Abstract
Purpose
Empty follicle syndrome (EFS) is a complex reproductive disorder characterized by the repeated failure to aspirate oocytes from mature ovarian follicles during in vitro fertilization (IVF). In addition to some cases caused by iatrogenic problems and known genetic factors, there are still many unexplained aspects of EFS. Here, we aimed to assess the clinical and genetic characteristics of two EFS patients.
Methods
We have characterized two primary infertility patients with EFS in a nonconsanguineous family from China. Both the patients presented similar clinical phenotypes, that is a few granulosa cells but no oocytes could be retrieved during repeated cycles with normal follicular development, E2 levels, and bioavailable hCG plasma levels. Abnormal oocytes were obtained once or twice between multiple IVF cycles. We performed Sanger sequencing of the LHCGR and ZP1~ZP4 genes in the patients, and further bioinformatics analysis was performed to identify pathogenic elements in the genes.
Results
A novel mutation, c.181C>T (p.Arg61Cys), and a known mutation, c.1169_1176delTTTTCCCA (p.Ile390Thrfs*16), in the ZP1 gene were both identified in patient 2, but no mutations were identified in patient 1. The novel mutation inherited from her mother was absent in the control cohort and the ExAc database. The arginine residue is conserved at this position, and its replacement by cysteine was predicted to be deleterious. In another allele, a paternal frameshift mutation was predicted to introduce premature stop codons, resulting in the deletion of 234 amino acids from the C-terminus of the ZP1 protein.
Conclusions
Our findings presented compound heterozygous mutations in ZP1 associated with EFS and abnormal oocytes and provided further new evidence for the genetic basis of EFS and support for the genetic diagnosis of infertile individuals.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01404-1) contains supplementary material, which is available to authorized users.
Keywords: Empty follicle syndrome, ZP1, Mutation, Oocyte anomalies, Infertility
Introduction
Cumulus-oocyte complexes (COCs) generally consist of cumulus cells with a centrally located oocyte surrounded by the zona pellucida and are isolated from the individual’s follicular fluid. Cumulus cells are one of the two functionally divided classes of granulosa cells in the antral follicle. During in vitro fertilization (IVF) treatment, COCs can be retrieved with aspiration and flushing of mature ovarian follicles following the induction of ovulation. Sometimes, even if the patients have a normal ovarian reserve and follicular development and are subjected to ovum pick up (OPU) and repeated aspiration, the complexes including the granulosa cells cannot be obtained; in these cases, empty follicle syndrome (EFS) is considered [1]. “False” EFS can be improved by correcting errors in the administration of hCG or repeating cycles with other tactics triggering an endogenous luteinizing hormone (LH) surge; in this situation, treatment options are chosen according to the levels of beta hCG (β-hCG) on the day of oocyte retrieval [2, 3].
Moreover, some genetic defects associated with “genuine” EFS (GEFS) are characterized, and its prevalence is 0.016%. There are two known genes responsible for GEFS, the luteinizing hormone/chorionic gonadotropin receptor (LHCGR, MIM: 152790) and the zona pellucida glycoprotein 3 (ZP3, MIM: 182889) [4, 5]. These mutations indicate that loss-of-function not only in LH signaling but also in zona pellucida (ZP) formation induces EFS manifestations, although oocytes can be obtained from some patients with digenic mutations in the ZP2 and ZP3 genes and ZP abnormalities [6]. ZP1 as another gene combining with the other three genes (ZP2, ZP3, and ZP4) encodes the zona pellucida glycoproteins during human folliculogenesis, which was believed to be responsible for female infertility with a lacked ZP [7, 8]. In fact, it is different that the clinical manifestation of LHCGR-related GEFS is from that of ZP3-related GEFS; in the former case, neither oocytes nor COCs are retrieved, but in the latter case, COCs can always be obtained although the oocytes are usually not [4, 5]. However, the exact pathological mechanism is not clear. Furthermore, there are still many unexplained GEFS cases that need further investigation.
In this study, we present two patients with clinical manifestations suggestive of GEFS. In one patient, a novel mutation and a known pathogenic mutation in the ZP1 gene were found. On the basis of the genetic analysis, this study provides additional evidence for the role of ZP1 in GEFS and advances in our understanding of the clinical features of ZP1 mutations. This is the first study to report GEFS clinical effects associated with the mutations in the ZP1 gene.
Materials and methods
Case report
Patient 1
The patient was a 25-year-old woman with a 4-year history of primary infertility with regular menses since menarche at the age of 13. Her basic follicle stimulating hormone (FSH) level was moderately elevated, but her luteinizing hormone (LH), estradiol (E2) and anti-Müllerian hormone (AMH) levels were normal (Table 1). An ultrasound scan showed a normal uterus and ovaries with a total of eight antral follicles (2 to 5 mm in diameter). Repeated ultrasound scans during her natural menstrual cycles showed that a leading follicle developed to 20 mm in diameter and then ovulated for 2 cycles. Chromosomal analysis showed the patient to be 46, XX. Her husband was 37 years old, and his anamnesis, physical examination, ultrasound, and semen analysis were normal. His chromosome karyotype was 46, XY. Subsequently, the patient underwent controlled ovarian hyperstimulation and 5 cycles of IVF at our hospital, as shown in Table 1. The patient was the only child of a nonconsanguineous marriage, and her family members did not have infertility disorders.
Table 1.
Clinical features of cases with EFS and oocyte anomalies
| Characteristic | Patient 1 | Patient 2 | |
|---|---|---|---|
| Age (years) | 25 | 28 | |
| Length of primary infertility history (years) | 4 | 4 | |
| Basal sexual hormone | FSH (IU/L) | 10.24 | 8.11 |
| LH (IU/L) | 8.85 | 2.93 | |
| E2 (ng/L) | 29.00 | 20.00 | |
| AMH (ng/mL) | 1.33 | 6.23 | |
| Pelvic ultrasound | Normal uterus, ovaries of equal sizes (left, 7.83 mL; right, 7.49 mL) | Normal uterus, ovaries of equal sizes (left, 2.37 mL; right, 2.67 mL) | |
| Cycle 1 | Protocol | GnRH antagonist | Short |
| Total dose of gonadotropin (IU) | 1875 | 1650 | |
| E2 level on trigger day (ng/L) | 2126 | 4346 | |
| No .of follicles ≥ 18 mm on trigger day | 2 | 9 | |
| Time interval between hCG administration and oocyte retrieval (h) | 36 | 36 | |
| Ovulation triggering (dose) | 10,000 IU (hCG) | 4000 IU (hCG) | |
| No. of oocytes retrieved | 0 (only a little granulosa cells cumulus) | 0 (only some granulosa cumulus cells) | |
| Serum hCG level (IU/L) on the day of OPU | 207.08 | 65.55 | |
| Cycle 2 | Protocol | GnRH antagonist | Long |
| Total dose of gonadotropin (IU) | 3825 | 1800 | |
| E2 level on trigger day (ng/L) | 2257 | 5110 | |
| No. of follicles ≥ 18 mm on trigger day | 1 | 3 | |
| Time interval between hCG administration and oocyte retrieval (h) | 36 and 39 | 36, 38 and 41 | |
| Ovulation triggering (dose) | 10,000 IU (hCG) + 0.2 mg (GnRH agonist) | 5000 IU (hCG) | |
| No. of oocytes retrieved | 0 (only a little granulosa cells cumulus) | 0, 0 (only a little granulosa cells cumulus) and 5 degenerated oocytes | |
| Serum hCG level (IU/L) on the day of OPU | NA | 111.50 | |
| Cycle 3 | Protocol | Natural | |
| Total dose of gonadotropin (IU) | 0 | ||
| E2 level on trigger day (ng/L) | 242 | ||
| No. of follicles ≥ 18 mm on trigger day | 0 (one follicle ≥ 14 mm) | ||
| Time interval between hCG administration and oocyte retrieval (h) | 36 | ||
| Ovulation triggering (dose) | 0.1 mg (GnRH agonist) | ||
| No. of oocytes retrieved | 1 (cumulus-oocyte complexes) | ||
| Cycle 4 | Protocol | Mild | |
| Total dose of gonadotropin (IU) | 1312 | ||
| E2 level on trigger day (ng/L) | 658 | ||
| No. of follicles ≥ 18 mm on trigger day | 0 (one follicle≥ 14 mm) | ||
| Time interval between hCG administration and oocyte retrieval (h) | 36 | ||
| Ovulation triggering (dose) | 0.2 mg (GnRH agonist) | ||
| No. of oocytes retrieved | 1 (zona pellucida free) | ||
| Cycle 5 | Protocol | Luteal phase ovarian stimulation | |
| Total dose of gonadotropin (IU) | 1800 | ||
| E2 level on trigger day (ng/L) | 1083 | ||
| No. of follicles ≥ 18 mm on trigger day | 1 | ||
| Time interval between hCG administration and oocyte retrieval (h) | 36 | ||
| Ovulation triggering (dose) | 10,000 IU (hCG) + 0.2 mg (GnRH agonist) | ||
| No. of oocytes retrieved | 0 (without granulosa cells cumulus) | ||
| Serum hCG level (IU/L) on the day of OPU | 177.62 | ||
EFS empty follicle syndrome, FSH follicle stimulating hormone (normal range 3.85–8.78 IU/L), LH luteinizing hormone (normal range 2.12–10.89 IU/L), E2 estradiol (normal range 27–122 pg/mL), AMH anti-Mullerian hormone (normal range 0.24–11.78 ng/mL), OPU ovum pick up, NA no data available, GnRH agonist (Diphereline®, Ipsen, France), GnRH antagonist (Orgalutran®, Organon, Netherlands), hCG (Livzon, China)
Patient 2
The patient was a 28-year-old woman with a 4-year history of primary infertility with regular menses since menarche at the age of 13. Her basic FSH, LH, E2, and AMH levels were normal (Table 1). An ultrasound scan showed a normal uterus and ovaries with a total of 12 antral follicles (2 to 5 mm in diameter). Ultrasound scans during her natural menstrual cycles showed that a leading follicle developed to 20 mm in diameter and then ovulated for 1 cycle. Hysterosalpingography revealed a partial bilateral obstruction of her fallopian tubes. Chromosomal analysis showed the patient to be 46, XX. Her husband was 34 years old, and his anamnesis, physical examination, ultrasound, and semen analysis were normal. His chromosome karyotype was 46, XY. Subsequently, the patient underwent controlled ovarian hyperstimulation and 2 cycles of IVF at our hospital, as shown in Table 1. The patient was the child of a nonconsanguineous marriage, and her sister, who had offspring, was unaffected (Fig. 1a).
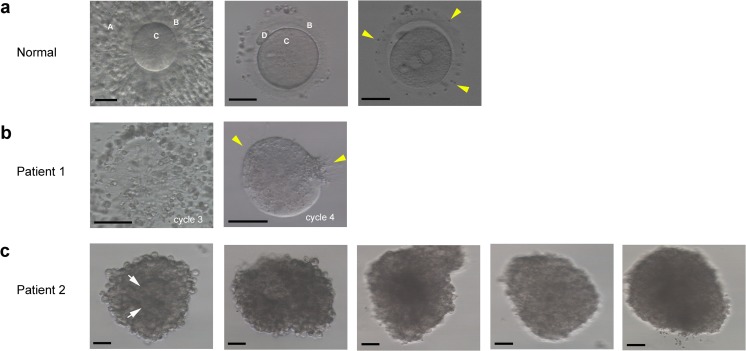
Fig. 1.
Oocyte characteristics examined by light microscopy in both of the patients. a Phenotypes of oocytes from a normal individual. Left panel: a cumulus-oocyte complex (COC) on day 0. Middle panel: a metaphase II oocyte on day 0. Right panel: a fertilized oocyte with two pronuclei on day 1. “A” indicates granulosa cells, “B” indicates zona pellucida, “C” indicates oocyte, and “D” indicates polar body. The yellow arrowheads indicate adhesive sperm. Scale bars: 50 μm. b Phenotypes of oocytes from patient 1 on day 1. Left panel: a COC without oocyte and zona pellucida in the first cycle. Right panel: an oocyte without zona pellucida but with adhesive sperm (yellow arrowheads). Scale bars: 50 μm. c Phenotypes of oocytes from patient 2 on day 1. Five oocytes were closely surrounded by granulosa cells, and an oocyte structure (white arrow) might be seen in COCs. Scale bars 50 μm
A control population of 200 unrelated Chinese fertile female volunteers was recruited. Written informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of our hospital.
Mutation detection and bioinformatics analysis
Mutation detection in the LHCGR gene and ZP-related genes (ZP1-ZP4) was performed on both of the patients by Sanger sequencing of polymerase chain reaction (PCR) products of all exons and flanking intronic regions using specific primers [4] (Supplementary file). The mutation was named according to the Human Genome Variation Society (HGVS) standards (http://www.hgvs.org/mutnomen/) with + 1 corresponding to the A of the ATG translation initiation codon in the GenBank cDNA sequence (LHCGR for NM_000233, ZP1 for NM_207341, ZP2 for NM_003460, ZP3 for NM_001110354, and ZP4 for NM_021186). Similar to other studies [9–12], we analyzed the first five exons of ZP3 to avoid false interpretation of the sequencing results due to a polymorphic locus, POM-ZP3 [13]. We also sequenced DNA from 200 control subjects.
CLUSTAL X (1.81) [14] was used to compare the human ZP1 amino acid sequence (Homo sapiens UniProt ID P60852) with those of five other species (Pan troglodytes, Macaca mulatta, Equus caballus, Oryctolagus cuniculus, and Mus musculus). The effects of the sequence variant were predicted using PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), Mutation Taster (http://www.mutationtaster.org/), and SIFT (http://sift.jcvi.org/). The database of the Exome Aggregation Consortium (ExAC) browser (http://exac.broadinstitute.org/) was used to determine the allele frequencies of the variant. Information on the motifs and domains of wild-type ZP1 protein was obtained from Interpro (http://www.ebi.ac.uk/interpro/) and the Human Protein Reference Database [15].
Results
Both patients had essentially normal ovarian reserve abilities, and their husbands were also normal. Owing to years of primary infertility, IVF was performed on these patients. Human chorionic gonadotropin (hCG) was administered 36 h before OPU was performed. In the first patient, no oocytes were obtained, and only a few cumulus granulosa cells were retrieved after 3 cycles under different simulation protocols; however, her serum hCG levels were normal on the day of OPU in 2 cycles (Table 1). With natural and mild protocols, 1 COC without an oocyte or ZP and 1 oocyte without ZP were retrieved. In the latter case, many sperm had adhered to the surface of the oocyte. However, no polar body or pronuclei were seen. After culturing to day 3, the oocyte did not cleave (Table 1) (Fig. 1b). In the second patient, no oocytes and only a few cumulus granulosa cells were retrieved in the first cycle with a normal hCG level on the day of OPU. However, in the second cycle, five oocytes were retrieved thorough repeated aspiration after we prolonged the interval between hCG stimulation and OPU to 41 h. However, the cumulus granulosa cells were extremely difficult to denude on day 1. Moreover, some oocyte structure was clearly seen in the COCs, and there was sperm adhesion around the granulosa cells (Fig. 1c). To avoid damaging the oocytes, we decided to allow the granulosa cells to detach naturally over time. However, up to day 3, some granulosa cells were still wrapped around the oocytes, which were degenerated (Table 1).
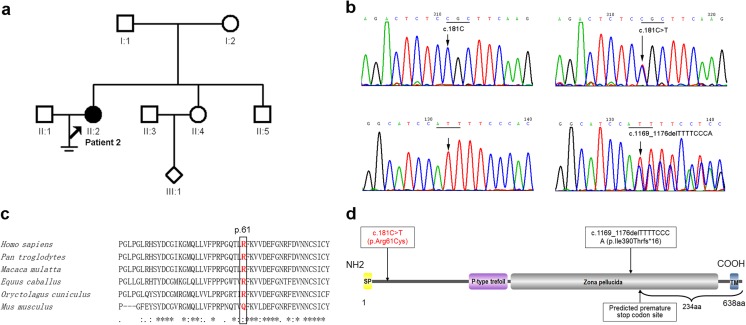
Direct sequencing of the LHCGR gene initially revealed no mutations in either of the patients. We further examined the ZP1~ZP4 genes in both of these patients. However, no mutations of ZP1~ZP4 were detected in patient 1. Sanger sequencing of four ZP-related genes in patient 2 revealed the compound heterozygous mutations of c.181C>T (p.Arg61Cys) and c.1169_1176delTTTTCCCA (p.Ile390Thrfs*16) in the ZP1 gene (Fig. 2a, b). The former, c.181C>T, is a novel missense mutation, but c.1169_1176delTTTTCCCA has been reported previously [8]. Sequencing of the parental DNA showed that the father was the carrier of c.1169_1176delTTTTCCCA (p.Ile390Thrfs*16) and the mother of c.181C>T (p.Arg61Cys), but genetic information of other family members is not available (Fig. 2a). Furthermore, we examined 200 healthy control subjects (400 alleles) and searched the ExAC database, and we found that this novel variant in ZP1 was absent from our cohort and the database, suggesting that it might not be a benign polymorphism. The computational programs SIFT, PolyPhen-2, and Mutation Taster predicted that the effect of this variant was deleterious and probably damaging and disease causing. Alignment of the ZP1 protein across six species showed that the arginine residue at position 61 was conserved among the majority of species, except for rodents such as mouse (Fig. 2c). The paternal mutation, c.1169_1176delTTTTCCCA (p.Ile390Thrfs*16), occurred in exon 7 and caused a frameshift in the reading frame that was predicted to introduce a premature stop codon at position 405, truncating the C-terminus of the ZP1 protein by 234 amino acids and probably resulting in the total loss of zona pellucida function, and the maternal mutation, c.181C>T (p.Arg61Cys), occurred in exon 1 near the signal peptide motif of the ZP1 protein (Fig. 1d).
Fig. 2.
Genetic and bioinformatic analysis of ZP1 mutations in the family of patient 2. a Pedigree of the family. The filled circle indicates the EFS patient 2 (II:2). The arrow indicates the proband. Open squares or circles indicate normal family members. The open rhombus indicates that II:4 was pregnant but with a baby of unknown gender. b Partial forward nucleotide sequences in the ZP1 gene. Upper panel: The arrow points to the wild-type c.181C in a control sample and the wild-type codon is underlined (left); the arrow points to the heterozygous c.181C>T (p.Arg61Cys) mutation in the patient (II:2), and the mutated codon is underlined (right). Bottom panel: The left arrow points to the wild-type in a control sample, and the right arrow points to the frameshift c.1169_1176delTTTTCCCA (p.Ile390Thrfs*16) mutation in the patient (II:2). c R61 was conserved among the majority of species, except for rodents such as the mouse. The arginine residue is at position 61 in Homo sapiens, Pan troglodytes, Macaca mulatta, Equus caballus, Oryctolagus cuniculus, and Mus musculus. d Schematic illustration of the motif and domains in ZP1. Wild-type ZP1 protein has 638 amino acids and contains a signal peptide motif (SP, yellow), a P-type trefoil domain (purple), a zona pellucida domain (gray), and a transmembrane domain (TM, blue). The arrows indicate the locations of the novel mutation (red) and a known frameshift mutation (black), the latter of which is predicted to delete 234 amino acids from the C-terminus of the ZP1 protein
Discussion
The etiology of EFS is complex. At present, the genetic factors associated with GEFS, including pericentric inversion of chromosome 2 and single gene defects (LHCGR and ZP3 genes), are involved [4, 5, 16]. In this study, we present two patients with similar clinical phenotypes: No oocytes and only a few granulosa cells could be retrieved during repeated IVF cycles from the women of childbearing age with normal follicular development, E2 levels, and bioavailable hCG plasma levels. Abnormal oocytes were obtained once or twice between multiple cycles. Furthermore, their condition was not due to poor ovarian response or failure of the hCG injection. Therefore, genetic factors were considered. To our knowledge, this is the first report to describe a GEFS patient with compound heterozygous mutations in the ZP1 gene. Including our study, a total of four cases with four different ZP1 mutations have been identified [8, 12]. In the previous studies, patients with ZP1 mutations displayed abnormal eggs that lacked ZP or degenerated or cracked oocytes [8, 12]. Our patient 2, who carries a novel mutation and a known mutation in ZP1 and exhibits GEFS manifestation, also has degenerated oocytes after prolonged OPU time in the second cycle. This finding demonstrates that ZP1 mutations could influence the formation and development of eggs. However, the clinical manifestations in patients are diverse and could be associated with different mutations. As in ZP3-related female infertility disorders, the ZP3 c.400G>A mutation was associated with EFS, and the c.1045_1046insT allele of ZP3 produced very thin ZP [5, 6].
During follicular development, ZP genes are expressed exclusively in growing and fully grown oocytes. The ZP matrix is crucial to oogenesis, fertilization, and early embryonic development in mammals [17]. In mice, the ZP matrix consists of three glycoproteins, namely, ZP1, ZP2, and ZP3. ZP1, which interconnects ZP2 and ZP3, and is considered an important component necessary for the integrity of the ZP structure. In Zp2-null or Zp3-null female mice, the oocytes completely lack ZP whose feature is like the human oocytes with no or thinner ZP due to digenic mutations of the ZP2 and ZP3 genes [6, 18, 19]. However, Zp1-null mice reveal diverse features of abnormal zonae pellucida associated with early embryonic loss or subfertility, especially ectopic localization of granulosa cells within the perivitelline space [20]. It has been proposed that the zona pellucida of mice and humans are similar in structure, although human zona pellucida is composed of four glycoproteins (ZP1, ZP2, ZP3, and ZP4) [21]. In this study, the same frameshift mutation in ZP1 was found in patient 2, who also carried another novel mutation (p.Arg61Cys). The positively charged arginine (R61) is near the N-terminal signal sequence of the ZP1 protein, which is composed of highly hydrophobic amino acids. We speculate that the substitution of the neutral residue C61 would affect the electrostatic distribution of the surrounding region and induce subtle conformational changes that would adversely affect the direct shuttling of nascent glycoproteins to the secretory pathway [22]. Different from the previous report of ZP1-null human patients with ZP-free [8], although we could not identify the ZP in patient 2 due to tight granulosa cells, we predict that the abnormal ZP (none or thin) would lead to a tighter gap junction between oocytes and granulosa cells and that the microvilli of the granulosa cells would stretch into the oocytes resulting in the cracking of the eggs during forceful denuding of granulosa cells [12, 23], as ectopic localization of granulosa cells within the perivitelline space extend in between them in Zp1-null mice [20].
In our study, mutations in ZP1 have an autosomal recessive inheritance pattern. Patient 2 carried the compound heterozygous mutations, whereas the fertile parents were heterozygous carriers of one or the other mutation. Although no genetic data are available for the fertile sister, it is reasonable to assume that it probably had no mutations or the same heterozygous mutation as her parents. However, patient 1 displayed GEFS and abnormal oocytes with no ZP, but no mutations were detected in LHCGR or ZP1~ZP4. In fact, mutations in ZP-related genes have been identified in approximately 9% of oocyte morphologic defects [12]. On the basis of our previous study and others’ descriptions of the clinical features with LHCGR-related and ZP-related GEFS [4, 5], the oocytes are usually not retrieved which does not indicate that there are not any oocytes in ovaries, whereas the pathologies display degenerated oocytes in human ovaries [5]. Therefore, we suggested that abnormal signaling pathway of LH/hCG receptor might result in strong adherence of cumulus granulosa cell complexes to the follicular wall, and abnormal zonae pellucida might cause ectopic localization of granulosa cells and a subsequent tighter gap junction between oocytes and granulosa cells. The in-depth study of pathological mechanisms and other genetic factors requires further investigation.
In conclusion, we report two EFS patients from China. The primary infertility patients exhibited typical GEFS manifestations and abnormal oocytes. One novel mutation and one known mutation were identified in the ZP1 gene. Our findings provide new evidence for the genetic basis of GEFS and support for the genetic diagnosis of infertile individuals with abnormal oocyte phenotypes.
Electronic supplementary material
(DOCX 23 kb)
Acknowledgments
The authors thank the patients and the control subjects for their participation in this study.
Funding information
This work was partially supported by the National Natural Science Foundation of China (No. 81801431).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval and consent to participate
The study protocol and all subjects who participated in this study were approved by the Institutional Review Board of our institute, and informed consent was obtained from all patients prior to their participation in accordance with institutional and national guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ping Yuan and Ruiqi Li contributed equally to this work.
References
- 1.Zreik TG, Garcia-Velasco JA, Vergara TM, Arici A, Olive D, Jones EF. Empty follicle syndrome: evidence for recurrence. Hum Reprod. 2000;15:999–1002. doi: 10.1093/humrep/15.5.999. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson TL, Lashen H. Empty follicle syndrome:the reality of a controversial syndrome, a systematic review. Fertil Steril. 2008;90:691–698. doi: 10.1016/j.fertnstert.2007.07.1312. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Fruchter R, Weiss A, Lavee M, Geslevich Y, Shalev E. Empty follicle syndrome: successful treatment in a recurrent case and review of the literature. Hum Reprod. 2012;27:1357–1367. doi: 10.1093/humrep/des037. [DOI] [PubMed] [Google Scholar]
- 4.Yuan P, He Z, Zheng L, Wang W, Li Y, Zhao H, Zhang VW, Zhang Q, Yang D. Genetic evidence of ‘genuine’ empty follicle syndrome: a novel effective mutation in the LHCGR gene and review of the literature. Hum Reprod. 2017;32:944–953. doi: 10.1093/humrep/dex015. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Bian Y, Liu X, Zhao S, Wu K, Yan L, Li M, Yang Z, Liu H, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet. 2017;101:459–465. doi: 10.1016/j.ajhg.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Li K, Bai D, Yin J, Tang Y, Chi F, Zhang L, Wang Y, Pan J, Liang S, Guo Y, Ruan J, Kou X, Zhao Y, Wang H, Chen J, Teng X, Gao S. Dosage effects of ZP2 and ZP3 heterozygous mutations cause human infertility. Hum Genet. 2017;136:975–985. doi: 10.1007/s00439-017-1822-7. [DOI] [PubMed] [Google Scholar]
- 7.Gook DA, Edgar DH, Borg J, Martic M. Detection of zona pellucida proteins during human folliculogenesis. Hum Reprod. 2008;23:394–402. doi: 10.1093/humrep/dem373. [DOI] [PubMed] [Google Scholar]
- 8.Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med. 2014;370:1220–1226. doi: 10.1056/NEJMoa1308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pökkylä RM, Lakkakorpi JT, Nuojua-Huttunen SH, Tapanainen JS. Sequence variations in human ZP genes as potential modifiers of zona pellucida architecture. Fertil Steril. 2011;95:2669–2672. doi: 10.1016/j.fertnstert.2011.01.168. [DOI] [PubMed] [Google Scholar]
- 10.Margalit M, Paz G, Yavetz H, Yogev L, Amit A, Hevlin-Schwartz T, Gupta SK, Kleiman SE. Genetic and physiological study of morphologically abnormal human zona pellucida. Eur J Obstet Gynecol Reprod Biol. 2012;165:70–76. doi: 10.1016/j.ejogrb.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Ferre M, Amati-Bonneau P, Moriniere C, Ferre-L’Hotellier V, Lemerle S, Przyrowski D, et al. Are zona pellucida genes involved in recurrent oocyte lysis observed during in vitro fertilization? J Assist Reprod Genet. 2014;31:221–227. doi: 10.1007/s10815-013-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P, Luan X, Peng Y, Chen T, Su S, Zhang C, Wang Z, Cheng L, Zhang X, Wang Y, Chen ZJ, Zhao H. Novel zona pellucida gene variants identified in patients with oocyte anomalies. Fertil Steril. 2017;107:1364–1369. doi: 10.1016/j.fertnstert.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Kipersztok S, Osawa GA, Liang L-F, Modi WS, Dean J. POM-ZP3, a bipartite transcript derived from human ZP3 and a POM121 homologue. Genomics. 1995;25:354–359. doi: 10.1016/0888-7543(95)80033-I. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshava Prasad TS, Goel R, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human protein reference database—2009 update. Nucleic Acids Res. 2009;37:D767–D772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vujisic S, Stipoljev F, Bauman R, Dmitrovic R, Jezek D. Pericentric inversion of chromosome 2 in a patient with the empty follicle syndrome: case report. Hum Reprod. 2005;20:2552–2555. doi: 10.1093/humrep/dei083. [DOI] [PubMed] [Google Scholar]
- 17.Wassarman PM, Litscher ES. Biogenesis of the mouse egg’s extracellular coat, the zona pellucida. Curr Top Dev Biol. 2013;102:243–266. doi: 10.1016/B978-0-12-416024-8.00009-X. [DOI] [PubMed] [Google Scholar]
- 18.Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J. Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development. 2001;128:1119–1126. doi: 10.1242/dev.128.7.1119. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Litscher ES, Mortillo S, Sakai Y, Kinloch RA, Stewart CL, Wassarman PM. Targeted disruption of the mZP3 gene results in production of eggs lacking a zona pellucida and infertility in female mice. Proc Natl Acad Sci U S A. 1996;93:5431–5436. doi: 10.1073/pnas.93.11.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin T, Talbot P, Lee E, Dean J. Abnormal zonae pellucidae in mice lacking ZP1 result in early embryonic loss. Development. 1999;126:3847–3855. doi: 10.1242/dev.126.17.3847. [DOI] [PubMed] [Google Scholar]
- 21.Lefièvre L, Conner SJ, Salpekar A, Olufowobi O, Ashton P, Pavlovic B, Lenton W, Afnan M, Brewis IA, Monk M, Hughes DC, Barratt CL. Four zona pellucida glycoproteins are expressed in the human. Hum Reprod. 2004;19:1580–1586. doi: 10.1093/humrep/deh301. [DOI] [PubMed] [Google Scholar]
- 22.Wassarman PM, Litscher ES. Influence of the zona pellucida of the mouse egg on folliculogenesis and fertility. Int J Dev Biol. 2012;56:833–839. doi: 10.1387/ijdb.120136pw. [DOI] [PubMed] [Google Scholar]
- 23.Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–686. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 23 kb)