Abstract
In this study, we introduce a simple and green method for synthesis of gold nanoparticles (AuNPs) using microbial glycolipid mannosylerythritol lipid (MEL) produced from Ustilago maydis CGMCC 5.203 and to evaluate their biomedical activities. MEL was found 10.3 g/L using sunflower oil. The formation of MEL-AuNPs was verified using UV–visible spectrum, XRD, TEM, FTIR, SEM, and EDX. In the biomedical examinations, MEL-AuNPs demonstrated potential cytotoxicity against HepG2 cells, and IC50 values were found to be 100 and 75 µg/mL for 24 h and 48 h of exposure, respectively, which indicates its good performance against cancer cells. The IC50 value of MEL-AuNPs was found to be 115 and 124 µg/mL for DPPH and ABTS scavenging activities, respectively. The biosynthesized MEL-AuNPs significantly inhibited cell growth of pathogenic Gram-positive and Gram-negative bacteria. These findings indicated that MEL plays a crucial role in the rapid biofabrication method of metallic NPs possessed the potential of biomedical activities.
Keywords: Mannosylerythritol lipids, Gold nanoparticles, Anticancer, Antioxidant activity, Antibacterial activity
Introduction
In recent years, gold nanoparticles (AuNPs) have gained considerable attention due to their wide range of potential applications including anticancer (Rajeshkumar 2016) antimicrobial (Dorosti and Jamshidi 2016), antioxidants (Muthuvel et al. 2014), and agriculture (Mahakham et al. 2016). Besides, they have widely used in applications of the biolabelling, photothermal therapy, tissue/tumor imaging, biosensors, drug delivery and detection of the pathogens (Ganesh Kumar et al. 2011). Nowadays, considerable attention has been paid to the green synthesis of nanoparticles through biosurfactants and biological substances, because of hazardous and toxic by-products are usually paired with chemical methods (Kasture et al. 2008; Kumar et al. 2010). As evident from earlier studies, biosurfactants can act as both reducing and stabilizing agents in the synthesis of NPs, such as lipopeptide biosurfactant, sophorolipids, and rhamnolipid (Kasture et al. 2008; Reddy et al. 2009; Priyadarshini et al. 2016). Synthesis of nanoparticles by biosurfactant is superior to the biological methods, due to the (Kiran et al. 2011). Previous researchers indicated that surfactants mediated nanoparticles might improve antibacterial activities, such as SDS, PVP 360, Tween 80 and CTAB have significantly improved the antibacterial activity for most bacteria species (Kvitek et al. 2008; Alkilany et al. 2009).
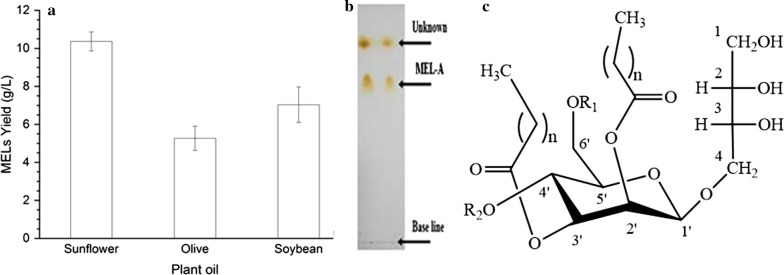
Mannosylerythritol lipids (MELs, Fig. 1c) are microbial glycolipid biosurfactants comprised of 4-O-β-d-mannopyranosyl-d-erythritol and a fatty acid or an acetyl group acting as hydrophilic and hydrophilic moieties, respectively Kitamoto (2008). MEL has been exploited in many fields such as potent antimicrobial activity and interfacial properties (Morita et al. 2006). As well, MEL shows numerous bioactivities, such as, induce cells of apoptosis and differentiation of mouse melanoma, rat pheochromocytoma and extensively applied in food, pharmaceutical, cosmetic and environmental protection (Zhao et al. 1999; Wakamatsu et al. 2001; Fan et al. 2016).
Fig. 1.
a Comparison of crude MEL production from different Carbone source; b analysis of MEL production by TLC; c structure of mannosylerythritol lipid (MEL)
The present work aimed to produce MEL from Ustilago maydis CGMCC 5.203 by resting cell fermentation under the limited nitrogen using different carbon sources. A further aim was to develop highly stabilized AuNPs using the produced MEL via one-step technique and verified by different techniques such as UV–vis absorption spectrum, TEM, SEM, EDX, FT-IR, and XRD (X-ray diffraction). Finally, the potential bioactivities of MEL-AuNPs in antibacterial, antioxidant, and cancer cell cytotoxicity were evaluated.
Materials and methods
Materials
All media components and chemicals were purchased from Sangon Biotech (Shanghai) Co., Ltd. (China). Other reagents and solvents used in this work were used as received. All reagents used were of the highest purity available.
Microorganisms
Ustilago maydis CGMCC 5.203 was obtained from China General Microbiological Culture Collection Center. Moreover, this fungus was growing at 28 °C in liquid YEPS (1% yeast extract, 2% peptone, 2% sucrose). U. maydis CGMCC 5.203 was preserved in 50% glycerol (v/v) at − 80 °C. Staphylococcus aureus CCTCC AB91093 and Escherichia coli CCTCC AB91112 were obtained from China Center for Type Culture Collection (China, Wuhan).
MEL production by resting cells cultivation
Seed cultures were prepared by inoculating cells previously grown on a YEPS, into 250 ml Erlenmeyer flasks containing 50 mL of a growth medium [0.1% (w/v) KH2PO4, 0.02% (w/v) citric acid, 0.04% (w/v) MgSO4·7H2O, 0.003% (w/v) FeSO4·7H2O, 0.06% (v/v) corn-steep liquor, 0.06% (w/v) urea, 5% (w/v) glucose and distilled water]. Then the cultures were incubated at 28 °C on a rotary shaker (120 rpm) for 3 days. After it, the cells were harvested by centrifugation at 3500 g for 15 min and washed twice with 0.9% NaCl solution under sterile conditions. The obtained cells were transferred into 250 mL Erlenmeyer flasks containing 50 mL of a fermentation medium, containing the same composition as the main culture except for glucose which was replaced by 1.0% (v/v) plant oil (sunflower, olive, and soybean oil). After that, the culture flasks incubated on a rotary shaker (120 rpm) at 28 °C for 8 days, unless otherwise indicated.
Extraction and purification of MELs
At the end of fermentation, the culture broth containing MELs was extracted with an equal amount of ethyl acetate and centrifuged for 15 min at 4000 rpm. The organic layer was separated, evaporated, and crude mixtures of MELs were washed with methanol and cyclohexane to remove the residual oil. The crude MEL extracted was diluted with chloroform and then purified by silica-gel column chromatography using chloroform–acetone (Onghena et al. 2011). The purified MEL was confirmed by using thin layer chromatography (TLC) (Silica gel 60 F, chloroform: methanol: water = 65:15:2, v/v) as the solvent system. The FTIR spectrometer (Vector 22, Bruker, Germany) was used to verify various functional groups existed in MEL.
Biosynthesis of gold nanoparticles
In the present study, mannosylerythritol lipid (MEL) was utilized as both the reducing and capping agents in the synthesis of gold nanoparticles (AuNPs). Briefly, 200 μL of MEL in 1 mL of methanol diluted to 10 mL of distilled water then added to 50 mL an aqueous solution of 3 mM HAuCl4 under magnetic stirring at 60 °C for 30 min. The pH value of the mixture was adjusted to 8 using 0.1 M of KOH. The change of color to purple visually observed the formation of AuNPs.
Characterization of the biosynthesized MEL-AuNPs
The bioreduction of the gold ion by MEL was verified by UV–Vis spectrophotometer (UV-2550 Shimadzu, Japan) at a resolution of 1 nm in the wavelength of 300–800 nm. For further characterization, an aqueous solution of MEL-AuNPs was centrifuged at 14,000 rpm for 20 min. Repeated rinses were achieved to remove impurities and were lyophilized. The FTIR spectrum analysis was collected at the resolution of 4 cm−1 in the range of 500–4000 cm−1 region using Fourier transform infrared spectrometer model (Vector 22, Bruker, Germany). The crystalline nature of MEL-AuNPs was evaluated via Siemens X-ray diffractometer (XRD) analysis, where XRD patterns were measured by drop coated film of dried powder of MEL-AuNPs onto glass slides. The operation conditions were at a voltage of 45 keV and a current of 20 mA with Cu-Ka radiation as an X-ray source in the range of 20–80 at the 2-theta angle. The structural characterization of MEL-AuNPs was carried out by transmission electron microscopy (TEM) (JEM-1230, JEOL, Akishima, Japan). The extra sample was removed from the carbon-coated copper grid using the cone of a blotting paper and samples were placed on the carbon-coated copper grid to make a thin film of the sample, and then it was reserved in a grid box sequentially. Scanning Electron Microscopic (SEM) (TM-1000, Hitachi, Japan) analyzed the size of the synthesized AuNPs. Thin film samples were prepared on a carbon-coated copper grid by dropping the sample on the grid, and an excess solution was removed via a blotting paper. Then, the film on the SEM grid was allowed to dry by putting the grid under a mercury lamp for 5 min. The device was supplemented with an energy-dispersive X-ray spectrum (EDX) to verify the existence of AuNPs.
In vitro assessment of biological activities of MEL-AuNPs
Antibacterial activity
The antibacterial activity of newly synthesized MEL-AuNPs was evaluated against Gram-positive S. aureus CCTCC AB91093 and Gram-negative E. coli CCTCC AB91112 by well diffusion technique (Srinivasan et al. 2001). Briefly, bacterial suspensions at concentrations (approximately 5 × 105 CFU/mL) were uniformly spread on Mueller–Hinton Agar (MHA) plates. Three wells about 6 mm diameter were created in each of these plates using sterile borer. 50 µL of the biosynthesized MEL-AuNPs solution were added at various concentrations (25, 50 and 100 µg/mL) to the wells at aseptic conditions, and the tested plates were incubated at 37 ± 2 °C for 24 h. After it, the diameter of an inhibitory zone was measured. This study was carried out in triplicates.
Anticancer activity against HepG2 cell line
The cytotoxicity potential of MEL-AuNPs was investigated by MTT assay using human liver hepatoma HepG2 cells. The cells were procured from Shanghai Cell Bank of China and maintained in DMEM (Dulbecco’s Modification Eagle Medium) containing 10% fetal bovine serum (FBS) and 1% antibiotics solution (105 U/L penicillin and 100 mg/L streptomycin) in a humidified incubator with 5% CO2 at 37 °C.
The HepG2 cells were placed into 96-well plates at a density of 1.25 × 105 cells/well and incubated for 24 h. Afterwards, the cells were treated with different concentrations of MEL-AgNPs (10–150 µg/mL) and MEL, the proliferation activity of the cells was determined by adding 5.0 mg/mL MTT reagent after 24 h and 48 h of incubation. Finally, the absorbance was scanned at 570 nm in a microtitre plate reader (Thermo Electron Corp, Asheville, NC). The viability of HepG2 cells was expressed as a percentage of the control culture value, which was considered to be 100% viable.
Antioxidant capacity
DPPH and ABTS examinations were exploited to evaluate the total antioxidant capacity of the nanoparticle. The DPPH radical scavenging activity of MEL-AuNPs samples was determined using DPPH assay method described by Saratale et al. (2017). About 0.2 mL of different concentrations of MEL-AuNPs were mixed with 2 mL of DPPH solution. The mixture was vortexed and kept at 24 °C for half an hour in the dark. The absorbance of the reaction mixture was read spectrophotometrically at 517 nm using BHT as a standard.
For ABTS scavenging activity of MEL-AuNPs was evaluated following the method described by (Moldovan et al. 2016) with some modifications. The ABTS radical cation was produced by mixing an ABTS stock solution (7.0 µM) and potassium persulphate (2.45 µM) and allowing the mixture to react in the dark at ambient temperature for 16 h. The ABTS.+ solution was diluted with ethanol 80% (v/v) to an absorbance of 0.700 ± (0.05) at 734 nm on the microplate reader (Thermo Electron Corp, Asheville, NC). About 200 µL of the ABTS.+ solution was mixed with 20 µL of the MEL and MEL-AuNPs solution at different concentrations. After 6 min in the dark, the absorbance at 734 nm was measured using ascorbic acid as a standard. The absorbance of a blank solution (without samples) was also measured. The radical scavenging activity was expressed as the IC50 values. The Free radical scavenging percentages of DPPH and ABTS+ radicals by MEL-AuNPs were calculated according to the following formula:
where AC is the absorbance control of DPPH and ABTS in two different assays, while AT is the absorbance of DPPH and ABTS free radical in the existence of Au nanoparticles at 517 nm and 734 nm, respectively.
Statistical analysis
The data were expressed as the standard deviation (mean ± SD) obtained from at least three independent experiments. The statistical analysis was evaluated by one-way ANOVA test (SPSS 16) followed by Tukey’s HSD test (p < 0.05).
Results
Production and characterization of MELs
Figure 1a shows the production of MELs from different carbon sources by the resting cells biotransformation of Ustilago maydis CGMCC 5.203 under growth-limiting nitrogen conditions. After fermentation, extracted and purified by silica-gel column chromatography, then it was confirmed by using thin layer chromatography (TLC) (Fig. 1b). In this work, the total yield of MELs from sunflower, olive, and soybean oil were 10.36, 5.26, and 7.03 g/L, respectively (Fig. 1a).
Visual observations and UV–vis absorption spectra (UV–vis) studies
The reduction of HAuCl4 to AuNPs was successfully achieved after optimization of the experimental parameters, by the aqueous solution of 3 mM HAuCl4 and aqueous MEL solution, which employed as both the reducing and capping agents. The change of color to purple was visually observed. This step was followed by UV–vis absorption spectroscopy as shown in Fig. 2. The strong surface plasmon resonance (SPR) was located at 566 nm.
Fig. 2.
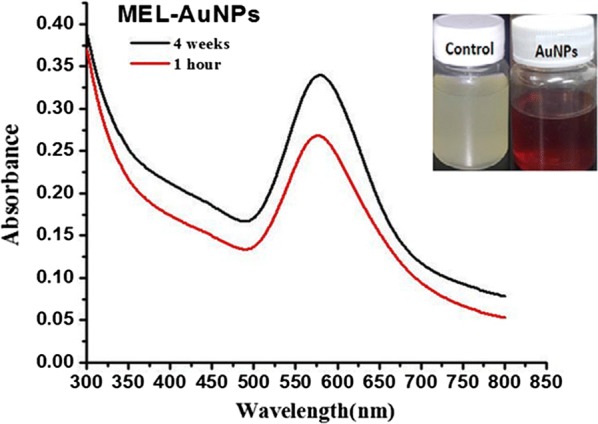
UV–Vis spectra of biosynthesized MEL-AuNPs using MEL
Morphological characterization of biosynthesized MEL-AuNPs
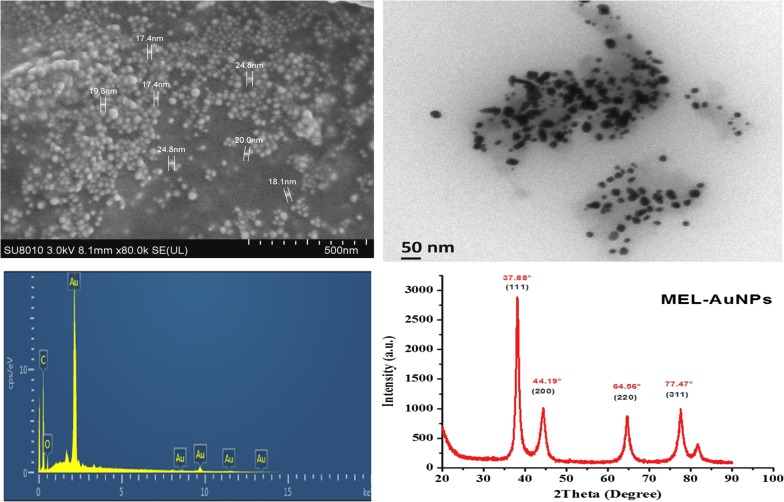
SEM image of the biosynthesized MEL-AuNPs exhibited a spherical shape as shown in Fig. 3a. Besides, the EDX Spectrum analysis has demonstrated the absorption of strong Au signal (Fig. 3b). The absorption peak of Au was observed at 2.2 keV, which is typical of the crystalline nature of the AuNPs. Other signals corresponding to C, O have also appeared. Furthermore, TEM image of MEL-AuNPs (Fig. 3c) confirmed that the sample is mainly comprised of spherical and uniform in shape. Moreover, the X-ray diffraction patterns of the biosynthesized MEL-AuNPs are shown in Fig. 3d. The diffraction peaks of MEL-AuNPs are centered at 2θ = 37.88°, 44.19°, 64.56°, and 77.47° corresponding to the planes of (111), (200), (220) and (311), respectively.
Fig. 3.
Characterization of biosynthesized AuNPs using MEL a SEM image; b TEM image; c EDX pattern; and d XRD pattern
Fourier transformed infrared spectroscopy (FTIR)
The FTIR spectra illustrated the possible interactions between the MEL and AuNPs (Fig. 4). The FTIR spectrum of MEL displayed transmittance peaks at 3406, 2926, 2854, 1743, 1464, 1377 and 1171 cm−1 corresponding to (O–H), (C–H), (C=O), (C–H) and (C–O), respectively that implying the complex nature of MEL, Fukuoka et al. reported similar findings (Fukuoka et al. 2007). The broadband at 3275 cm−1 attributed to O–H stretching vibrations. The distinct absorption bands at 1395 cm−1 and 2921 cm−1 demonstrated the existence of a long fatty acid chain. The strong and broad bands at 2921–2854 cm−1 and 1395 cm−1 denote the presence of C–H stretching, matching to CH2 and CH3 groups of aliphatic chains. The absorbance bands at 1635 cm−1 and 1179 cm−1 indicate the presence of fatty acids.
Fig. 4.
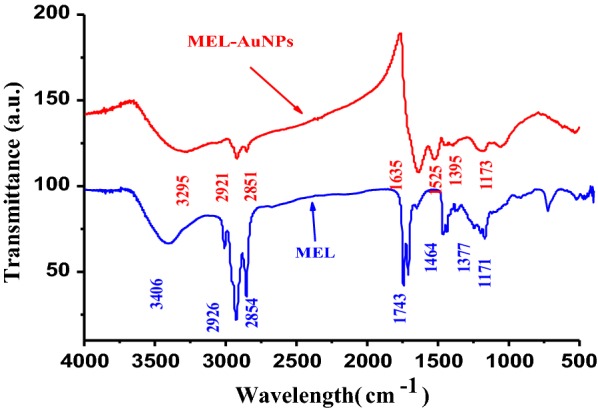
FTIR spectrum of MEL-AuNPs and MEL
Antibacterial activity of biosynthesized MEL-AuNPs
Figure 5 shows the antibacterial potential of freshly biosynthesized MEL-AuNPs against Gram-positive and Gram-negative bacteria at the concentration of 25, 50 and 100 µg/mL using well diffusion method, and results are presented in Table 1. MEL-AuNPs displayed a noticeable antibacterial effect against E. coli and S. aureus at the minimum concentration. The average inhibition zone of MEL-AuNPs against bacteria cells ranged from 12 to 17.6 mm.
Fig. 5.
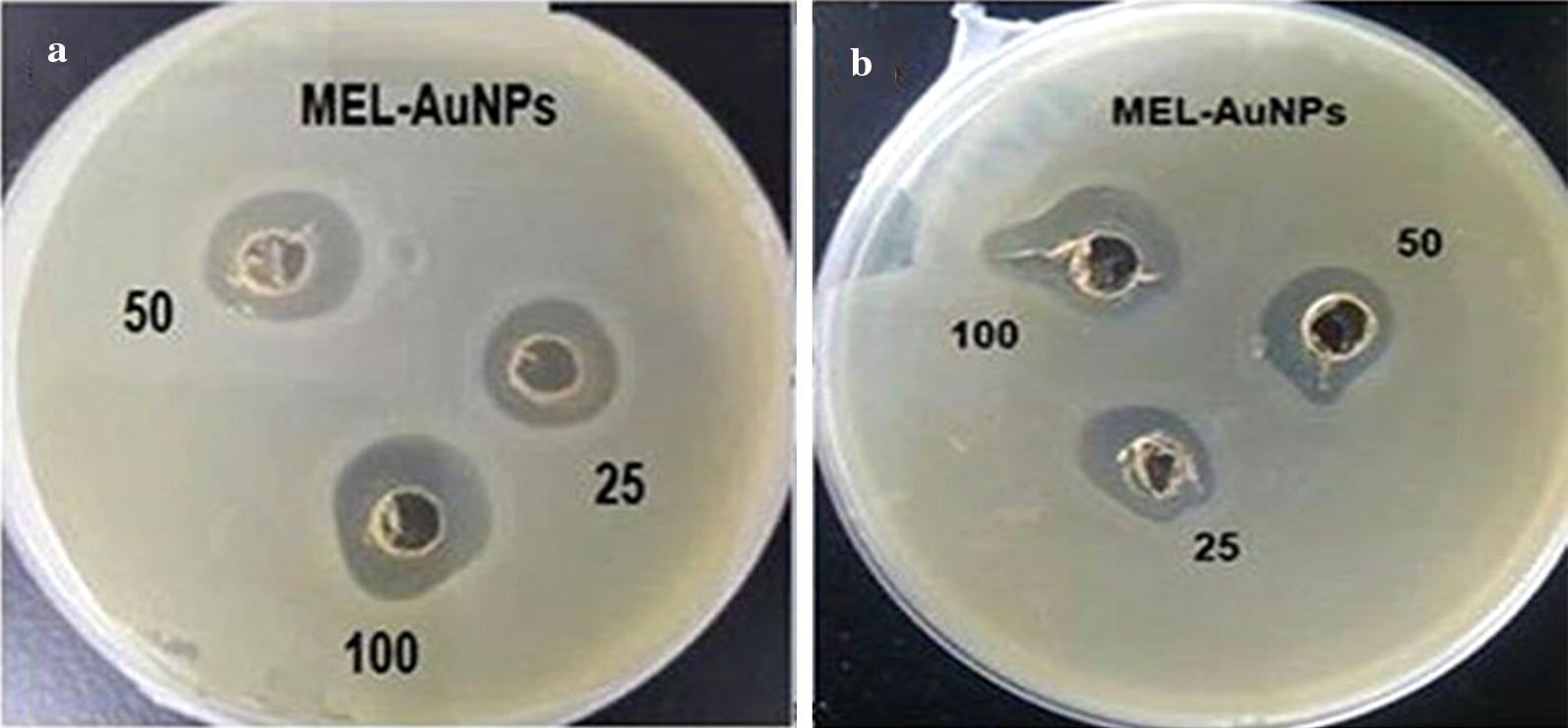
Antibacterial activity of biosynthesized MEL-AuNPs against a Escherichia coli and b Staphylococcus aureus
Table 1.
Antibacterial activity of biosynthesized MEL-AuNPs
| Pathogenic bacteria | Inhibition zone (mm) for various concentrations of MEL-AuNPs | ||
|---|---|---|---|
| 25 µg/mL | 50 µg/mL | 100 µg/mL | |
| E. coli | 12.5b ± 0.10 | 16.3a ± 0.11 | 17.6a ± 0.15 |
| S. aureus | 12c ± 0.05 | 14.5b ± 0.05 | 17a ± 0.18 |
Different superscript letters in each row indicate significant differences
Anticancer activity of MEL-AuNPs against the HepG2 cell line
The potential cytotoxicity of MEL-AuNPs against human liver cancer cells (HepG2) was examined by the MTT assay using different concentrations (10, 25, 50, 75, 100, 125 and 150 µg/mL) for 24 h and 48 h exposure as shown in Fig. 6. The HepG2 cell population was gradually decreased with an increased MEL-AuNPs concentration and treating time. Considerably, MEL-AuNPs demonstrated potential cytotoxicity on HepG2 cells, the IC50 value were 75 and 100 µg/mL for 24 h and 48 h, respectively. The maximum concentration of MEL-AuNPs has inhibited cell growth about 89%.
Fig. 6.
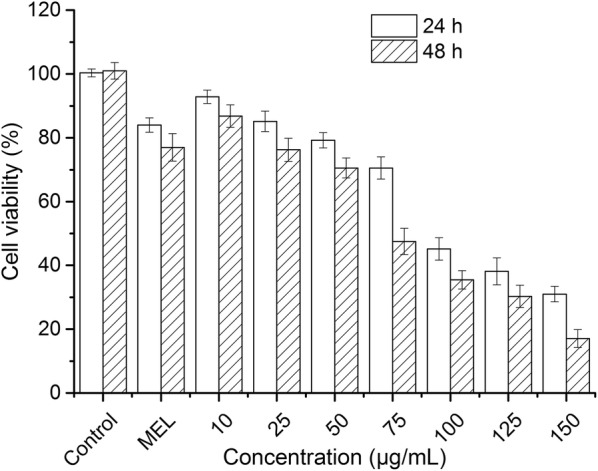
Anticancer activity of the biosynthesized MEL-AuNPs against HepG2 cells after 24 h and 48 h. Each result represented the mean ± standard deviation (SD) and performed in triplicate
Antioxidant capacity of MEL-AuNPs
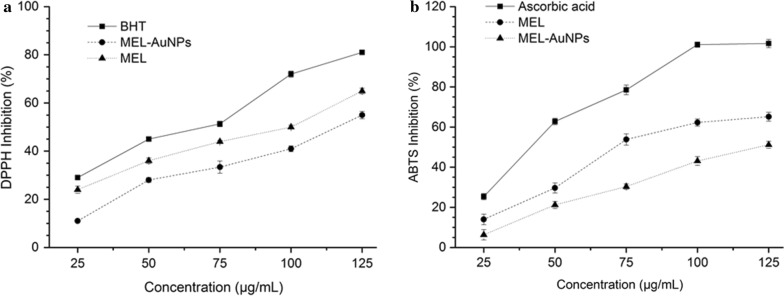
The antioxidant activity of MEL-AuNPs was evaluated against DPPH and ABTS radicals at various concentrations ranging from 25 to 125 µg/mL. Figure 7a revealed that the DPPH radical scavenging activity was increased with increasing in concentration and showing the highest inhibition of 81%, 65%, and 55% at a maximum concentration corresponding to BHT, MEL, and MEL-AuNPs, respectively. As seen in Fig. 7a, the IC50 value of MEL-AuNPs for DPPH activity was found to be 115 µg/mL. The antioxidant capacity of MEL-AuNPs was further investigated by ABTS scavenging using ascorbic acid as a positive control. The MEL-AuNPs showed a dose-dependent activity, and the ABTS scavenging percentages were increased from 6.33% to 51.16%, with an IC50 value of about 124 µg/mL (Fig. 7b). We also observed that MEL exhibits superior antioxidant activity than MEL-AuNPs in both assays.
Fig. 7.
Antioxidant potential of the biosynthesized MEL-AuNPs by use of DPPH and ABTS scavenging evaluation
Discussion
In this study, three types of vegetable oils were used as carbon sources for the production of MEL under growth-limiting nitrogen conditions, using Ustilago maydis CGMCC 5.203. The sunflower oil gave a higher yield of MEL than olive and soybean oil. Morita et al. have obtained 2.62 g/L MELs by U. maydis NBRC5346 from olive oil (Morita et al. 2009). The present data demonstrated that sunflower oil is the beneficial substrate for MEL formation by U. maydis CGMCC 5.203.
Recently, biosurfactants have received growing interest in the area of nanotechnology, owing to their electrostatic force that could uniform the size and shape of particles as well as prevent the aggregation (Kitamoto et al. 2009; Kiran et al. 2011). In the current work, the reduction of HAuCl4 to AuNPs was successfully achieved by the aqueous solution of 3 mM HAuCl4 and MEL was employed as both the reducing and capping agents in alkaline medium. The strong surface plasmon resonance (SPR) was located at 566 nm, suggesting the fabrication of stable AuNPs by MEL. Typical AuNPs show peaks at wavelengths between 540 to 570 nm (Das et al. 2014; Gómez-Graña et al. 2017; Ramkumar et al. 2017; Singh et al. 2017). There is no noticeable change at the peak after 30 days; we deduced that MEL was acting as an excellent stabilizing agent and hinder the aggregation.
The SEM image shows surface morphology of the biosynthesized MEL-AuNPs in a spherical shape and mostly uniform that may be due to the electrostatic force of biosurfactant MEL. Besides, the TEM image has also confirmed the MEL-AuNPs are mainly composed of spherical and uniform in shape. Furthermore, the EDX Spectrum analysis of MEL-AuNPs proved the existence of Au in the sample, which indicates that Au is the dominant element and confirmed that MELs are capable of synthesizing AuNPs. Comparable peak has been previously reported by (Wang et al. 2016). The crystallinity of MEL-AuNPs was verified by X-ray diffraction technique. The lattice planes of the face-centered cubic (fcc) structure demonstrated that MEL-AuNPs were crystalline. The findings are in accordance with a recent study (Chahardoli et al. 2017).
FTIR spectroscopy was utilized to explore the role of biofunctional groups of MEL that are responsible for the formation of AuNPs. FTIR spectra revealed the possible interactions between the MEL and AuNPs. The bands observed at 3406, 2926–2854, 1743, 1464–1377 and 1171 cm−1 corresponding to (O–H), (C–H), (C=O), (C–H) and (C–O), respectively, which are emphasized the structure of MEL, Fukuoka et al. reported similar findings (Fukuoka et al. 2007). The broadband at 3275 cm−1 attributed to O–H stretching vibrations. The distinct absorption bands at 1395 cm−1 and 2921 cm−1 demonstrated the existence of a long fatty acid chain. The strong and broad bands at 2921–2854 cm−1 and 1395 cm−1 denote the presence of C–H stretching, matching to CH2 and CH3 groups of aliphatic chains. The absorbance bands at 1635 cm−1 and 1179 cm−1 indicate the presence of fatty acids. From the results as mentioned above, the existence of fatty acid chain and carbohydrates functional groups represented that it is a MEL composition as well as confirms the interaction between MEL and AuNPs (Gómez-Graña et al. 2017).
In this study, the potential antibacterial activity of MEL-AuNPs displayed a noticeable antibacterial effect against E. coli and S. aureus at the minimum concentration. Similar results were recently reported by the previous literature (Muthuvel et al. 2014). Previous researchers indicated that the antibacterial activity of nanoparticles facilitated by surfactants, like SDS, Tween 80, PVP 360 and CTAB have significantly improved the antibacterial activity for most species of the bacteria (Kvitek et al. 2008; Alkilany et al. 2009). The supposed mechanism of the antibacterial action by AuNPs is mostly due to; the positive charge of Au+ ions linked with the negatively charged bacterial cell wall, inhibits the cellular enzymes and causes disorder in membrane permeability. Additionally, it damages the protein and DNA by releasing reactive oxygen species and thereby leading to cell death (Lee et al. 2003; Sondi and Salopek-Sondi 2004). Besides, nanoparticles with MEL (IL-CS-Nano-MEL) exhibited stronger antibacterial effects against S. aureus than IL-CS-Nano, suggesting the antibacterial effects of MEL (Wu et al. 2018).
Moreover, the potential cytotoxicity of MEL-AuNPs on human liver cancer cells (HepG2) was examined by the MTT assay using different concentrations for 24 h and 48 h exposure as shown in Fig. 6. Our findings exhibited a significant inhibitory effect at the low concentration of MEL-AuNPs in comparison with that Muthukumar et al. (2016) has reported an IC50 value of 150 µg/mL against HepG2 cells for AuNPs prepared by Catharanthus roseus leaf extract. According to the several reports, The cytotoxicity of AuNPs was ascribed to the interactions between cells and NPs, which relied on physiochemical properties of NPs such as size and shape of particles, surface charge, the nature of cells, the NPs concentration and treating time (Lee et al. 2015; Muthukumar et al. 2016; Chahardoli et al. 2017).
Finally, the antioxidant activity of MEL-AuNPs was evaluated against DPPH and ABTS radicals at various concentrations. Figure 7a, b revealed that the DPPH and ABTS radical scavenging activity was increased with increase in concentration. The IC50 value of MEL-AuNPs for DPPH and ABTS activity was found to be 115 µg/mL and 124 µg/mL, respectively. The results thus obtained are compatible with that Muthuvel et al. (2014) have got a similar effect on DPPH activity by AuNPs synthesized using Solanum nigrum leaf extract. We also observed that MEL exhibits superior antioxidant activity than MEL-AuNPs in both assays. This results might be possible due to that the unsaturated fatty acids in MEL lead to increase the antioxidant activity by working as donors of hydrogen atoms (Takahashi et al. 2012).
In summary, a promising mannosylerythritol lipids (MELs) produced from U. maydis CGMCC 5.203 was utilized as green reducing/stabilizing agents to synthesize AuNPs. Based on the results, we concluded that the biosynthesized MEL-AuNPs had good physical characteristic and proved potential bioactivities such as antibacterial activity against pathogenic bacteria, antioxidant, and toxicity against HepG2 cells. The present data can be used for diagnosis and cancer therapy as drug delivery as well as an antibiotic compound.
Authors’ contributions
AB designed and executed the experiments, collected and analyzed all data, wrote the manuscript. QHC was responsible for initiation and supervision of the study. YWN, HK participated in the provided critical review of the manuscript. HK and YWN provided technical support. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the conclusions are presented in the main article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This article does not contain any studies with human participants performed by any of the authors.
Funding
This study was financially supported by Public Projects of Zhejiang Province (LGF18C200003) and Nature Science Foundation of Zhejiang Province (LR13C200002), China.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelmoneim Bakur, Email: bakur888@yahoo.com.
Yongwu Niu, Email: 11713002@zju.edu.cn.
Hui Kuang, Email: 11813032@zju.edu.cn.
Qihe Chen, Email: chenqh@zju.edu.cn.
References
- Alkilany AM, Nagaria PK, Hexel CR, Shaw TJ, Murphy CJ, Wyatt MD. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small. 2009;5(6):701–708. doi: 10.1002/smll.200801546. [DOI] [PubMed] [Google Scholar]
- Chahardoli A, Karimi N, Sadeghi F, Fattahi A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif Cell Nanomed B. 2017;46:1–10. doi: 10.1080/21691401.2017.1332634. [DOI] [PubMed] [Google Scholar]
- Das A, Chadha R, Maiti N, Kapoor S. Role of surfactant in the formation of gold nanoparticles in aqueous medium. J Nanop. 2014;2014:9297. [Google Scholar]
- Dorosti N, Jamshidi F. Plant-mediated gold nanoparticles by Dracocephalum kotschyi as anticholinesterase agent: synthesis, characterization, and evaluation of anticancer and antibacterial activity. J Appl Biomedicine. 2016;14(3):235–245. doi: 10.1016/j.jab.2016.03.001. [DOI] [Google Scholar]
- Fan L, Li H, Niu Y, Chen Q. Characterization and inducing melanoma cell apoptosis activity of mannosylerythritol lipids-A produced from Pseudozyma aphidis. PLoS ONE. 2016;11(2):e0148198. doi: 10.1371/journal.pone.0148198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Morita T, Konishi M, Imura T, Kitamoto D. Characterization of new types of mannosylerythritol lipids as biosurfactants produced from soybean oil by a basidiomycetous yeast, Pseudozyma shanxiensis. J Oleo Sci. 2007;56(8):435–442. doi: 10.5650/jos.56.435. [DOI] [PubMed] [Google Scholar]
- Ganesh Kumar V, Dinesh Gokavarapu S, Rajeswari A, Stalin Dhas T, Karthick V, Kapadia Z, Shrestha T, Barathy IA, Roy A, Sinha S. Facile green synthesis of gold nanoparticles using leaf extract of antidiabetic potent Cassia auriculata. Colloid Surface B. 2011;87(1):159–163. doi: 10.1016/j.colsurfb.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Gómez-Graña S, Perez-Ameneiro M, Vecino X, Pastoriza-Santos I, Perez-Juste J, Cruz JM, Moldes AB. Biogenic synthesis of metal nanoparticles using a biosurfactant extracted from corn and their antimicrobial properties. Nanomaterials. 2017;7(6):139. doi: 10.3390/nano7060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasture M, Patel P, Prabhune A, Ramana C, Kulkarni A, Prasad B. Synthesis of silver nanoparticles by sophorolipids: effect of temperature and sophorolipid structure on the size of particles. J Chem Sci. 2008;120(6):515–520. doi: 10.1007/s12039-008-0080-6. [DOI] [Google Scholar]
- Kiran GS, Selvin J, Manilal A, Sujith S. Biosurfactants as green stabilizers for the biological synthesis of nanoparticles. Crit Rev Biotechnol. 2011;31(4):354–364. doi: 10.3109/07388551.2010.539971. [DOI] [PubMed] [Google Scholar]
- Kitamoto D. Naturally engineered glycolipid biosurfactants leading to distinctive self-assembling properties. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan. 2008;128(5):695–706. doi: 10.1248/yakushi.128.695. [DOI] [PubMed] [Google Scholar]
- Kitamoto D, Morita T, Fukuoka T, Konishi M-A, Imura T. Self-assembling properties of glycolipid biosurfactants and their potential applications. Curr Opin Colloid In. 2009;14(5):315–328. doi: 10.1016/j.cocis.2009.05.009. [DOI] [Google Scholar]
- Kumar CG, Mamidyala SK, Das B, Sridhar B, Devi GS, Karuna MS. Synthesis of biosurfactant-based silver nanoparticles with purified rhamnolipids isolated from Pseudomonas aeruginosa BS-161R. J Microbiol Biotechnol. 2010;20(7):1061–1068. doi: 10.4014/jmb.1001.01018. [DOI] [PubMed] [Google Scholar]
- Kvitek L, Panáček A, Soukupova J, Kolář M, Večeřová R, Prucek R, Holecova M, Zbořil R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs) J Phys Chem C. 2008;112(15):5825–5834. doi: 10.1021/jp711616v. [DOI] [Google Scholar]
- Lee H, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mater Sci. 2003;38(10):2199–2204. doi: 10.1023/A:1023736416361. [DOI] [Google Scholar]
- Lee KD, Nagajyothi PC, Sreekanth TVM, Park S. Eco-friendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J Ind Eng Chem. 2015;26:67–72. doi: 10.1016/j.jiec.2014.11.016. [DOI] [Google Scholar]
- Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- Moldovan B, David L, Achim M, Clichici S, Filip GA. A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L. fruits extract and their antioxidant activity. J Mol Liq. 2016;221:271–278. doi: 10.1016/j.molliq.2016.06.003. [DOI] [Google Scholar]
- Morita T, Konishi M, Fukuoka T, Imura T, Kitamoto HK, Kitamoto D. Characterization of the genus Pseudozyma by the formation of glycolipid biosurfactants, mannosylerythritol lipids. FEMS Yeast Res. 2006;7(2):286–292. doi: 10.1111/j.1567-1364.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Morita T, Ishibashi Y, Fukuoka T, Imura T, Sakai H, Abe M, Kitamoto D. Production of glycolipid biosurfactants, mannosylerythritol lipids, using sucrose by fungal and yeast strains, and their interfacial properties. Biosci Biotech Bioch. 2009;73(10):2352–2355. doi: 10.1271/bbb.90439. [DOI] [PubMed] [Google Scholar]
- Muthukumar T, Sambandam B, Aravinthan A, Sastry TP, Kim J-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016;51(3):384–391. doi: 10.1016/j.procbio.2015.12.017. [DOI] [Google Scholar]
- Muthuvel A, Adavallan K, Balamurugan K, Krishnakumar N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed Prevent Nutr. 2014;4(2):325–332. doi: 10.1016/j.bionut.2014.03.004. [DOI] [Google Scholar]
- Onghena M, Geens T, Goossens E, Wijnants M, Pico Y, Neels H, Covaci A, Lemiere F. Analytical characterization of mannosylerythritol lipid biosurfactants produced by biosynthesis based on feedstock sources from the agrofood industry. Anal Bioanal Chem. 2011;400(5):1263–1275. doi: 10.1007/s00216-011-4741-9. [DOI] [PubMed] [Google Scholar]
- Priyadarshini E, Pradhan N, Pradhan AK, Pradhan P. Label free and high specific detection of mercury ions based on silver nano-liposome. Spectrochim Acta A Mol Biomol Spectr. 2016;163:127–133. doi: 10.1016/j.saa.2016.03.040. [DOI] [PubMed] [Google Scholar]
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Eng Biotechnol. 2016;14(1):195–202. doi: 10.1016/j.jgeb.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar R, Balasubramani G, Raja RK, Raja M, Govindan R, Girija EK, Perumal P. Lantana camara Linn root extract-mediated gold nanoparticles and their in vitro antioxidant and cytotoxic potentials. Artif Cell Nanomed Biotech. 2017;45(4):748–757. doi: 10.1080/21691401.2016.1276923. [DOI] [PubMed] [Google Scholar]
- Reddy AS, Chen C-Y, Baker SC, Chen C-C, Jean J-S, Fan C-W, Chen H-R, Wang J-C. Synthesis of silver nanoparticles using surfactin: a biosurfactant as stabilizing agent. Mater Lett. 2009;63(15):1227–1230. doi: 10.1016/j.matlet.2009.02.028. [DOI] [Google Scholar]
- Saratale GD, Saratale RG, Benelli G, Kumar G, Pugazhendhi A, Kim D-S, Shin H-S. Anti-diabetic potential of silver nanoparticles synthesized with Argyreia nervosa leaf extract high synergistic antibacterial activity with standard antibiotics against foodborne bacteria. J Clust Sci. 2017;28(3):1709–1727. doi: 10.1007/s10876-017-1179-z. [DOI] [Google Scholar]
- Singh H, Du J, Singh P, Yi TH. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cell Nanomed Biotech. 2017;46(6):1–8. doi: 10.1080/21691401.2017.1362417. [DOI] [PubMed] [Google Scholar]
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Inter Sci. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Nathan S, Suresh T, Perumalsamy PL. Antimicrobial activity of certain Indian medicinal plants used in folkloric medicine. J Ethnopharmacol. 2001;74(3):217–220. doi: 10.1016/S0378-8741(00)00345-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Morita T, Fukuoka T, Imura T, Kitamoto D. Glycolipid biosurfactants, mannosylerythritol lipids, show antioxidant and protective effects against H2O2-induced oxidative stress in cultured human skin fibroblasts. J Oleo Sci. 2012;61(8):457–464. doi: 10.5650/jos.61.457. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Zhao X, Jin C, Day N, Shibahara M, Nomura N, Nakahara T, Murata T, Yokoyama KK. Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. FEBS J. 2001;268(2):374–383. doi: 10.1046/j.1432-1033.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Singh P, Kim YJ, Mathiyalagan R, Myagmarjav D, Wang D, Jin C-G, Yang DC. Characterization and antimicrobial application of biosynthesized gold and silver nanoparticles by using Microbacterium resistens. Artif Cell Nanomed Biotech. 2016;44(7):1714–1721. doi: 10.3109/21691401.2015.1089253. [DOI] [PubMed] [Google Scholar]
- Wu J, Shu Q, Niu Y, Jiao Y, Chen Q. Preparation, characterization, and antibacterial effects of chitosan nanoparticles embedded with essential oils synthesized in an ionic liquid containing system. J Agric Food Chem. 2018;66(27):7006–7014. doi: 10.1021/acs.jafc.8b01428. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wakamatsu Y, Shibahara M, Nomura N, Geltinger C, Nakahara T, Murata T, Yokoyama KK. Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999;59(2):482–486. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the conclusions are presented in the main article.