Abstract
OBJECTIVE:
To evaluate second primary cancer (SPC) risk following endometrial cancer (EC) according to histological subtype.
METHODS:
Using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 13 Registries we identified women diagnosed with a primary EC between 1992 and 2014. We calculated standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for SPC risk (all anatomical sites combined and for individual anatomical sites) among EC cases compared with the general population, in the overall study population and according to histological subtype.
RESULTS:
Among 96,256 women diagnosed with EC, 8.4% (n=8,083) developed an SPC. SPC risk was higher among EC cases than in the general population (SIR=1.05, 95% CI=1.03–1.07). We observed significantly higher SPC risk among women with high-grade endometrioid (SIR=1.12, 95% CI=1.05–1.19), serous (SIR=1.24, 95% CI=1.11–1.38), carcinosarcoma (SIR=1.18, 95% CI=1.02–1.35), mixed epithelial (SIR=1.22, 95% CI=1.06–1.40), and sarcoma (SIR=1.28, 95% CI=1.12–1.45) compared to the general population, but not for women with low-grade endometrioid (SIR=1.01, 95% CI=0.98–1.03) or clear cell (SIR=1.09, 95% CI=0.88–1.33) EC. Women with low-grade endometrioid EC had significantly lower SPC risks in gum and other mouth (SIR=0.57, 95% CI=0.30–0.97), lung and bronchus (SIR=0.72, 95% CI=0.660.77), and lymphocytic leukemia (SIR=0.71, 95% CI=0.54–0.93), while women with high-risk EC histological subtypes experienced significantly higher SPC risk at several anatomical sites.
CONCLUSIONS:
Risk of developing SPCs at all anatomical sites combined and at individual anatomical sites varied according to histological subtype. Clinicians should be aware that women with different histological subtypes carry different risks of developing SPCs.
Keywords: endometrial cancer, second primary cancer, histology, standardized incidence ratio, cancer registry
INTRODUCTION
Uterine cancer is the most common gynecological cancer in the United States (U.S.) and the fourth most common cancer overall in women. In 2018, 63,230 new cases are expected with an estimated 11,000 deaths [1]. Endometrial cancer (EC) comprises approximately 90% of all uterine cancers. Since 2000, the annual incidence of EC has risen, mainly driven by increases in the prevalence of obesity [2]. Moreover, changes in the prevalence of other established risk factors including increases in the incidence of diabetes, declining use of combination menopausal hormone use, and decreases in parity and smoking have also contributed to recent increases in EC incidence. Overall, prognosis for women with EC is favorable due to the large proportion of cases detected when disease is localized to the uterus [3]. With more women developing EC and surviving the disease, long-term health concerns, including the development of second primary cancers (SPCs), are increasingly important.
There are conflicting reports on the association between an EC diagnosis and overall risk of developing an SPC, possibly reflecting heterogeneous risks for particular anatomic sites. For instance, risks for SPCs in the colon, breast, bladder, and soft tissue are consistently elevated after EC while risks for lung and esophageal cancers are usually lower among women with EC compared to the general population [4–14]. These patterns of anatomic site-specific SPC risk likely reflect overlapping etiology, late effects of cancer treatment, and genetic susceptibility [15]. As an example, obesity is strongly related to risk of developing endometrial, colon, and breast cancers [16] – therefore, obese women who develop and survive their EC are also at risk of developing other obesity-related cancers.
EC is recognized as etiologically heterogeneous, with notable differences in the magnitude of risk factor associations according to histological subtype [17–19]. As such, it is plausible that the risk and distribution of SPCs varies according to the primary EC histological subtype. Additionally, histological subtype guides adjuvant treatment decisions, [20] resulting in differences in exposures to chemotherapy and/or radiation according to histological subtype. While patients with early stage, well-differentiated endometrioid disease undergo surveillance or radiation, those with high-risk histologies, such as serous, typically receive platinum-based chemotherapy with or without radiation [20]. These treatment patterns could impact SPC risk as chemotherapy and radiation are carcinogenic and have been shown to increase risks of SPCs at various sites including colon, bladder and leukemias [15, 21].
Few studies have addressed the risk of developing an SPC after EC according to histological subtype. Understanding these patterns could provide further insights into the epidemiology of EC and guide clinical decisions regarding surveillance and adjuvant treatment after EC. We addressed this gap in the literature using data from the Surveillance, Epidemiology, and End Results (SEER) Program with diverse representation of EC histological subtypes.
MATERIALS AND METHODS
Data source
The SEER database is the source of population-based cancer information provided by the National Cancer Institute (NCI). For the current analysis, we used data from 13 SEER registries which include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. Information on all incident cancers (excluding basal or squamous cell skin cancer) occurring within these regions is collected by SEER.
Study population
We included women ≥18 years of age at the time of a first primary EC (C54.0-C54.9, C55.9) diagnosed between 1992 and 2014, without a history of malignant cancer prior to EC (not including benign and borderline tumors of the brain and central nervous system), and women whose primary EC was not identified by death certificate or autopsy only. We defined SPC as a new cancer occurrence diagnosed greater than two months from the time of EC diagnosis not considered to be metastatic disease or recurrence of the first neoplasm.
We created a case listing that included the following variables: age at diagnosis, race, calendar year of diagnosis, International Classification of Diseases for Oncology, Third Edition (ICD-O-3) morphology, SEER summary stage, grade, type of surgery, radiation, and chemotherapy. We categorized ICD-O-3 codes into the following groups: endometrioid and mucinous adenocarcinoma (8380–8383, 8140, 8210, 8211, 8560, 8260, 8262, 8263, 8570, 8261, 8480–8482), serous (8441, 8460, 8461), carcinosarcoma (8950, 8951, 8980, 8981), clear cell (8310), mixed epithelial (8323, 8255), and sarcoma (8800–8935). This categorization is in line with other histology-specific EC analyses [18, 19]. Endometrioid and mucinous adenocarcinoma were further classified as low-grade (well- differentiated or moderately differentiated) or high-grade (poorly differentiated, undifferentiated, or anaplastic) endometrioid. By definition, serous, carcinosarcoma, clear cell, mixed epithelial, and sarcoma histologies are considered poorly differentiated tumors; therefore, additional categorization based on grade was unnecessary [22].
Statistical Analysis
We examined distributions of categorical variables related to the primary EC diagnosis (age at diagnosis, race, calendar year of diagnosis, SEER registry, histology, grade, stage at diagnosis, surgical treatment status, radiation therapy, and chemotherapy) among all women with a first primary EC and separately among EC patients who developed an SPC.
Standardized incidence ratios (SIRs) were calculated for all women with a first diagnosis of EC to determine risk associated with SPC development. We used the Multiple Primary-Standardized Incidence Ratio (MP-SIR) program of the SEER*Stat software to calculate the SIRs for SPC for all sites combined and for individual cancer sites. We excluded second cancers of the uterus, ovaries, and cervix from all analyses as EC surgery typically involves removal of these organs, precluding development of cancers at these sites. SIRs were also calculated separately according to the primary EC histological subtypes mentioned above. For each case group defined by the primary EC histological subtype, we calculated SIRs for SPCs at all sites combined and for each of the most common non-EC cancer sites. Follow-up time was counted from two months after the initial EC diagnosis until the diagnosis of a SPC, death, or end of the study (December 31, 2014). A single outcome analysis was used which caused individuals to exit the study when a SPC or death occurred; thus, these individuals contributed no additional person time at risk. For each tumor site, the SIR was calculated with the following equation: observed number of cancer cases at a particular tumor site/expected number of cancer cases at that particular tumor site [23]. The observed number of cancer cases refers to the number of SPCs in this collection of registries. Expected numbers of SPCs were estimated by multiplying sex-, race-, age-, and calendar year–specific SEER cancer incidence rates (available at http://seer.cancer.gov ) by the accumulated person-years at risk.
All analyses were completed using SEER Stat and SAS/STAT software (version 9.4 of the SAS System for Windows, SAS Institute, Cary, NC, USA). This study was considered exempt by the Institutional Review Board of the Ohio State University as all data are de-identified and intended for public use. All P values were two-sided, and a P value of less than 0.05 was considered statistically significant.
RESULTS
Study population
Overall, 96,256 women with a primary EC were included. Median age at the primary EC diagnosis was 62 years (interquartile range, 54–71). Eight percent of women (n=8,083) developed an SPC, with a mean latency to development of SPC of 7.1 years. Additional baseline characteristics of patients with primary EC and among those who developed an SPC are shown in Table 1. The majority of women with a primary EC were white (81.3%), with diagnoses of endometrioid tumors (80.7%) and localized stage disease (71.3%). Almost all women were treated with hysterectomy, one-quarter received radiation therapy, and approximately 13% received some type of chemotherapy. We observed approximately similar distributions of these variables among the subsets of EC cases who developed SPCs. Among women who did not develop SPCs, distributions were similar to all EC cases.
Table 1.
Characteristics of women with a first primary endometrial cancer in the Surveillance, Epidemiology, and End Results 13 Registries, 1992–2014
| All first primary EC cases | First primary EC cases who developed an SPC | First primary EC cases who did not develop an SPC | |
|---|---|---|---|
| N=96,256 | N=8,083 | N=88,173 | |
| N (%) | |||
| Age at EC diagnosis (years) | |||
| 18–45 | 7,851 (8.2) | 314 (3.9) | 7,537 (8.5) |
| 45–54 | 18,120 (18.8) | 1,216 (15.0) | 16,904 (19.2) |
| 55–64 | 30,164 (31.3) | 2,487 (30.8) | 27,677 (31.4) |
| 65–74 | 23,596 (24.5) | 2,599 (32.2) | 20,997 (23.8) |
| 75+ | 16,525 (17.2) | 1,467 (18.2) | 15,058 (17.1) |
| Race | |||
| Black | 7,848 (8.2) | 487 (6.0) | 7,361 (8.3) |
| White | 78,216 (81.3) | 6,968 (86.2) | 71,248 (80.8) |
| American Indian/Alaska Native | 590 (0.6) | 41 (0.5) | 549 (0.6) |
| Asian/Pacific Islander | 9,027 (9.4) | 583 (7.2) | 8,444 (9.6) |
| Unknown | 575 (0.6) | 4 (0.05) | 571 (0.6) |
| Calendar year of EC diagnosis | |||
| 1992–1996 | 17,480 (18.2) | 2,605 (32.2) | 14,875 (16.9) |
| 1997–2001 | 18,960 (19.7) | 2,271 (28.1) | 16,689 (18.9) |
| 2002–2006 | 20,092 (20.9) | 1,661 (20.6) | 18,431 (20.9) |
| 2007–2011 | 24,347 (25.3) | 1,285 (15.9) | 23,062 (26.2) |
| 2012–2014 | 15,377 (16.0) | 261 (3.2) | 15,116 (17.1) |
| Histology | |||
| Low-grade endometrioid | 57,225 (59.5) | 5,517 (68.3) | 51,708 (58.6) |
| High-grade endometrioid | 11,120 (11.6) | 963 (11.9) | 10,157 (11.5) |
| Endometrioid with unknown grade | 9,241 (9.6) | 526 (6.5) | 8,715 (9.9) |
| Serous | 5,017 (5.2) | 319 (4.0) | 4,698 (5.3) |
| Carcinosarcoma | 4,025 (4.2) | 209 (2.6) | 3,816 (4.3) |
| Clear cell | 1,302 (1.4) | 94 (1.2) | 1,208 (1.4) |
| Mixed epithelial | 3,722 (3.9) | 210 (2.6) | 3,512 (4.0) |
| Sarcoma | 4,604 (4.8) | 245 (3.0) | 4,359 (4.9) |
| Tumor stage | |||
| Localized | 68,615 (71.3) | 6,354 (78.6) | 62,261 (70.6) |
| Regional | 16,739 (17.4) | 1,270 (15.7) | 15,469 (17.5) |
| Distant | 8,230 (8.6) | 287 (3.6) | 7,943 (9.0) |
| Unknown | 2,672 (2.8) | 172 (2.1) | 2,500 (2.8) |
| Surgical status | |||
| No surgery | 6,138 (6.4) | 250 (3.1) | 5,888 (6.7) |
| Simple excision | 706 (0.7) | 37 (0.5) | 669 (0.8) |
| Hysterectomy or exenteration | 89,368 (92.8) | 7,796 (96.5) | 81,572 (92.5) |
| Unknown | 44 (0.1) | 44 (0.0) | |
| Radiation treatment | |||
| None/unknown | 71,261 (74.0) | 5,811 (71.9) | 65,450 (74.2) |
| Any radiation | 24,995 (26.0) | 2,272 (28.1) | 22,723 (25.8) |
| Chemotherapy treatment | |||
| None/Unknown | 83,339 (86.6) | 7,513 (93.0) | 75,826 (86.0) |
| Any chemotherapy | 12,917 (13.4) | 570 (7.1) | 12,347 (14.0) |
| Vital status | |||
| Alive | 63,915 (66.4) | 3,917 (48.5) | 59,998 (68.0) |
| Dead | 32,341 (33.6) | 4,166 (51.5) | 28,175 (32.0) |
SPC risks in the overall study population
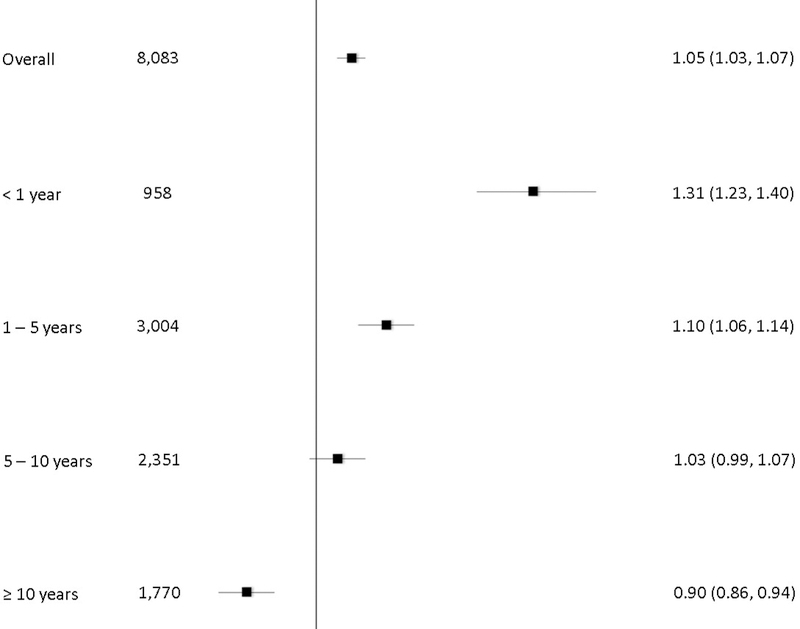
Overall, we observed a significantly higher risk for the development of an SPC at all anatomical sites combined comparing EC patients with the general population (Figure 1, SIR=1.05, 95% CI=1.03–1.07), with SPC risk highest within the one year interval after EC diagnosis (SIR=1.31, 95% CI=1.23–1.40). SPC risk decreased over time, with EC cases experiencing a significantly lower second cancer risk than the general population 10 years after the initial diagnosis (SIR-0.90, 95% CI=0.86–0.94).
Figure 1.
Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for second primary cancer (SPC) risk overall and by latency
Figure 1 shows significantly higher risk for the development of an SPC at all anatomical sites combined comparing EC patients with the general population. SPC risks were highest within the one year interval after EC diagnosis and decreased with increasing time since EC diagnosis.
As shown in Supplemental Table 1, SPC risk varied according to the anatomical site of the second cancer. Overall, we observed significantly increased SPC risks in small intestine (SIR=1.36, 95% CI=1.02–1.79), colorectal (SIR=1.27, 95% CI =1.20–1.35), bladder (SIR=1.20, 95% CI=1.07–1.35), kidney (SIR=1.46, 95% CI=1.29–1.64), and vaginal (SIR=6.12, 95% CI=4.97–7.57) cancers was observed. In addition, there were increased risks of many types of leukemia, including acute non-lymphocytic leukemia (ANLL) (SIR=1.34, 95% CI=1.09–1.62) and acute myeloid leukemia (AML) (SIR=1.37–1.10–1.68), while there were decreased risks associated with other types such as lymphocytic leukemia (SIR=0.73, 95% CI=0.57–0.91). Pancreatic (SIR=0.87, 95% CI=0.76–0.98) and lung and bronchus (SIR=0.81, 95% CI=0.76–0.86) cancers were all significantly lower among women with EC than the general population.
SPC risks according to histological subtype
When stratified by histological subtype, women diagnosed with high-grade endometrioid (SIR=1.12, 95% CI=1.05–1.19), serous (SIR=1.24, 95% CI=1.11–1.38), carcinosarcoma (SIR=1.18, 95% CI=1.02–1.35), mixed epithelial (SIR=1.22, 95% CI=1.06–1.40), and sarcoma (SIR=1.28, 95% CI=1.12–1.45) tumors had significantly higher SPC risks at all sites combined compared to the general population. We also noted heterogeneous SPC risks at individual anatomical sites according to histological subtype. Among women diagnosed with low-grade endometrioid EC, risks of vaginal (SIR=4.82, 95% CI=3.62–6.29) and kidney (SIR=1.38, 95% CI=1.19–1.60) cancers were increased while gum and other mouth (SIR=0.57, 95% CI=0.30–0.97), lung and bronchus (SIR=0.72, 95% CI=0.66–0.77), and lymphocytic leukemia (SIR=0.71, 95% CI=0.54–0.93) were lower than the general population.
Among women with poor prognosis EC histological subtypes (high-grade endometrioid, serous, carcinosarcoma, clear cell, mixed epithelial, and sarcoma), SPC anatomical sites were either significantly elevated or not significantly different than the general population. SPC in the vagina was elevated among all histological subtypes, and was particularly higher among women with poor prognosis tumors. Colorectal SPC risk was elevated among women with high-grade endometrioid (SIR=1.40, 95% CI=1.19–1.63), and clear cell (SIR=1.79, 95% CI=1.11–2.74), tumors. Kidney SPC risks were elevated among women with low-grade endometrioid (SIR=1.38, 95% CI=1.19–1.60) mixed carcinosarcomas (SIR=2.77, 95% CI=1.43–4.84) and sarcomas (SIR=2.68, 95% CI=1.43–4.58), while bladder SPC risks were higher among women with high-grade endometrioid (SIR=1.42, 95% CI=1.01–1.95) or carcinosarcomas (SIR=2.48, 95% CI=1.36–4.16). Women with high-grade endometrioid (SIR=1.87, 95% CI=1.02–3.13) and carcinosarcoma (SIR=3.87, 95% CI=1.42–8.43) had higher risks of AML.
Several SPC anatomical sites that were elevated in the overall analysis (e.g. breast, myeloid and monocytic leukemia (MML), and lung and bronchus) were only elevated for one histological subtype. For example, only women with low-grade endometrioid EC experienced a higher risk of SPC of the breast (SIR=1.05, 95% CI= 1.00–1.10) while women with carcinosarcoma had an elevated risk of myeloid and monocytic leukemia (SIR=3.03, 95% CI=1.22–6.24).
Histology-specific SPC risks according to race, stage, radiation, and chemotherapy
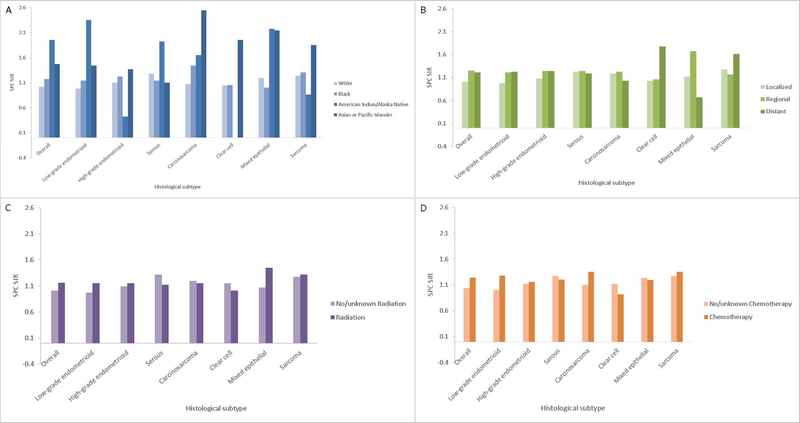
We examined EC histology-specific patterns of SPC risk for all sites combined across strata defined by race, stage, radiation therapy, and chemotherapy (Figure 2A–D). With a few exceptions, SPC risk was higher among non-white EC cases compared to white cases and among women with a regional or distant stage tumor than those with localized stages for all histological subtypes. Among women diagnosed with a primary endometrioid EC, mixed epithelial, or sarcoma histology, radiation treatment was associated with a higher SPC risk compared to no radiation treatment. Conversely, women with an index serous, carcinosarcoma, or clear cell histological subtype showed slightly higher SPC risks when radiation treatment was not used. There were higher SPC risks in women with endometrioid, carcinosarcoma and sarcoma treated with chemotherapy compared to no chemotherapy.
Figure 2.
Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for second primary cancer (SPC) risk according to histological subtype and A.) race, B.) stage, C.) radiation treatment, or D.) chemotherapy.
Figure 2A shows SPC risk was higher among non-white EC cases compared to white cases.
Figure 2B shows SPC risk was higher among women with a regional or distant stage tumor than those with localized stages for all histological subtypes.
Figure 2C shows higher SPC risks among women diagnosed with a primary endometrioid EC, mixed epithelial, or sarcoma histology who received radiation treatment compared to no or unknown radiation treatment.
Figure 2D shows higher SPC risks in women with endometrioid, carcinosarcoma and sarcoma treated with chemotherapy compared to no or unknown chemotherapy.
DISCUSSION
This population-based analysis adds to the existing body of literature related to EC heterogeneity by demonstrating that SPC risk varies according to the histological subtype of the primary EC. Compared with the general population, overall SPC risk was higher among women diagnosed with non-endometrioid histological subtypes, which carry a poor prognosis. Further, although the overall SPC risk for women with low-grade endometrioid tumors did not differ from that expected in the general population, we observed higher SPC risks at individual anatomical sites for these women. While additional confirmatory studies are needed – specifically, studies with available information on important confounders such as lifestyle factors, genetic susceptibility, and detailed treatment data – our analysis highlights the importance of parsing EC according to histological subtype, not just for analyses of etiology, molecular biology, and treatment, but also to examine outcomes such as SPC risk.
Our findings of higher SPC risk in the overall population of EC patients are in agreement with prior literature [4–6, 8, 10–12, 14, 24]. Further, we confirmed higher SPC risks at specific anatomical sites, including small intestine, breast, colon, kidney, and bladder [4–9, 11–14] as well as elevated risks of soft tissue and ANLL SPCs [6, 11]. Related to the breast SPC finding, the risk we observed in the current analysis was lower in magnitude compared with prior studies, potentially reflecting the overall decline in breast cancer incidence that has been described since 2000 [25].
Patterns of SPC risk following an EC diagnosis likely reflect several overlapping mechanisms including shared risk or prevention factors, genetic predisposition, and treatment effects [15]. Analysis of histological subtype-specific SPCs allows us to further comment on the potential for these mechanisms to contribute to SPCs after EC. For example, our observation that lung/bronchus and esophageal SPCs were significantly lower among women with low-grade endometrioid EC is likely related to the well-established link between cigarette smoking and decreased risk of EC, particularly of the endometrioid type [18, 26]. Similarly, increased risks of colorectal SPC following low- and high-grade endometrioid and clear cell ECs could partially be related to obesity [18, 27].
Heritable causes could also underlie links between EC and increased risk of subsequent cancers, particularly colorectal, ovarian, bladder, and small intestine cancers, as risk of these malignancies is elevated among individuals with Lynch syndrome, a familial disorder caused by mutations in DNA mismatch repair genes [28]. Although Lynch syndrome confers a high absolute risk of cancer development, the low frequency of highly penetrant mutations in Lynch syndrome genes likely accounts for a small proportion of SPCs [15].
Treatment effects may also contribute to the observed patterns of SPC risk. Adjuvant radiation therapy and chemotherapy are commonly prescribed to women diagnosed with high-risk EC histologies (e.g. serous, clear cell, mixed epithelial, carcinosarcoma, and sarcomas) and could contribute to higher SPC risk among these women [12, 20]. Radiation-induced second cancers can arise through perturbation of healthy tissue within the field of radiation [29]. In particular, increased risks of leukemia, colon, rectum, vulva, skin, and bladder SPCs among EC patients receiving radiation therapy have been observed [30], particularly when women are younger at diagnosis [31]. In our analysis, we observed higher risks of leukemias and bladder SPCs among women with high-grade endometrioid EC or carcinosarcoma and higher colorectal SPCs following diagnosis of high-grade endometrioid, serous, clear cell, and mixed epithelial tumors, in line with potential radiation effects. Still, others have not observed radiation-related increases in SPCs after EC. In the Post-Operative Radiation Therapy in Endometrial Carcinoma 1 trial, ten-year rates of SPCs did not differ between EC patients who received external-beam radiotherapy compared to those who did not receive radiation (17.3% vs. 16.9%, respectively) [32]. Inconsistences in the literature are likely driven by variable radiation doses and use of intensity-modulation radiation therapy as well as differences in follow-up procedures, patient populations, and the absence of stratification by SPC anatomical site or histology of the primary EC. Of note, Berrington de Gonzalez and colleagues [33] demonstrated a 14% higher risk of adult SPCs among women with primary EC who received radiation compared to women who did not.
Chemotherapy is also associated with increased SPC risk. Currently, platinum-containing regimens, particularly carboplatin, are preferred for EC patients undergoing chemotherapy [20]. In our study, if patients received chemotherapy they most likely received cisplatin due to the years of diagnosis included in the analysis. While a recent meta-analysis did not show an increased odds of overall SPC among patients receiving cisplatin, there was a trend towards increased odds of secondary leukemias or myelodysplastic syndromes, in line with our observations of higher rates of several types of leukemias overall and for certain histological subtypes [34].
Few other studies have examined SPC risk according to the histological subtype of the primary EC. Hinshaw and colleagues explored site-specific SPCs among women diagnosed with serous or clear cell EC [13]. Increased risks of colorectal, renal, bladder, AML and soft tissue SPCs were observed among serous EC patients (n=8,045), whereas women with clear cell EC (n=1,740) did not experience increased SPC risk at any anatomical site. Chen and colleagues [24] recently examined anatomical site-specific SPCs according to EC histological subtype among German and Swedish patients. They observed increased lung and ovarian SPCs among women with endometrioid EC, increased cervical and kidney SPCs after clear cell EC, and no increased risk of any SPC following a serous EC diagnosis. Our histology-specific EC findings contrast with this scant literature; however, differences in treatment or the underlying study populations may underpin dissimilar results.
The major strength of this analysis is the consideration of SPC risks according to EC histological subtypes given differences in etiology, molecular aberrations and recommended treatment. Additionally, we included a large sample size and a multi-institutional population representative of the U.S. Limitations of this study are related to the data available within the SEER Program. For example, the SEER Program does not collect detailed information regarding dose of radiation, type of chemotherapy, lifestyle factors, family history of cancer, or genetic factors, and thus our commentary on the etiology of SPCs in EC patients is speculative. Further, no central pathology review was conducted which could result in misclassification of EC histological subtype, particularly for the non-endometrioid subtypes where poor intra-observer reproducibility has been reported [35]. Finally, we cannot rule out that some SPCs – particularly vaginal cancers, which are rare – were actually recurrences of the primary EC. Moreover, the higher risk of SPCs within the first year of diagnosis suggests that some cancers were either present at diagnosis of the EC or these second tumors developed rapidly after the EC diagnosis. If confirmed, our observations could benefit population health in several ways. First, given our observations of higher risk of obesity-related cancers (e.g. colorectal) following diagnosis of certain EC subtypes, counseling regarding weight loss along with provision of resources to reduce barriers could be implemented. Epidemiological studies that test the impact of weight loss on second cancer risk will be important for creating an evidence-base around this recommendation. In addition, our findings could be useful in the eventual development of evidence-based guidelines aimed at early detection of second cancers among EC survivors. For example, the consistently elevated risk of vaginal SPC could prompt additional research on the efficacy of vaginal cancer screening modalities among EC survivors.
In summary, our population-based study of the relationship between EC histological subtype and risk of subsequent malignancy in a U.S. population revealed histology-specific patterns. Furthermore, we confirmed increased risks of SPCs in specific anatomical sites regardless of histology. Although the exact etiology of SPCs after EC is unknown, our data should prompt physician awareness of the development of SPCs in EC survivors, particularly obesity-related cancers.
Supplementary Material
Table 2.
Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for second primary cancer (SPC) risk among women with a first primary EC according to histologic subtype
| Low-grade endometrioid | High-grade endometrioid | Serous | Carcinosarcoma | Clear cell | Mixed epithelial | Sarcoma | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | Observed | SIR (95% CI) | |
| All Sites | 5,517 | 1.01 (0.98, 1.03) | 963 | 1.12 (1.05, 1.19) | 319 | 1.24 (1.11, 1.38) | 209 | 1.18 (1.02, 1.35) | 94 | 1.09 (0.88, 1.33) | 210 | 1.22 (1.06, 1.40) | 245 | 1.28 (1.12, 1.45) |
| Gum and Other Mouth | 13 | 0.57 (0.30, 0.97) | 3 | 0.82 (0.17, 2.39) | 2 | 1.85 (0.22, 6.68) | 1 | 1.33 (0.03, 7.42) | 0 | 0.00 (0.00, 10.02) | 0 | 0.00 (0.00, 5.48) | 0 | 0.00 (0.00, 5.43) |
| Tonsil | 5 | 0.60 (0.20, 1.41) | 0 | 0.00 (0.00, 3.02) | 0 | 0.00 (0.00, 10.12) | 0 | 0.00 (0.00, 14.34) | 0 | 0.00 (0.00, 31.01) | 0 | 0.00 (0.00, 12.37) | 0 | 0.00 (0.00, 10.18) |
| Esophagus | 29 | 0.85 (0.57, 1.22) | 2 | 0.36 (0.04, 1.29) | 1 | 0.57 (0.01, 3.18) | 1 | 0.83 (0.02, 4.60) | 0 | 0.00 (0.00, 6.31) | 3 | 2.95 (0.61, 8.62) | 0 | 0.00 (0.00, 3.58) |
| Stomach | 71 | 0.80 (0.63, 1.01) | 11 | 0.73 (0.36, 1.31) | 8 | 1.68 (0.73, 3.31) | 3 | 0.94 (0.19, 2.75) | 0 | 0.00 (0.00, 2.21) | 2 | 0.74 (0.09, 2.69) | 5 | 1.74 (0.57, 4.07) |
| Small Intestine | 33 | 1.24 (0.85, 1.74) | 8 | 1.89 (0.82, 3.72) | 1 | 0.71 (0.02, 3.97) | 1 | 1.05 (0.03, 5.85) | 0 | 0.00 (0.00, 8.14) | 3 | 3.14 (0.65, 9.16) | 2 | 2.03 (0.25, 7.33) |
| Colorectal | 836 | 1.23 (1.15, 1.32) | 157 | 1.40 (1.19, 1.63) | 40 | 1.18 (0.85, 1.61) | 27 | 1.15 (0.76, 1.67) | 21 | 1.79 (1.11, 2.74) | 25 | 1.33 (0.86, 1.96) | 25 | 1.21 (0.78, 1.78) |
| Pancreas | 174 | 0.89 (0.76, 1.03) | 34 | 1.05 (0.73, 1.47) | 6 | 0.59 (0.21, 1.27) | 2 | 0.29 (0.03, 1.04) | 2 | 0.58 (0.07, 2.10) | 8 | 1.28 (0.55, 2.52) | 4 | 0.70 (0.19, 1.78) |
| Lung and Bronchus | 638 | 0.72 (0.66, 0.77) | 147 | 1.04 (0.88, 1.22) | 55 | 1.25 (0.94, 1.62) | 29 | 0.98 (0.65, 1.40) | 11 | 0.77 (0.38, 1.37) | 33 | 1.19 (0.82, 1.68) | 42 | 1.58 (1.14, 2.13) |
| Soft Tissue including Heart | 35 | 1.13 (0.78, 1.57) | 5 | 1.04 (0.34, 2.42) | 4 | 2.75 (0.75, 7.05) | 0 | 0.00 (0.00, 3.68) | 0 | 0.00 (0.00, 7.62) | 1 | 0.96 (0.02, 5.37) | 16 | 13.51 (7.72, 21.94) |
| Breast | 1,851 | 1.05 (1.00, 1.10) | 275 | 1.03 (0.91, 1.16) | 95 | 1.22 (0.99, 1.49) | 56 | 1.03 (0.78, 1.34) | 24 | 0.92 (0.59, 1.37) | 61 | 1.07 (0.82, 1.37) | 53 | 0.74 (0.55, 0.96) |
| Vagina | 54 | 4.82 (3.62, 6.29) | 13 | 7.24 (3.86, 12.38) | 5 | 9.10 (2.95, 21.23) | 7 | 18.30 (7.36, 37.71) | 5 | 27.15 (8.82, 63.36) | 6 | 16.96 (6.22, 36.91) | 2 | 5.17 (0.63, 18.67) |
| Vulva | 43 | 1.13 (0.82, 1.52) | 12 | 1.99 (1.03, 3.48) | 1 | 0.58 (0.01, 3.22) | 2 | 1.64 (0.20, 5.93) | 0 | 0.00 (0.00, 6.20) | 0 | 0.00 (0.00, 3.13) | 1 | 0.84 (0.02, 4.67) |
| Urinary System | 397 | 1.22 (1.10, 1.34) | 75 | 1.45 (1.14, 1.82) | 27 | 1.71 (1.13, 2.49) | 31 | 2.87 (1.95, 4.08) | 5 | 0.95 (0.31, 2.22) | 15 | 1.46 (0.82, 2.40) | 16 | 1.58 (0.91, 2.57) |
| Urinary Bladder | 194 | 1.14 (0.99, 1.31) | 39 | 1.42 (1.01, 1.95) | 14 | 1.71 (0.93, 2.87) | 14 | 2.48 (1.36, 4.16) | 4 | 1.43 (0.39, 3.67) | 4 | 0.8 (0.22, 2.04) | 3 | 0.65 (0.13, 1.90) |
| Kidney | 182 | 1.38 (1.19, 1.60) | 28 | 1.38 (0.92, 1.99) | 11 | 1.73 (0.86, 3.10) | 12 | 2.77 (1.43, 4.84) | 0 | 0.00 (0.00, 1.79) | 9 | 1.97 (0.90, 3.75) | 13 | 2.68 (1.43, 4.58) |
| Endocrine System | 152 | 1.21 (1.02, 1.42) | 15 | 0.86 (0.48, 1.41) | 13 | 2.64 (1.41, 4.52) | 6 | 1.72 (0.63, 3.75) | 4 | 2.42 (0.66, 6.19) | 9 | 1.82 (0.83, 3.45) | 31 | 4.44 (3.02, 6.30) |
| Lymphocytic Leukemia | 54 | 0.71 (0.54, 0.93) | 11 | 0.92 (0.46, 1.65) | 4 | 1.14 (0.31, 2.91) | 1 | 0.41 (0.01, 2.27) | 1 | 0.84 (0.02, 4.69) | 2 | 0.86 (0.10, 3.11) | 1 | 0.45 (0.01, 2.53) |
| Acute NonLymphocytic Leukemia (ANLL) | 69 | 1.29 (1.00, 1.63) | 14 | 1.63 (0.89, 2.73) | 5 | 1.93 (0.63, 4.50) | 6 | 3.39 (1.25, 7.39) | 1 | 1.14 (0.03, 6.33) | 2 | 1.18 (0.14, 4.28) | 2 | 1.20 (0.15, 4.34) |
| Myeloid and Monocytic Leukemia | 86 | 1.22 (0.98, 1.51) | 15 | 1.34 (0.75, 2.21) | 5 | 1.48 (0.48, 3.45) | 7 | 3.03 (1.22, 6.24) | 1 | 0.88 (0.02, 4.88) | 2 | 0.90 (0.11, 3.25) | 2 | 0.89 (0.11, 3.23) |
| Acute Myeloid Leukemia | 61 | 1.29 (0.99, 1.66) | 14 | 1.87 (1.02, 3.13) | 3 | 1.31 (0.27, 3.83) | 6 | 3.87 (1.42, 8.43) | 1 | 1.30 (0.03, 7.25) | 2 | 1.31 (0.16, 4.74) | 2 | 1.35 (0.16, 4.88) |
Acknowledgments
Funding Information: This work was supported by the National Cancer Institute (K01CA21845701A1 to ASF)
Footnotes
Conflicts of interest
None.
Author Disclosure: the authors have no financial relationships to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Onstad MA, Schmandt RE, Lu KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol. 2016;34(35):4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER. SEER Cancer Stat Facts: Endometrial Cancer. Bethesda, MD2017 [Available from: http://seer.cancer.gov/statfacts/html/corp.html.
- 4.Schottenfeld D, Berg J. Incidence of miltiple primary cancers. IV. Cancers of the female breast and genital organs. J Natl Cancer Inst. 1971;46(1):161–70. [PubMed] [Google Scholar]
- 5.Annegers JF, Malkasian GD Jr. Patterns of other neoplasia in patients with endometrial carcinoma. Cancer. 1981;48(3):856–9. [DOI] [PubMed] [Google Scholar]
- 6.Curtis RE, Hoover RN, Kleinerman RA, et al. Second cancer following cancer of the female genital system in Connecticut, 1935–82. National Cancer Institute monograph. 1985;68:113–37. [PubMed] [Google Scholar]
- 7.Storm HH, Lynge E, Osterlind A, et al. Multiple primary cancers in Denmark 1943–80; influence of possible underreporting and suggested risk factors. The Yale journal of biology and medicine. 1986;59(5):547–59. [PMC free article] [PubMed] [Google Scholar]
- 8.Bergfeldt K, Einhorn S, Rosendahl I, et al. Increased risk of second primary malignancies in patients with gynecological cancer. A Swedish record-linkage study. Acta oncologica (Stockholm, Sweden). 1995;34(6):771–7. [DOI] [PubMed] [Google Scholar]
- 9.Re A, Taylor TH, DiSaia PJ, et al. Risk for breast and colorectal cancers subsequent to cancer of the endometrium in a population-based case series. Gynecol Oncol. 1997;66(2):255–7. [DOI] [PubMed] [Google Scholar]
- 10.Hemminki K, Aaltonen L, Li X. Subsequent primary malignancies after endometrial carcinoma and ovarian carcinoma. Cancer. 2003;97(10):2432–9. [DOI] [PubMed] [Google Scholar]
- 11.Curtis RE, Freedman DM, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute, NIH Publ. No. 05–5302; Bethesda, MD, 2006. [Google Scholar]
- 12.Brown AP, Neeley ES, Werner T, et al. A population-based study of subsequent primary malignancies after endometrial cancer: genetic, environmental, and treatment-related associations. International journal of radiation oncology, biology, physics. 2010;78(1):127–35. [DOI] [PubMed] [Google Scholar]
- 13.Hinshaw HD, Smith A, Rungruang B, et al. The risk of subsequent malignancies in women with uterine papillary serous or clear cell endometrial cancers. Int J Gynecol Cancer. 2013;23(6):1044–9. [DOI] [PubMed] [Google Scholar]
- 14.Lee KD, Chen CY, Huang HJ, et al. Increased risk of second primary malignancies following uterine cancer: a population-based study in Taiwan over a 30-year period. BMC cancer. 2015;15:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nature reviews Clinical oncology. 2013;10(5):289–301. [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Peterson LL. Obesity and Cancer: Evidence, Impact, and Future Directions. Clinical chemistry. 2018;64(1):154–62. [DOI] [PubMed] [Google Scholar]
- 17.Brinton LA, Felix AS, McMeekin DS, et al. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129(2):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31(20):2607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang HP, Wentzensen N, Trabert B, et al. Endometrial cancer risk factors by 2 main histologic subtypes: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2013;177(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Guidelines for Treatment of Cancer by Site (Uterine Neoplasms Version 2.2016) [Available from: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 21.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nature reviews Cancer. 2005;5(12):943–55. [DOI] [PubMed] [Google Scholar]
- 22.Lax SF. Pathology of Endometrial Carcinoma. Advances in experimental medicine and biology. 2017;943:75–96. [DOI] [PubMed] [Google Scholar]
- 23.Rose Ragin CC, Taioli E. Second primary head and neck tumor risk in patients with cervical cancer--SEER data analysis. Head & neck. 2008;30(1):58–66. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Brenner H, Fallah M, et al. Risk of second primary cancers in women diagnosed with endometrial cancer in German and Swedish cancer registries. Int J Cancer. 2017. [DOI] [PubMed] [Google Scholar]
- 25.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA: a cancer journal for clinicians. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 26.Zhou B, Yang L, Sun Q, et al. Cigarette smoking and the risk of endometrial cancer: a metaanalysis. The American journal of medicine. 2008;121(6):501–8 e3. [DOI] [PubMed] [Google Scholar]
- 27.Jochem C, Leitzmann M. Obesity and Colorectal Cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2016;208:17–41. [DOI] [PubMed] [Google Scholar]
- 28.Win AK, Lindor NM, Young JP, et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J Natl Cancer Inst. 2012;104(18):1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braunstein S, Nakamura JL. Radiotherapy-induced malignancies: review of clinical features, pathobiology, and evolving approaches for mitigating risk. Frontiers in oncology. 2013;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lonn S, Gilbert ES, Ron E, et al. Comparison of second cancer risks from brachytherapy and external beam therapy after uterine corpus cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(2):46474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onsrud M, Cvancarova M, Hellebust TP, et al. Long-term outcomes after pelvic radiation for early-stage endometrial cancer. J Clin Oncol. 2013;31(31):3951–6. [DOI] [PubMed] [Google Scholar]
- 32.Wiltink LM, Nout RA, Fiocco M, et al. No Increased Risk of Second Cancer After Radiotherapy in Patients Treated for Rectal or Endometrial Cancer in the Randomized TME, PORTEC-1, and PORTEC-2 Trials. J Clin Oncol. 2015;33(15):1640–6. [DOI] [PubMed] [Google Scholar]
- 33.Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12(4):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang F, Zhang S, Xue H, et al. Risk of second primary cancers in cancer patients treated with cisplatin: a systematic review and meta-analysis of randomized studies. BMC cancer. 2017;17(1):871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013;37(6):874–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.