Abstract
Purpose
β-defensins are antimicrobial peptides expressed at mucosal level of male and female genito-urinary tract, where they exert protective functions against infections, possibly preserving human health and fertility. In our study, we investigated the possible involvement of β-defensins in female and male infertility in Italian infertile couples (i) evaluating the presence of human β-defensin 1 (hBD-1) in follicular fluid (FF) and its correlation with in vitro fertilization (IVF) outcomes; (ii) investigating the relationship between hBD-1 levels in semen and IVF outcomes (comprising correlation with sperm parameters); and (iii) exploring the effect of hBD-1 peptide on spermatozoa motility in vitro.
Methods
A perspective observational analytic pilot study was conducted. hBD-1 concentration was measured with ELISA assay in FF and semen from 50 couples that underwent assisted procreation technique procedures due to infertility status. Moreover, hBD-1 exogenous peptide was administered to 29 normozoospermic semen and their motility was recorded.
Results
hBD-1 was detected in FF and its levels were significantly higher in women with good fertilization rate (≥ 75%), respect to those with a poor fertilization rate (< 75%). The hBD-1 semen concentrations in oligo-asthenozoospermic subjects were significantly lower than that in normozoospermic men. Instead, hBD-1 level in sperm and FF not correlated with pregnancy rate. Finally, incubation of sperm with exogenous hBD-1 significantly increased progressive motility after 1 h and 24 h.
Conclusions
Being aware of the relatively small sample size and medium power, our results possibly suggest that hBD-1 could influence oocyte and sperm quality, and could improve, when exogenously added, sperm motility.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01409-w) contains supplementary material, which is available to authorized users.
Keywords: Antimicrobial peptides, β-defensin 1, Innate immunity, Infertility, In vitro fertilization, Sperm motility
Introduction
It is well-known that genito-urinary tract infections may affect male and female infertility in different ways [1]. The microorganisms involved in such infections include bacteria, yeasts, parasites, and viruses, being these pathogens able to infect and colonize the reproductive system, inducing inflammation, inflammatory disorders, and anatomic abnormalities, possibly influencing the fertility status. Therefore, the protection against pathogens conferred by an efficient immune system could prevent persistent infections and reduce the associated tissue damage. The innate immune system is the first line of defense against pathogens in the genito-urinary trait and it can be involved in the protection against infections, thus playing a possible role in the risk of infertility [2, 3]. Within the innate immune system, defensins are antimicrobial peptides produced by leucocytes and epithelial cells active on gram positive and negative bacteria, yeast, and viruses [4, 5]. So, defensins, reducing susceptibility towards infections could be involved in maintaining genito-urinary health and subsequently in protecting the fertility status and potentiality to conceive [6].
The constitutive expression of human β-defensin 1 (hBD-1) in all human epithelia indicates its important role in the innate immunity [7].
In females, hBD-1 is present in the vagina, cervix, endometrium, fallopian tube, and in the pregnant uterus with expression in the amnion, decidua, chorion, and placental trophoblast; hBD-1 is differentially expressed during the menstrual cycle and is important during pregnancy for preserving the health of both the uterus and the fetus [2, 8]. Thus, innate immunity molecules, including hBDs, play a pivotal role in the defense of the endometrium against pathogens. However, the presence of hBD-1 in the ovary has not been so far reported; moreover, intra-follicular environment is devoid of professional immune cells and it is unknown whether innate immunity has a role within the ovarian follicle [2].
In males, the expression of β-defensins has been reported in prostate, testis, and sperm where these molecules probably exert a protective activity against infections and other functions directly on sperm functionality; actually, it has been hypothesized that hBD-1 may create an antimicrobial shield around the sperm; thus, in the transit through the female reproductive tract, they may stop premature hyperactivation, reduce the toxicity due to excessive cytokine production, and protect sperm motility against a negative effect caused by lipopolysaccharide [9, 10].
Moreover, recently, it has been also demonstrated that treatment with recombinant hBD-1 can restore hBD-1 expression, bactericidal activity, sperm motility, and improve egg-penetrating ability in asthenozoospermic and leukocytospermic patients [11]. The increase of sperm-fertilizing capacity was observed in a human sperm–hamster oocyte hybrid system [11], which has been shown to have prognostic value on the fecundity rate [12]. However, no data on human oocytes are, so far, available.
All this considered, we can hypothesize a role of hBD-1 in the preservation of a healthy status both in the female and male genital regions; moreover, as reported by Diao et al. [11], hBD-1 could have other functions than those related to antimicrobial activity; indeed they reported that hBD-1 could affect sperm motility. In the current study, infertile couples were recruited and the follicular fluid and semen were collected and analyzed; therefore, the aims of this study are (i) to evaluate the presence of hBD-1 in the follicular fluid and to assess the relationship between hBD-1 levels in follicular fluid and in vitro fertilization (IVF) outcomes; (ii) to investigate the relationship between hBD-1 levels in semen and IVF outcomes (comprising correlation with sperm parameters); and (iii) to explore the effect of hBD-1 peptide on spermatozoa motility in vitro.
Materials and methods
Study design
(i) and (ii) are perspective observational analytic pilot studies. (iii) is an in vitro experimental study on spermatozoa.
Study population
One hundred and seven couples were submitted to assisted reproductive technology procedure at the Assisted Reproduction Unit of Institute for Maternal and Child Health IRCCS Burlo Garofolo (Trieste, Italy) from February to April 2015. A written informed consent was signed by all subjects and all study experiments and procedures have been performed in accordance with the ethical standards of the Declaration of Helsinki and the Bio-Ethical Committee of IRCCS Burlo Garofolo approved the study (RC03/04, L1055, protocol number 118/10).
The eligibility criteria for the recruitment were: presence of infertility status, documented as the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse; availability to undergo assisted reproductive techniques to achieve pregnancy; absence of genitourinary infections, follicular-stimulating hormone (FSH) < 15 UI/L at the third day of cycle, women age ≤ 43 years old, and absence of chronic diseases.
All partners of the infertile couples were Caucasian, asymptomatic for genitourinary infections at the moment of enrollment, and had been screened for chlamydia, mycoplasma, and other bacterial infections; all the individuals were negative for HIV infection.
Semen samples were collected on the same day of women oocyte pick-up. Azoospermic men were excluded from the study (n = 2). Sperm evaluation was performed according to World Health Organization criteria [13]. Specimens were collected into a sterile container by masturbation after 2–7 days of sexual abstinence. After liquefaction, semen was evaluated for volume, viscosity, sperm concentration, total and progressive motility (percentage and concentration as millions/ml) and round cells concentration. Semen samples were processed by using swim-up method either for conventional IVF or intracytoplasmatic sperm injection (ICSI) [14].
All recruited women were not smokers and reported no use of alcohol; they did not undergo hospitalization and they did not follow systemic medication for chronic diseases or antibiotics/probiotics (oral or topic) within the 6 months previous to sample collection, they were not affected by hydrosalpinx or chronic diseases including endocrinopathies (e.g., diabetes mellitus), cardiovascular diseases, dyslipidemia, and rheumatologic diseases (e.g., systemic lupus erythematosus). All patients were treated according to standard conventional IVF/ICSI protocols, as previously described [15]. In two patients, the cycle was canceled due to high risk of ovarian hyperstimulation syndrome and they were excluded from the study. Briefly, a long gonadotrophin-releasing hormone (GnRH) analogue suppression protocol or flexible GnRH antagonist protocol with oral contraceptive pill pre-treatment were used. Recombinant FSH (rec-FSH) 150–225 IU/day was started when pituitary downregulation was established or from day 2 of cycle, in GnRH agonist and antagonist protocol, respectively. The rec-FSH dose was adjusted and/or highly purified menotropin (hphMG) was added, after 5 days of stimulation, depending on the ovarian response, as assessed by serum estradiol (E2) levels and through ultrasound. In three patients, the cycle was canceled due to poor ovarian response. Human chorionic gonadotropin (hCG) 5000 or 10000 IU was administered when two or more follicles were > 18 mm in diameter. Trans-vaginal ultrasound guided oocyte retrieval under general anesthesia was generally performed 36 h later; the most accessible first follicles were selected for the aspiration and then, the follicular fluid was collected. In 32 women, the clinicians used fluid to flush the follicles, so they were excluded from the study and hBD-1 measurements not performed.
Poor ovarian response was defined as the collection of ≤ 3 oocytes at retrieval and good ovarian response as the collection of > 3 oocytes [16]. In one woman, the oocyte retrieval failed; meanwhile in two women, the oocytes were not suitable for conventional IVF and they were excluded from the study. The IVF procedures were carried out using ICSI and/or conventional IVF techniques. Fertilization was assessed the morning after fertilization and was defined by the presence of two pronuclei. The IVF cycles were classified into two categories according to the fertilization rate: “good” fertilization rate (≥ 75%), and “poor” fertilization rate (< 75%). The threshold value of 75% fertilization rate was assumed according to a previous study [17] and it represents the mean fertilization rate of 1120 patients of our institute undergoing ICSI with at least three oocytes retrieved (unpublished data).
One to two embryos or blastocysts were transferred on days 3 or 5 after oocyte pick-up. The luteal phase was supported by vaginal administration of micronized progesterone 600 mg/day. Cleavage-stage embryos were graded based on the Veeck criteria [18], while the blastocyst-stage embryos were graded based on criteria described by Gardner and Schoolcraft [19]. Serum hCG levels were measured 14 days after embryo transfer and, if positive, an ultrasound scan was scheduled 2 weeks later to assess the number and status of the implanted embryos. Successful pregnancy was considered as a positive clinical pregnancy where one or more gestational sacs were visualized with an ultrasound scan [20, 21]. The live birth was defined as the expulsion or extraction of a product of fertilization from a woman, after 22 weeks of gestation; which breathes, presents heart beating, umbilical cord pulsation, or movement of muscles [20, 21].
The follicular and semen samples used for the hBD-1 analysis derived from discarded specimens. The fluid from the first follicle retrieved (the most accessible one) was used for the analysis.
Seminal and follicular fluid hBD-1
Each sample (semen and follicular fluid) was centrifugated (200×g for 10 min), the supernatant collected, immediately stored at − 20 °C, and carried to the laboratories. Follicular fluid with blood contamination were excluded from the analysis (n = 15).
The hBD-1 concentration was measured with a commercially validated Human Beta Defensin 1 ELISA kit, (Cat. No. 100-240-BD1, Alpha Diagnostic, San Antonio, TX, USA; sensitivity range = 0–800 pg/ml) using manufacturer’s instructions: the semen samples were diluted 1:100, while the follicular samples at 1:50 in sample dilution buffer (provided by the kit). Each assay sample was conducted in duplicate. The absorbance was measured with GloMax®-Multi Detection System (Promega, Fitchburg, WI, USA).
Sperm motility
Recombinant hBD-1 protein (mature secreted form including pro-peptide: 22–88 amino acids; ab191781, Abcam®, Cambridge, UK) was used for in vitro test of sperm motility. Briefly, freshly ejaculated semen samples were obtained from 29 normozoospermic men attending the Assisted Reproduction Unit of the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy, by masturbation after 2–7 days of sexual abstinence and processed according to WHO guidelines [13] from October 2016 to February 2017. The criteria for eligibility were the same of the other population above described.
Spermatozoa were incubated with 800 ng/ml (final concentration) of hBD-1 [11] at room temperature for 1 or 24 h before the examination: progressive and total motility (as percentage and concentration millions/ml) were recorded [13]. Not treated spermatozoa from the same sample were used as control for the comparison. The 1 h time point was chosen to replicate Diao et al. findings [11] and to observe a precocious effect of hBD-1 supplementation, while the 24 h were selected to determine if the effect is a late one and/or maintained over time.
Sample size description
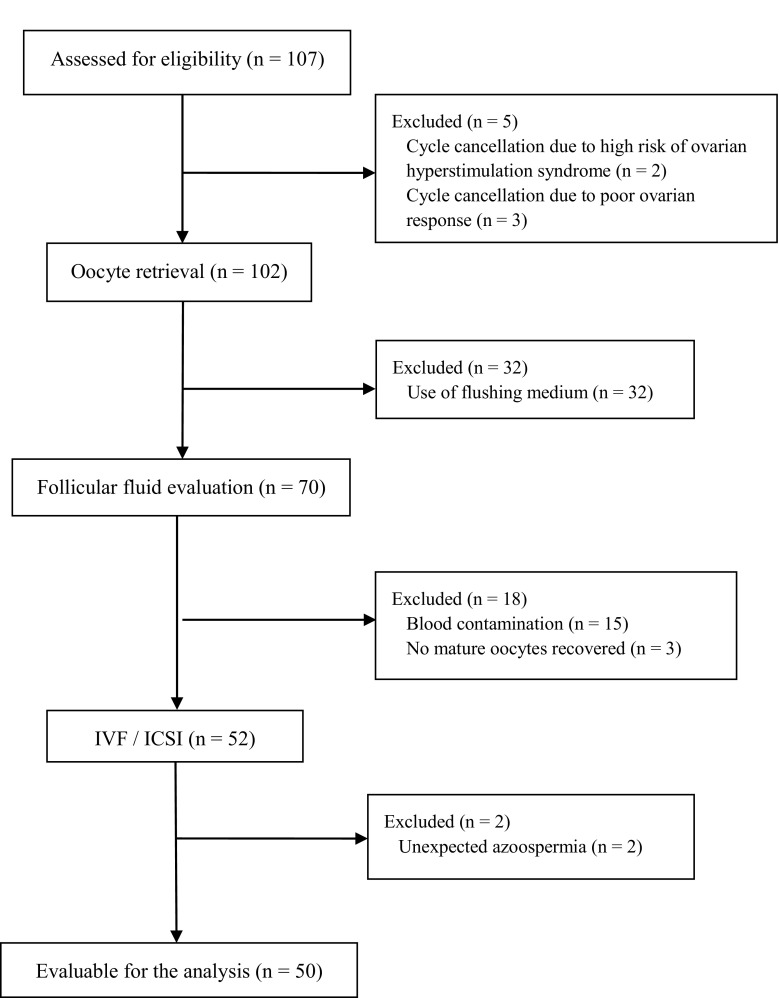
In (i) and (ii), from the initial 107 couples evaluated for eligibility, 2 couples were excluded for cycle cancelation due to high risk of ovarian hyperstimulation syndrome, 3 for cycle cancelation due to poor ovarian response, 32 for the utilization of flushing media during oocyte retrieval by clinicians, 15 for blood contamination of the follicular fluid, 3 for no mature oocytes recovered, and 2 for no sperm count in the ejaculate; finally, a total of 50 couples were selected and their samples were included in the analysis (the flowchart of the enrollment is reported in Fig. 1).
Fig. 1.
Flow chart with the numbers of individuals at each stage of the study: the perspective observational analytic pilot study on 50 infertile couples
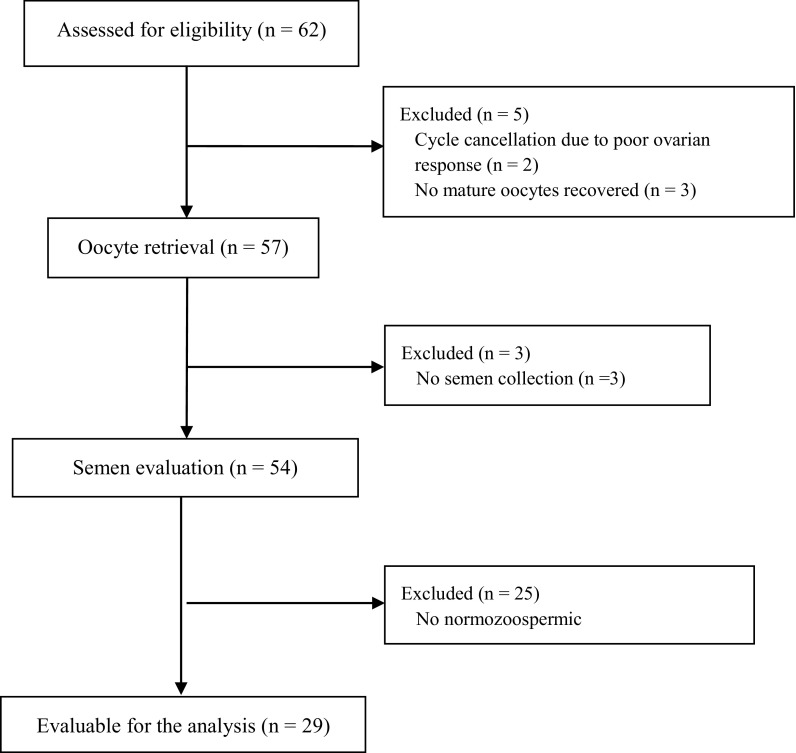
In (iii), from the initial 62 men from couples evaluated for eligibility, 2 couples were excluded for cycle cancelation due to poor ovarian response, 3 for no mature oocytes recovered, 3 for no semen collection, and 25 for alteration in semen parameters (motility and/or count); finally, 29 normozoospermic men were selected for the in vitro analysis of hBD-1 supplementation on spermatozoa (the flowchart of the enrollment is reported in Fig. 2).
Fig. 2.
Flow chart with the numbers of individuals at each stage of the study: the in vitro experimental study on spermatozoa from 29 normozoospermic men
Statistical analysis
The statistical analyses were performed using the R software (version 3.5.0) [22]. Pearson’s product-moment correlation was used to determine if estradiol, female age, numbers of oocyte retrieved, spermatozoa concentration, total and progressive motility (percentage), total and progressive motile spermatozoa concentration (millions/ml), techniques employed, number of embryos obtained, number of embryos transferred, and cause of infertility impacted the fertilization and pregnancy rate and could be considered as confounders in the statistical analysis. Then, linear regression analysis for continuous variable and logistic regression analysis for binomial data were performed in order to test the correlation between hBD-1 and different parameters related to infertility status: correlation was evaluated between FF hBD-1 and estradiol, numbers of oocyte retrieved, number of embryos obtained, number of embryos transferred, and fertilization and pregnancy rate; moreover, correlation was examined between semen hBD-1 and spermatozoa concentration, total and progressive motility (percentage), total and progressive motile spermatozoa concentration (millions/ml), fertilization rate, number of embryos obtained, number of embryos transferred, and pregnancy rate. Moreover, a regression analysis was performed, where the impact of hBD-1 levels of follicular fluid and semen on fertilization and pregnancy rate were considered together.
Mann-Whitney test was performed using software Prism™ 5 version 5.0 (GraphPad, La Jolla, CA, USA) and it was used to investigate the differences of hBD-1 level in follicular fluid between women with good or poor fertilization rate and in semen between oligo-asthenozoospermic and normozoospermic men. Moreover, Mann-Whitney test was used to detect the effect of exogenous hBD-1 protein on sperm motility comparing hBD-1 supplemented spermatozoa and not treated ones.
The figures were created using software Prism™ 5 version 5.0 (GraphPad). Only p value of ≤ 0.05 were considered as statistically significant.
Since different outcomes were considered and some of them were not available at the moment of enrollment, it was not possible to estimate a priori the sample size to obtain; however, post hoc power calculations were performed with GPower software version 3.1.9.3 using post hoc calculation employing the Mann-Whitney test and regression analysis tests [23]. The manuscript followed the STROBE guidelines [24].
Results
The characteristics of the study population as well as the reproductive outcome are shown in Table 1. Briefly, 43 couples underwent ICSI, 5 conventional IVF, and 2 were submitted to both techniques (some oocytes were treated with conventional IVF, some with ICSI); the etiology of infertility was determined as due to male factors (n = 18), idiopathic (n = 15), pelvic/tubal factor (n = 12), and ovulatory factor (n = 5). The implantation rate was 13 on 63 embryos transferred in the women; meanwhile, the clinical pregnancy rate was 10 on the 50 couples submitted to IVF (in 3 women there were two fetuses), totally 13 fetuses developed, while the live births were 9, and the live birth delivery rate was 7 on 38 embryo transfer cycles. These data were comparable to those reported by the Italian register of PMA (available at http://old.iss.it/rpma/index.php?lang=1).
Table 1.
Study population and cycle characteristics, and reproductive outcome (n = 50)
| Partners’ age (years) | |
| Female | Median = 38 (range = 26–43) |
| Male | Median = 39 (range = 28–58) |
| Etiology of infertility | |
| Male factor | n = 18 (36%) |
| Unexplained | n = 15 (30%) |
| Pelvic/tubal factor | n = 12 (24%) |
| Ovulatory factor | n = 5 (10%) |
| Sperm concentration (million/ml) | median = 34 (range = 0.1–180) |
| oligo-asthenozoospermic < 5 × 106/ml sperm with progressive motility | n = 16 |
| Normozoospermic ≥ 5 × 106/ml sperm with progressive motility | n = 34 |
| Sperm total motility rate | Median = 60 (range = 4–80) |
| Sperm progressive motility rate | Median = 32.5 (range = 1–65) |
| Estradiol on hCG day (pg/ml) | Median = 1576 (range = 227–4682) |
| Number of oocytes recovered | Median = 8 (range = 1–24) |
| Type of IVF technique | |
| ICSI | n = 43 (86%) |
| Conventional IVF | n = 5 (10%) |
| Conventional IVF/ICSI | n = 2 (4%) |
| Fertilization rate (excluding patients with poor ovarian response, oocytes ≤ 3) | Median: 67 (range = 0–100) |
| Fertilization rate ≥ 75% (excluding women with oocytes retrieved ≤ 3) | n = 15 |
| Fertilization rate < 75% (excluding women with oocytes retrieved ≤ 3) | n = 27 |
| Total number of embryos transferred | n = 63 (Median for woman = 1; range = 0–3) |
| Grade of embryos transferred | Median = 1 (range = 1–3) |
| Implantation rate (on the of embryos transferred) | 13/63 |
| Pregnancy | |
| Clinical pregnancy | n = 10 |
| Clinical pregnancy rate (on the number of couples submitted to IVF cycles) | 10/50 |
| Clinical pregnancy rate (on the number of embryo transfer cycles) | 10/38 |
| Number of fetuses | n = 13 |
| Live birth | n = 9 |
| Live birth delivery rate (on the number of embryo transfer cycles) | n = 7/38 |
The effect of estradiol, female age, numbers of oocyte retrieved, spermatozoa concentration, total and progressive motility (percentage), total and progressive motile spermatozoa concentration (millions/ml), techniques employed, number of embryos obtained, number of embryos transferred, cause of infertility on fertilization, and pregnancy rate was checked: only the females age correlated significantly with pregnancy rate, the other variables had not effect.
Follicular fluid hBD-1
The concentration of hBD-1 in the follicular fluid ranged from 2.84 to 17.93 ng/ml, median 7.91 ng/ml, and average 8.60 ng/ml, while in the semen, the hBD-1 measurement displayed a range between 7.15 and 137.06 ng/ml, median 24.52 ng/ml, and average 29.31 ng/ml. hBD-1 concentrations in follicular fluid were not significantly different in women with good (> 3 oocytes at retrieval) and poor ovarian response (≤ 3 oocytes). Then, the correlation between hBD-1 levels in follicular fluid and fertilization rate has been evaluated. Since the low number of oocytes retrieved (≤ 3) may represent a potential confounder in calculating fertilization rate, the patients with poor ovarian response were excluded from this analysis (n = 8) and the cutoff of 75% for the fertilization rate was chosen. Considering together the women submitted to FIVET and/or ICSI, hBD-1 levels in the follicular fluid were significantly higher in the group of women with good fertilization rate (≥ 75%, n = 15), respect to those with a poor fertilization rate (< 75%, n = 27) (Mann-Whitney test: p value = 0.04, power = 0.61, Fig. 3). A similar result was observed also in the sub-group of patients submitted to ICSI technique with a borderline statistical significance (p = 0.05, data not shown). The patients were also sub grouped accordingly with the type of infertility, but no hBD-1 concentration difference was observed between those with good and poor fertilization rate, probably due to the too small sample size of the subgroups. Then, a correlation analysis was performed comparing hBD-1 level in each woman with the respective fertilization and pregnancy rate, but no association was found (Supplementary Table 1).
Fig. 3.
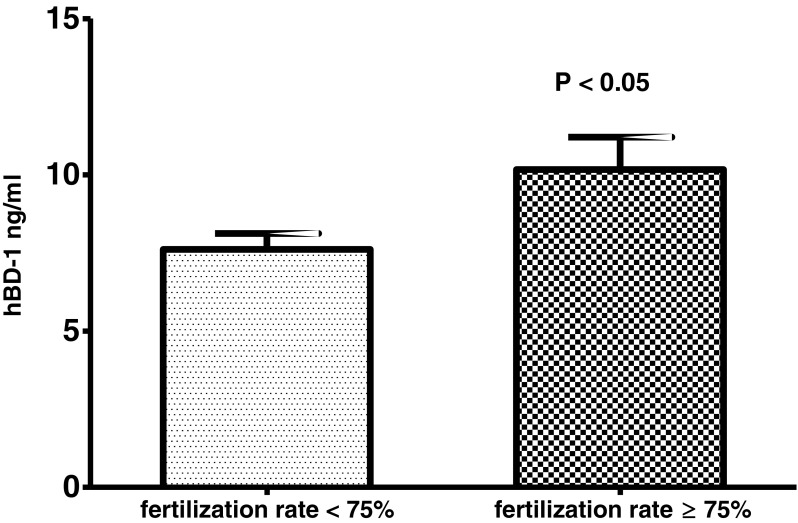
follicular fluid hBD-1 levels in subjects with poor (< 75%, n = 27) and good (≥ 75%, n = 15) oocyte fertilization rate. Results are represented as mean ± standard error of the mean.
Finally, there was no correlation between hBD-1 follicular fluid levels and other outcomes, such as peak estradiol levels, number of oocytes recovered, number of embryos obtained, quality of embryos, implantation rate, pregnancy rate, and also after correction for women age.
Seminal hBD-1
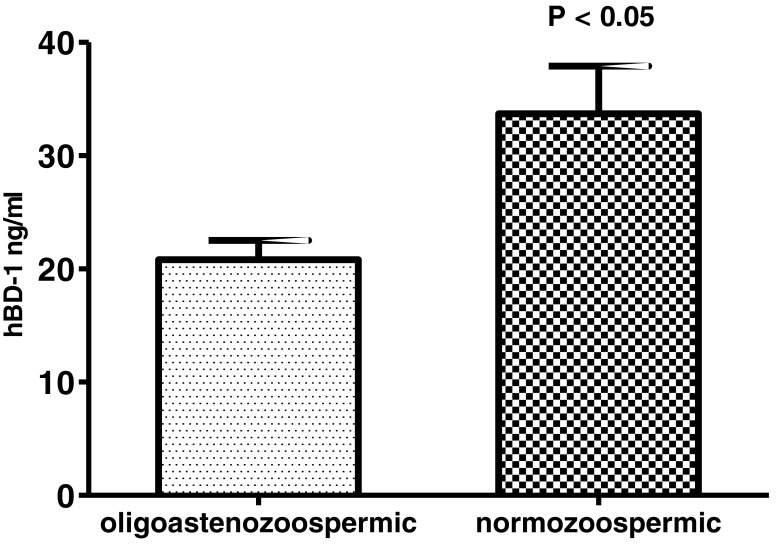
We compared the seminal hBD-1 levels in men with normal and abnormal sperm concentration and motility, evaluated according to the WHO criteria [13]. The hBD-1 semen concentrations in patients with oligo-asthenozoospermia (< 5 × 106/mL sperm with progressive motility, n = 14) were significantly lower than that in patients with normozoospermia (≥ 5 × 106/mL sperm with progressive motility, n = 28) (Mann-Whitney test: p value = 0.02, power = 0.63, Fig. 4). The men were also sub-grouped based on the type of infertility: no different hBD-1 level was detected between oligo-asthenozoospermic and normozoospermic individuals, probably due to the too small sample size of the subgroups.
Fig. 4.
Seminal hBD1 levels in oligo-asthenozoospermic (n = 16) and normozoospermic (n = 34) subjects. Results are represented as mean ± standard error of the mean
Instead, hBD-1 did not correlate with total and progressive motility (percentage), total and progressive motile spermatozoa concentration (millions/ml), or with the concentration of spermatozoa (Supplementary Table 1).
Regarding the parameters linked to IVF outcomes, the hBD-1 levels were not significantly different in the group with good fertilization rate (≥ 75%), respect to those with a poor fertilization rate (< 75%). There was no significant correlation between hBD-1 semen levels and other IVF outcomes, such as number of embryos obtained, quality of embryos, implantation rate, pregnancy rate, also after adjustment for sperm total and progressive motility (as percentage and concentration millions/ml) and spermatozoa concentration.
Finally, there was no correlation between follicular and semen hBD-1, considered together, and fertilization or pregnancy rate. The power analysis of the data without statistically significant results are reported in Supplementary Table 2.
Recombinant-hBD-1 and sperm motility
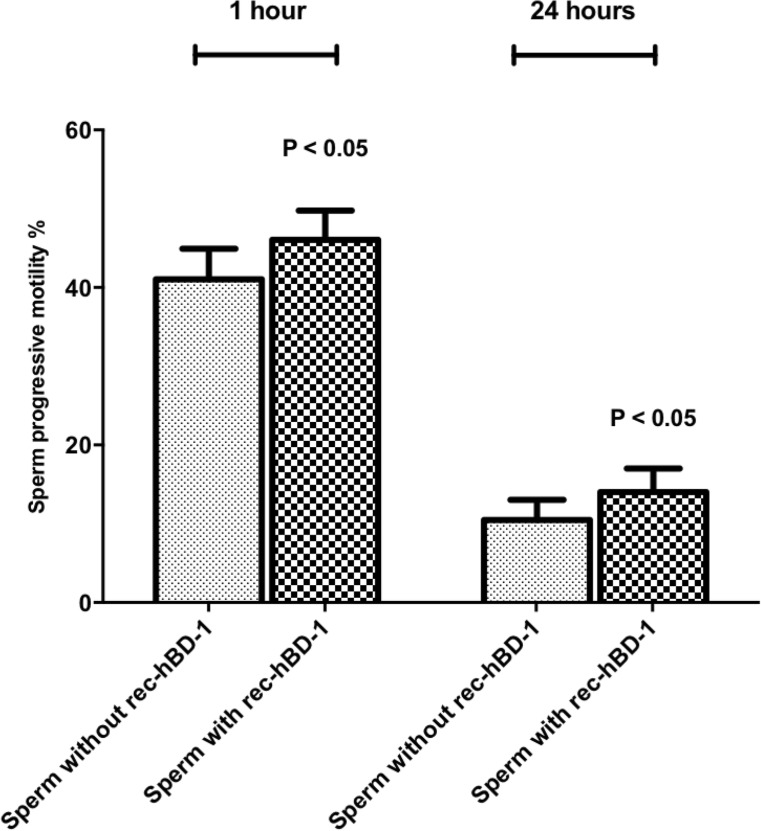
The incubation with hBD-1 exogenous peptide (800 ng/ml) [11] increased significantly the progressive motility after 1 h (Mann-Whitney test: p value = 0.03, power = 0.50, Fig. 5) and 24 h (Mann-Whitney test: p value = 0.02, power = 0.46) (Fig. 5) respect to not treated spermatozoa.
Fig. 5.
Sperm progressive motility after 1 h and 24 h of incubation with and without recombinant-hBD-1 (rec-hBD-1) in 29 normozoospermic subjects. Results are represented as mean ± standard error of the mean
Discussion
To our knowledge, this is the first study that reports on the presence of hBD-1 in the follicular fluid. There are no studies on the expression of β-defensin in human ovarian tissue. However, it has been shown that in animal models β-defensins are expressed in the follicle, actually avian-β-defensin have been identified in interstitial, theca interna, and granulosa cells, where their amounts are likely increased with follicular growth [25]. So, it can be hypothesized that β-defensin are mainly produced by granulosa cells, being the most metabolically active epithelial line during follicle growth [26]. The role of this defensin in the follicular environment is not clear. β-defensins are a group of small secretory peptides with antimicrobial activity but they have also immunomodulatory properties and are involved in many processes, such as fertility, development, wound healing, and cancer [27].
In the last decades, many data have been accumulated on the interactions between innate immunity, inflammation, and reproduction [2, 3, 6]. It has been shown that physiological events within the ovary, such as follicle development, use several inflammatory mediators, and that an impaired inflammation may contribute to infertility status [28, 29]. Thus, the presence of hBD-1 in the follicular fluid might be related with the inflammatory response that has been demonstrated to be necessary for appropriate folliculogenesis as well as for inducing both ovulation and its associated tissue remodeling [30]. Our findings do not support this hypothesis: we observed no correlation between hBD-1 levels and number of oocytes retrieved as well as 17β-estradiol levels, which may be considered as marker of ovarian inflammation [31, 32]. Moreover, it has been shown that 17β-estradiol do not influence hBD-1 production in vaginal epithelial cells where this defensin is constitutively produced [33].
In our study, we observed that the hBD-1 follicular levels of women with a good oocyte fertilization rate (≥ 75%) were significantly higher than those of women with poor fertilization rate (< 75%); this finding suggests a potential fertilization-promoting effect of hBD-1. Our data failed to observe an association between hBD-1 concentration and pregnancy achievement; however, it is reasonable to think that the successful embryo implantation and pregnancy, both complex events, reasonably depend upon a major number of variables, therefore, hBD-1 should play a minor role in this context respect to the one played in a more controlled condition such as the in vitro fertilization. Prior to our work Das et al. [34] measured α-defensin 1–3 production in the follicular fluid without finding a correlation with fertilization or IVF outcome. The same authors [35] observed no difference in expression (using immunohistochemistry) of α-defensin 1–3 and hBD-1 in endometrium from patients who did or did not reach pregnancy following subsequent IVF. To note, the endometrial samples were collected at least two cycles before the IVF and therefore may not directly reflect the endometrial status of the treatment cycle accurately.
It has been suggested that β-defensins modulate sperm–oocyte interaction [36]. However, about 86% of our patients were treated using ICSI that bypasses sperm–egg surface interaction. Therefore, alternative fertilization promoting mechanism by hBD-1 should be hypothesized.
β-defensins could guarantee a better quality of oocyte, protecting it from microbial agents. Animal studies suggest that alterations in the follicular environment due to pathogen exposure results in diminished oocyte quality, further affecting fertility [29]. Alternatively, immunomodulatory properties of hBD-1 on cytokine inflammatory response [27] might explain its positive association with fertilization rate. Actually, it has been shown that follicular fluid cytokines may influence, negatively or positively, oocyte fertilization and thus embryo quality [37, 38]. Thus, the favorable effect of hBD-1 on oocyte fertilization might be mediated through cytokine system. Unfortunately, we have no experimental data supporting these hypotheses and further studies are needed.
Several animal studies showed that β-defensins influence sperm functions, with the most of the research conducted on rodents [10], but there are very scanty data for humans. The primary evidence for a role of defensins in human fertility has been provided by the study of Tollner et al. [39] who described that a mutation in DEFB126 (chromosome 20p13, encoding for human β-defensin 126) was associated with a reduced ability to successfully reproduce. Later, Diao et al. [11] demonstrated in a Chinese population that the amount of hBD-1 in sperm from infertile men with asthenozoospermia and leukocytospermia, was lower when compared to that in normal fertile sperm.
In the present study, we were able to confirm these results in a European population: we observed that subjects with oligoasthenospermia, defined by WHO criteria [13], had hBD-1 levels significantly lower than subjects with normal sperm concentration and motility. Moreover, we found that recombinant-hBD may improve sperm motility in normozoospermic subjects. These data do not completely agree with the observation of Diao et al. showing that recombinant hBD-1 may increase sperm motility in asthenozoospermic, and leukocytospermic patients but not in healthy fertile donors [11]. However, it should be noticed that we used a different method to detect hBD-1 and, that our population was different from the population investigated by Diao et al. [11], as a matter of fact, in Italy, due to legal restrictions, healthy donors were not available, we recruited male partner of infertile couples referring to our andrology laboratory for routine semen analysis.
Taken together, our findings confirm an important role of hBD-1 in regulating sperm motility and the possibility to use recombinant hBD-1 to improve male fertility, as firstly suggested by Diao et al. [11].
Since the relationship between hBD-1 and sperm fertilization capacity have never been evaluated on human oocytes, we have also correlated hBD-1 levels in samples collected on the day of in vitro oocyte insemination and fertilization rate and IVF outcomes. We did not find any correlation between hBD-1 levels and oocyte fertilization or IVF outcomes. Although these results need of further confirmation, they can be explained considering that performing ICSI sperm defects are bypassed; thus, β-defensin may influence natural sperm-egg interaction but not oocyte fertilization after ICSI.
We are aware that our study presented some limitations, such as the restricted sample size; the heterogenicity inside the population with the presence of different subgroups when considering the IVF techniques or the type of infertility.
The power analysis values were medium for the statistically significant results here reported, while in the correlation analysis, the power is very low, so it should not be excluded that the lack of association could be due to the insufficient sample size. The small sample size is a limitation of our study that we fully acknowledge. Nevertheless, this is a preliminary pilot study aiming at depicting a picture, although not complete, of the role of hBD-1 in infertile couples. In conclusion, our study suggests that hBD-1 could play a role also in ovarian dynamics, although the precise mechanisms need to be further elucidated in a larger cohort. The presence of hBD-1 in the follicular fluid could indicate that this peptide might influence oocyte maturation, as it has already been indicated for sperm maturation and has been corroborated by our data. Further studies are needed to define the possible role of follicular and seminal β-defensin in egg–sperm interaction. The preliminary positive correlation between hBD-1 and fertilization rate and the property of recombinant hBD-1 to improve sperm motility observed in our study, should be investigated in larger clinical experimentation to evaluate the possible role of β-defensin in the treatment of infertility.
Electronic supplementary material
(DOCX 14 kb)
Authors’ contributions
LZ performed the statistical analyses and wrote the manuscript. VP performed hBD-1 ELISA experiments. MM, SL, and EG collected the samples, participated in patients enrollment, and revised the manuscript. GZ and FR enrolled the participants, were responsible of patients clinical management, and collected the samples. LS supervised the experiments, revised the manuscript, and contributed in the study design. SC conceived the study and critically revised the manuscript. GR conceived the study, was responsible for the management of patients, and critically revised the manuscript.
Funding
This work was supported by University of Trieste – “University funding for scientific research project “(U22SCFRA15) and by IRCCS Burlo Garofolo/Italian Ministry of Health (RC 15/2017 and RC 18/2016).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baecher-Lind LE, Miller WC, Wilcox AJ. Infectious disease and reproductive health: a review. Obstet Gynecol Surv. 2010;65:53–65. doi: 10.1097/OGX.0b013e3181c9e7a1. [DOI] [PubMed] [Google Scholar]
- 2.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–749. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 3.Sheldon IM, Owens S-E, Turner ML. Innate immunity and the sensing of infection, damage and danger in the female genital tract. J Reprod Immunol. 2017;119:67–73. doi: 10.1016/j.jri.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarczak J, Kościuczuk EM, Lisowski P, Strzałkowska N, Jóźwik A, Horbańczuk J, Krzyżewski J, Zwierzchowski L, Bagnicka E. Defensins: natural component of human innate immunity. Hum Immunol. 2013;74:1069–1079. doi: 10.1016/j.humimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon IM, Bromfield JJ. Innate immunity in the human endometrium and ovary. Am J Reprod Immunol. 2011;66(Suppl 1):63–71. doi: 10.1111/j.1600-0897.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 7.Raschig J, Mailänder-Sánchez D, Berscheid A, Berger J, Strömstedt AA, Courth LF, Malek NP, Brötz-Oesterhelt H, Wehkamp J. Ubiquitously expressed human beta defensin 1 (hBD1) forms bacteria-entrapping nets in a redox dependent mode of action. PLoS Pathog. 2017;13:e1006261. doi: 10.1371/journal.ppat.1006261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King AE, Kelly RW, Sallenave J-M, Bocking AD, Challis JRG. Innate immune defences in the human uterus during pregnancy. Placenta. 2007;28:1099–1106. doi: 10.1016/j.placenta.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, Jégou B, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- 10.Dorin JR, Barratt CLR. Importance of β-defensins in sperm function. Mol Hum Reprod. 2014;20:821–826. doi: 10.1093/molehr/gau050. [DOI] [PubMed] [Google Scholar]
- 11.Diao R, Fok KL, Chen H, Yu MK, Duan Y, Chung CM, et al. Deficient human β-defensin 1 underlies male infertility associated with poor sperm motility and genital tract infection. Sci Transl Med. 2014;6:249ra108. doi: 10.1126/scitranslmed.3009071. [DOI] [PubMed] [Google Scholar]
- 12.Corson SL, Batzer FR, Marmar J, Maislin G. The human sperm-hamster egg penetration assay: prognostic value. Fertil Steril. 1988;49:328–334. doi: 10.1016/S0015-0282(16)59724-6. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 14.Ricci G, Perticarari S, Boscolo R, Simeone R, Martinelli M, Fischer-Tamaro L, Guaschino S, Presani G. Leukocytospermia and sperm preparation--a flow cytometric study. Reprod Biol Endocrinol. 2009;7:128. doi: 10.1186/1477-7827-7-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci G, Granzotto M, Luppi S, Giolo E, Martinelli M, Zito G, Borelli M. Effect of seminal leukocytes on in vitro fertilization and intracytoplasmic sperm injection outcomes. Fertil Steril. 2015;104:87–93. doi: 10.1016/j.fertnstert.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of “poor response” to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26:1616–1624. doi: 10.1093/humrep/der092. [DOI] [PubMed] [Google Scholar]
- 17.Oehninger S, Coddington CC, Scott R, Franken DA, Burkman LJ, Acosta AA, et al. Hemizona assay: assessment of sperm dysfunction and prediction of in vitro fertilization outcome. Fertil Steril. 1989;51:665–670. doi: 10.1016/S0015-0282(16)60618-0. [DOI] [PubMed] [Google Scholar]
- 18.Veeck L. Preembryo grading and degree of cytoplasmic fragmentation. An atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999. [Google Scholar]
- 19.Gardner D, Schoolcraft W. Toward reproductive certainty: fertility and genetics beyond. London: Parthenon Publishing; 1999. In vitro culture of human blastocysts; pp. 378–388. [Google Scholar]
- 20.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID, Simpson JL, van der Poel S. The international glossary on infertility and fertility care, 2017†‡§. Hum Reprod. 2017;32:1786–1801. doi: 10.1093/humrep/dex234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.R core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria. 2018. Available from: http://www.R-project.org
- 23.Faul F, Erdfelder E, Lang A-G, Buchner A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura Y. Avian β-defensins expression for the innate immune system in hen reproductive organs. Poult Sci. 2015;94:804–809. doi: 10.3382/ps/peu021. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers RJ, Irving-Rodgers HF. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 27.Semple F, Dorin JR. β-defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheldon IM, Cronin JG, Healey GD, Gabler C, Heuwieser W, Streyl D, Bromfield JJ, Miyamoto A, Fergani C, Dobson H. Innate immunity and inflammation of the bovine female reproductive tract in health and disease. Reproduction. 2014;148:R41–R51. doi: 10.1530/REP-14-0163. [DOI] [PubMed] [Google Scholar]
- 30.Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med. 2015;33:270–275. doi: 10.1055/s-0035-1554928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abramov Y, Schenker JG, Lewin A, Friedler S, Nisman B, Barak V. Plasma inflammatory cytokines correlate to the ovarian hyperstimulation syndrome. Hum Reprod. 1996;11:1381–1386. doi: 10.1093/oxfordjournals.humrep.a019404. [DOI] [PubMed] [Google Scholar]
- 32.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 33.Han JH, Kim MS, Lee MY, Kim TH, Lee M-K, Kim HR, Myung SC. Modulation of human beta-defensin-2 expression by 17beta-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010;49:209–214. doi: 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Das S, Bates MD, Vince GS, Lewis-Jones I, Gazvani R. Follicular fluid expression of alpha-defensins and their role in ovulation. J Assist Reprod Genet. 2008;25:83–87. doi: 10.1007/s10815-007-9197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das S, Vince GS, Lewis-Jones I, Bates MD, Gazvani R. The expression of human alpha and beta defensin in the endometrium and their effect on implantation. J Assist Reprod Genet. 2007;24:533–539. doi: 10.1007/s10815-007-9173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caballero-Campo P, Buffone MG, Benencia F, Conejo-García JR, Rinaudo PF, Gerton GL. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J Cell Physiol. 2014;229:68–78. doi: 10.1002/jcp.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedaiwy M, Shahin AY, AbulHassan AM, Goldberg JM, Sharma RK, Agarwal A, et al. Differential expression of follicular fluid cytokines: relationship to subsequent pregnancy in IVF cycles. Reprod BioMed Online. 2007;15:321–325. doi: 10.1016/S1472-6483(10)60346-X. [DOI] [PubMed] [Google Scholar]
- 38.Sarapik A, Velthut A, Haller-Kikkatalo K, Faure GC, Béné M-C, de Carvalho BM, et al. Follicular proinflammatory cytokines and chemokines as markers of IVF success. Clin Dev Immunol. 2012;2012:606459. doi: 10.1155/2012/606459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tollner TL, Venners SA, Hollox EJ, Yudin AI, Liu X, Tang G, et al. A common mutation in the defensin DEFB126 causes impaired sperm function and subfertility. Sci Transl Med. 2011;3:92ra65. doi: 10.1126/scitranslmed.3002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)