton J Med. Res. Rev 1988, 8, 77–94. [DOI] [PubMed] [Google Scholar]
- (6).Zask A; Murphy J; Ellestad GA Chirality, 2013, 25, 265–274. [DOI] [PubMed] [Google Scholar]
- (7).Smith DE; Marquez I; Lokensgard ME; Rheingold AL; Hecht DA; Gustafson JL Angew. Chem. Int. Ed 2015, 54, 11754–11759. [DOI] [PubMed] [Google Scholar]
- (8).(a) For select recent examples, see: Chandrasekhar J; Dick R; Van Veldhuizen J; Koditek D; Lepist E-Ir; McGrath ME; Patel L; Phillips G; Sedillo K; Somoza JR; Therrien J; Till NA; Treiberg J; Villasenor AG; Zherebina Y; Perreault S J. Med. Chem 2018, 61, 6858–6868.. [DOI] [PubMed] [Google Scholar]; (b) Beutner G; Carrasquillo R; Geng P; Hsaio Y; Huang EC; Janey J; Katipally K; Kolotuchin S; La Porte T; Lee A; Lobben P; Lora-Gonzalez F; Mack B; Mudryk B; Qiu Y; Qian X; Ramirez A; Razler TM; Rosner T; Shi Z; Simmons E; Stevens J; Wang J; Wei C; Wisniewski SR; Zhu Y Org. Lett 2018, 20, 3736– 3740 [DOI] [PubMed] [Google Scholar]; (c) Fordyce EAF; Fraser Hunt S; Crepin D; Onions ST; Parra GF; Sleigh CJ; King-Underwood J; Finch H; Murray J Med. Chem. Commun 2018, 9, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]; For recent review, see: (d) Toenjes ST; Gustafson JL Future Med. Chem 2018, 10, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pauson PL Chem. Rev 1955, 55, 9–136. [Google Scholar]
- (10).Morita H; Matsumoto K; Takeya K; Itokawa H Chem. Pharm. Bull 1993, 41, 1478–1480. [DOI] [PubMed] [Google Scholar]
- (11).Williams RV; Edwards WD; Zhang P; Berg DJ; Mitchell RH J. Am. Chem. Soc 2012, 134, 16742–16752. [DOI] [PubMed] [Google Scholar]
- (12).Cavazza M; Cifelli M; Domenici B; Funaioli T; Munnucci B; Veracini CA; Zandomeneghi M Chirality, 2013, 25, 648–655. [DOI] [PubMed] [Google Scholar]
- (13).(a) For some lead examples, see: Sennari G; Saito R; Hirose T; Iwatsuki M; Ishayama A; Hokari R; Otoguro K; Omura S; Sunazuka T Sci. Rep 2017, 7, 7259. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Donlin MJ; Zunica A; Lipnicky A; Garimallaprabhakaran AK; Berkowitz AJ; Grigoryan A; Meyers MJ; Tavis JE; Murelli RP Antimicrob. Agents Chemother 2017, 61, e02574–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sato D Tayuka K; Kiminori O Bioorg. Med. Chem 2018, 26, 536–42. [DOI] [PubMed] [Google Scholar]; For a review, see: (d) Meck C; D’Erasmo MP; Hirsch DR; Murelli RP Med. Chem. Comm 2014, 5, 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Barrett KT; Metrano AJ; Rablen PR; Miller SJ Nature, 2014, 509, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Berkowitz AJ; Abdelmessih RG; Murelli RP Tetrahedron Lett 2018, 59, 3026–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16). See supporting information for details.
- (17).(a) Ahmed A; Bragg RA; Clayden J; Lai LW; McCarthy C; Pink JH; Westlund N; Yasin SA Tetrahedron, 1998, 54, 13277–13294. [Google Scholar]; (b) Bragg RA; Clayden J; Morris GA; Pink JH Chem. Eur. J 2002, 8, 1279–89. [DOI] [PubMed] [Google Scholar]; (c) Bragg RA; Clayden J Org. Lett 2000, 2, 3351–4; [DOI] [PubMed] [Google Scholar]; (d) Clayden J; Pink JH Angew. Chem., Int. Ed 1998, 37, 1937–9. [Google Scholar]
- (18).(a) For examples, see: Miyaji R; Asano K; Matsubara S J. Am. Chem. Soc 2015, 137, 6766–9. [DOI] [PubMed] [Google Scholar]; (b) Stodulski M; Kohlkepp SV; Raabe G; Gulder T Eur. J. Org. Chem 2016, 137, 6766–6769. [Google Scholar]
- (19).LaPlante SR; Fader LD; Fandrick DR; Hucke I; Kemper R; Miller SPF; Edwards PJ J. Med. Chem 2011, 54, 7005–7022. [DOI] [PubMed] [Google Scholar]
- (20).(a) Barrett KT; Miller SJ J. Am. Chem. Soc 2013, 135, 2963–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Diener ME; Metrano AJ; Kusano S; Miller SJ J. Am. Chem. Soc 2015, 137, 12369–12377. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gustafson JL; Lim D; Barrett KT; Miller SJ Angew. Chem. Int. Ed 2011, 50, 5125–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gustafson J; Lim D; Miller SJ Science, 2010, 328, 1251–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21). (−)-15 is the major enantiomer following demethylation of enantioenriched 10c acquired through the peptide-catalyzed atroposelective bromination. Thus, 10c and (−)-15 are drawn with their absolute stereochemistry established for that of benzamide 12 obtained using analogous conditions described in reference 20a.
- (22).Meck C; Mohd N; Murelli RP Org. Lett 2012, 14, 5988–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).(a) For recent studies, see: Hirsch DR; Schiavone DV; Berkowitz AJ; Morrison LA; Masaoka T; Wilson JA; Lomonosova E; Zhao H; Patel BS; Dalta SH; Majidi SJ; Pal R;K; Gallicchio E; Tang L; Tavis JE; Le Grice SFJ; Beutler JA; Murelli RP Org. Biomol. Chem 2018, 16, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dehghanpir SD; Birkenheuer CH; Yang K; Murelli RP; Morrison LA; Le Grice SFJ; Baines JD Vet. Microbiol 2018. 214, 125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Long KR; Lomonosova E; Li Q; Ponzar NL; Villa JA; Touchette E; Rapp S; Liley RM; Murelli RP; Grigoryan A; Buller RM; Wilson L; Bial J; Sagartz JE; Tavis JE Antiviral Res, 2018, 149, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Welch CJ; Biba M; Pye P; Angelaud R; Egertson MJ Chromatogr. B 2008, 875, 111–121. [DOI] [PubMed] [Google Scholar]
- (25).Yui N Sci. Rep. Tohoku Univ. First Ser 1956, 40, 114–120. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


