Abstract
Objective
To study the location and expression of receptors (SR-BI/CLA-1, SR-BII, and LDLr) and transporter (ABCA1) involved in uptake and efflux of cholesterol in human spermatozoa and assess whether obesity alters its location/expression and whether this could be related to infertility.
Design
Observational study.
Setting
None
Patient(s)
Ten controls and 20 obese patients.
Intervention(s)
Anthropometric parameters. Serum and semen samples were collected.
Main outcome measure(s)
Spermatozoon concentration, immunolocalization, and protein expression in semen.
Results
Spermatozoon concentration and motility was decreased in morbidly obese patients. SR-BI/CLA-1, SR-BII, LDLr, and ABCA1 are located in the spermatozoon cell membrane and the localization does not change between obese patients and controls. Control spermatozoa showed high SR-BI expression, and less expression for the rest of the receptors analyzed, indicating that SR-BI/CLA-1 is relevant in human spermatozoon cholesterol uptake/efflux. On the contrary, spermatozoa of obese patients showed less SR-BI/CLA-1 expression than controls, and more intense positive staining for SR-BII, LDLr, and ABCA1. Finally, human sperm expresses the 130- and 82-kDa hormone-sensitive lipase (HSL) isoforms. The 130-kDa isoform is expressed in the control sperm, and the expression disappears in the obese patients.
Conclusion(s)
The presence of lipid receptors/transporters and HSL in human spermatozoa suggests their role in the process of maturation/capacitation. The changes in the expression of lipid receptors/transporters and the lack of the 130-kDa HSL isoform in obese patients prevent the hydrolysis of cholesterol esters internalized by these receptors, and favor their accumulation in the cytoplasm of the spermatozoa that could contribute to lipotoxicity and infertility.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01406-z) contains supplementary material, which is available to authorized users.
Keywords: Lipid receptors and transporters, Human spermatozoa, Obesity, Fertility, Cholesterol
Introduction
Spermatogenesis is a complex process regulated by many factors [1]. After spermiogenesis, the immobile spermatozoa released into the lumen of the seminiferous tubule initiate the process of maturation [2]. Sperm maturation is defined as the development of the ability of spermatozoa to fertilize an oocyte as they progress through the epididymis. In the different regions of the epididymis, the spermatozoa present different levels of maturation and different capacity of fertilization, due to the numerous changes in the membrane glycoproteins and the loss of cholesterol, which decrease the anisotropy of the membrane, a prerequisite for capacitation, acrosomal reaction, and subsequent oocyte fertilization [3].
The capacitation occurs when sperm enters in contact with the fluids of the female genital tract, and multiple changes occur that activate the spermatozoa for the final processes of fertilization [4]. The first step of capacitation is the loss of cholesterol and desmosterol [2, 5]. Cholesterol is the most abundant sterol and the main modulator of membrane properties in sperm cells, followed by desmosterol; both have the capacity to inhibit the capacitation. Cholesterol’s ability to order saturated phospholipids may also be important in the formation of lipid rafts, sterol- and sphingolipid-enriched domains that have characteristic protein compositions and may modify signaling pathways [5], also may compartmentalize cellular processes and bring order to certain regions of the membrane [6, 7]. Some of the main receptors for cholesterol influx and efflux, such as scavenger receptors class B types I and II, SR-BI/CLA-1 and SR-BII, work through lipid rafts at the caveolar level (tested in animal models) [8, 9]. SR-BII is an isoform of SR-BI that makes up 12% of the total combined SR-BI + SR-BII and has fourfold lower efficiency for cholesterol flux than SR-BI.
Lipid homeostasis alterations of protein involved in cholesterol uptake, mobilization from stored intracellular pools, and synthesis significantly alter normal cellular function and, in turn, impact fertility. Most species, including humans, depend mostly on cellular uptake of lipoprotein cholesterol as the major precursor for steroidogenesis [10]. The two dominant mechanisms include uptake via the low-density lipoprotein receptor, LDLr [10, 11], and the lipoprotein-derived selective high-density lipoprotein (HDL)-cholesteryl esters uptake mediated by SR-BI/CLA-1 [12], which provide the majority of cholesterol in mouse testis [13]. LDLr-mediated uptake involves the binding of apolipoprotein B (ApoB)- and ApoE-enriched cholesterol particles with the subsequent endocytosis of the low-density lipoprotein (LDL) receptor and uptake of the cholesterol-containing lipoprotein particles into the cell. In contrast, SR-BI/CLA-1 mediates high-capacity selective uptake of cholesteryl esters from the core of HDL, LDL, and very-low-density lipoprotein (VLDL) [14]. Selective uptake refers to the process whereby lipoproteins dock on the extracellular loop of SR-BI, and the cholesteryl esters within the core of the lipoprotein are transferred into the interior of the cell via a channel formed by dimerization of SR-BI/CLA-1 receptors. The lipoprotein, now depleted of cholesteryl esters, dissociates from the receptor and is not internalized. The internalized cholesteryl ester is stored in lipid droplets (LDs) in the cytoplasm of the cells and is then hydrolyzed by cholesteryl ester hydrolases such as hormone-sensitive lipase (HSL) to generate free or unesterified cholesterol, which is then used for cellular processes such as steroidogenesis or membrane integrity [15].
ATP-binding cassette (ABC) transporters are members of a large and ubiquitous transmembrane protein family (e.g., ABCA1, ABCA3, and ABCG2) that exports free cholesterol to acceptor proteins like HDL [16, 17]. ABCA1 is as highly expressed in the testis as it is in the liver, suggesting that ABCA1 may also play a role in regulating testicular lipid transport [18]. Numerous ABCA cholesterol transporters have also been detected immunologically in spermatozoa from various species, and some analyses of their ability to control sperm cholesterol levels have been undertaken. ABCA1 has been detected in both the mouse and dog [19].
In clinical studies, high cholesterol levels in sperm are negatively correlated with sperm quality [2], and patients with idiopathic infertility display a higher cholesterol to phospholipid ratio [20]. Some cases of unexplained male infertility could also be the result of cholesterol and sterol intermediate perturbations in the testes due to genetic or environmental factors, as it may occur with obesity. In fact, it is well known that obesity is associated with male secondary hypogonadism and infertility [21, 22].
Therefore, the aim of this study was to describe the location and expression of receptors and lipid transporters involved in influx and efflux of cholesterol in spermatozoa and assess whether obesity alters its location/expression and whether this could be related to infertility in obese patients.
Materials and methods
We included 10 healthy controls and 20 male patients with moderate to severe obesity who gave written informed consent to participate in a substudy and the study was approved by the Institutional Review Board of our hospital. Data of the whole group and semen analysis characteristics were reported before [21].
Inclusion criteria required a body mass index (BMI) of at least 35 kg/m2 and the commitment to attend the scheduled visits. Exclusion criteria included previous vasectomy, diagnoses of hypogonadism, thyroid and heart disease, kidney or liver failure, and hyperprolactinemia or treatment for sexual dysfunction or use of drugs that could interfere with normal gonadal function or lipid metabolism.
Anthropometric parameters were recorded. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured as the smallest perimeter between the costal border and the anterior suprailiac spines. Ideal body weight was calculated as the weight corresponding for a BMI of 25, given the previous lack of consensus for a precise definition [23, 24]. Excess body weight (EBW) was calculated as the difference between body weight and ideal weight.
Serum glucose concentrations were measured by standard colorimetric methods using the Architect ci8200 analyzer (Abbot Diagnostics, Berkshire, UK). Serum concentrations of HDL-cholesterol were measured in supernatant after plasma precipitation with phosphotungstic acid and Mg2+ 110 (Boehringer Mannheim GmbH, Mannheim, Germany). Serum concentrations of total cholesterol and triglycerides were measured by enzymatic methods (Menarini Diagnostica, Florence, Italy). The LDL-cholesterol concentration was calculated by using Friedewald’s formula [25].
Fasting insulin, total testosterone (TT), sex hormone–binding globulin (SHBG), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol were also assayed as described [21]. Briefly, TT was measured by direct radioimmunoassay (Spectria, Orion Diagnostica, Espoo, Finland). Free testosterone (FT) concentration was calculated from total testosterone and SHBG concentrations [26]. LH, FSH, serum estradiol, and insulin were measured by immunochemiluminescence (Immulite 2000, Siemens Healthcare Diagnostics Inc., Gwynedd, UK). All these assays had coefficients of variation below 10%. Normal ranges were 10.4–31.2 nmol/L for TT and 13–71 nmol/L for SHBG as provided by the Central Laboratory of Hospital Universitario Ramón y Cajal. We used the 95% confidence interval of the mean of a group of 10 healthy men to obtain the reference range for FT, which was set at 225–635 pmol/L.
Sperm samples were produced by masturbation and ejaculated into a clean wide-mouthed container, following a standardized 4-day sexual abstinence period. Sperm concentration was determined in a Bürker hemocytometer. The number of motile sperms was analyzed using a Sperm Class Analyzer ® SCA 2002 (Microptic Inc., Barcelona, Spain). For semen analysis, we used the reference values proposed by the World Health Organization [27].
After evaluation of the seminal parameters in the laboratory of Andrology, the semen samples were transported to the laboratory of Biochemistry-Research for the studies of expression of proteins by western blot and cellular immunolocalization by immunohistochemistry. An aliquot of the semen samples was used to make the extension on the slide to evaluate the morphology of spermatozoa by staining with May Grünwald-Giemsa.
Immunohistochemistry technique used was streptavidin-biotin. The slides are prepared by padding them with poly-d-lysine, then incubated for 1 h at 37 °C and washed with distilled water. Fifty microliters of the total sperm is dispensed into each well and incubated overnight at 37 °C, then washed three times with PBS (phosphate-buffered saline) pH 7.4, and fixed with 4% formaldehyde for 20 min at room temperature. We washed with PBS three times and blocked the endogenous peroxidase with 3% hydrogen peroxide for 30 min at room temperature in the absence of light. We washed with distilled water and then with TBS (Tris-buffered saline), incubated with normal donkey serum at 3%, 0.01% Triton X-100, and 0.1% glycine in TBS at room temperature for 1 h. We then added 200 μL of the corresponding primary antibody (Supplemental table 1) diluted in 0.3% normal serum, 0.0001% Triton X-100, and 0.1% glycine in TBS and incubated overnight at 4 °C in a humid chamber. We washed with TBS to remove the unbound antibody and added 200 μL to each well of the corresponding secondary antibody (Supplemental table 1), conjugated with biotin, in the same diluent. We washed three times with TBS followed by incubation with the streptavidin-peroxidase complex (Zymed, Invitrogen), 30 min. After washing with TBS, peroxidase activity was revealed with 3-diaminobenzydinatetrahydrochloride (DAB + Dako substrate buffer) as chromogen. And they were contrasted with Carazzi’s hematoxylin (Panreac). Finally, they were dehydrated with increasing concentrations of ethanol, washed with xylol, mounted with DePex (BDH), and observed under an optical microscope.
The specificity of the immunohistochemical procedures was assessed by means of negative controls that were performed as follows: omitting the primary antibodies, using nonimmune serum (0.3% donkey normal serum 017-000-121; Jackson ImmunoResearch) instead of the primary antibodies, and incubating with an inappropriate secondary antibody (anti-goat biotin 1/200 from Millipore for SR-BI, SR-BII, and HSL; and anti-rabbit biotin 1/500 from Dako for LIMPII) after the incubation with the primary antibodies at optimal titers.
Western blot analysis was performed according to standard protocols as described previously [28–30]. Semen samples were homogenized in 10 mM Tris-HCL buffer pH 7.4 containing 0.5% Triton, 0.1% SDS, 10 mM CIK, 1 mM DTT, 1 mM EDTA, 1 mM PMSF-ethanol, 10 mg/mL leupeptin, 1 μg aprotinin/mL, 100 mM FIN, and 10 mM Na-orthovanadate. Total protein of samples was quantified by the BCA method (Pierce Biotechnology). Fifty micrograms of protein of each semen sample was loaded onto each well and the proteins were separated according to their size by electrophoresis on SDS-PAGE gels between 8 and 10% and transferred to nitrocellulose membranes (GE Healthcare Life Science Cat, 1060048) by wet transfer, overnight at 15 V in both cases at 4 °C. After blocking the membrane (TBS-Tw 10% skim milk), they were incubated overnight with the corresponding primary antibody (Supplemental table 2). After several washes with TBS-Tween the membranes were incubated with the secondary antibody (Supplemental table 2) conjugated to IRDye 800 CW or IRDye 680 LT (LI-COR, Lincoln, NE, USA). The membranes were analyzed using the Odyssey Infrared Imaging (LI-COR Bioscience) system. To correct for differences in protein loading, alpha-tubulin (Santa Cruz Biotechnology) was used as the internal control of protein expression. The densitometric analysis of the bands was performed using the Odyssey V 3.0 Infrared Imaging System (LI-COR Bioscience).
The immunohistochemistry technique used was streptavidin-biotin peroxidase. The method is described in the expanded discussion of the “Materials and methods” section. The primary and secondary antibodies employed are described in Supplemental table 2.
All statistical analyses were performed using Pad Prism 4.03 (GraphPad Software, San Diego, CA). Data from western blot results were statistically analyzed using the two-tailed Student t-test. Western blot data are expressed as means ± SD. Differences were considered statistically significant with values of P < 0.05 (one asterisk indicates P < 0.05; two asterisks, P < 0.01; and three asterisks, P < 0.001).
Results
The clinical, biochemical, hormonal, and seminal characteristics of the obese patients and controls are shown in Table 1. As expected, body weight and BMI were significantly higher in adult morbid obese patients compared with controls. Plasma total testosterone and sex hormone–binding globulin (SHBG) were significantly decreased in adult obese patients compared with controls, whereas plasma estradiol was increased.
Table 1.
Clinical, biochemical, and seminal characteristics of controls and obese patients
| Controls (n = 10) | Obese patients (n = 20) | P | |
|---|---|---|---|
| Age (years) | 34 ± 5 | 40 ± 8 | 0.055 |
| Weight (kg) | 77 ± 8 | 146 ± 27 | 0.000 |
| Body mass index (kg/m2) | 24 ± 2 | 48 ± 9 | 0.000 |
| Excess body weight (kg) | 3 ± 6 | 70 ± 26 | 0.000 |
| Total cholesterol (nmol/L) | 177 ± 33 | 192 ± 33 | 0.381 |
| Low-density lipoprotein cholesterol (nmol/L) | 2.90 ± 0.78 | 3.57 ± 0.78 | 0.038 |
| High-density lipoprotein cholesterol (nmol/L) | 1.11 ± 0.18 | 0.96 ± 0.18 | 0.063 |
| Triglycerides (nmol/L) | 1.23 ± 0.50 | 1.98 ± 0.79 | 0.025 |
| Total testosterone (nmol/L) | 21.6 ± 6.0 | 12.9 ± 5.8 | 0.001 |
| Sex hormone–binding globulin (nmol/L) | 4.1 ± 1.3 | 2.9 ± 0.8 | 0.011 |
| Free testosterone (pmol/L) | 439 ± 103 | 302 ± 137 | 0.008 |
| Estradiol (pmol/L) | 84 ± 15 | 147 ± 48 | 0.008 |
| Follicle-stimulating hormone (U/L) | 2.6 ± 1 | 3.6 ± 1.9 | 0.199 |
| Luteinizing hormone (U/l) | 4.1 ± 0.6 | 3.5 ± 1.3 | 0.123 |
| Fasting glucose (mmol/L) | 5.1 ± 0.7 | 6.8 ± 3.6 | 0.328 |
| Fasting insulin (pmol/L) | 49 ± 28 | 181 ± 83 | 0.000 |
| Semen volume (mL) | 3.7 ± 1.9 | 2.8 ± 1.3 | 0.281 |
| Sperm concentration (106/mL) | 72.5 ± 18.3 | 40.9 ± 34.9 | 0.038 |
| Total motility (%) | 58 ± 4 | 37 ± 18 | 0.001 |
| Progressive motility (%) | 43 ± 3 | 18 ± 7 | 0.001 |
| Immobile (%) | 43 ± 4 | 64 ± 18 | 0.001 |
*Data are shown as means ± SD
Spermatozoon concentration was 72.5 ± 18.3·106 spermatozoids/mL in controls and 40.9 ± 34.9·106 spermatozoids/mL in obese patients (Table 1). Total motility (%) and progressive motility (%) of the spermatozoa were significantly decreased in obese patients, increasing the percentage (27.5%) of immobile spermatozoa in obese patients.
When we analyzed the results of the whole groups, sperm concentration was inversely correlated with BMI (r = − 0.428, P = 0.006) and EBW (r = − 0.419, P = 0.007). Total sperm motility was inversely correlated with BMI (r = − 0.433, P = 0.005) and EBW (r = − 0.397, P = 0.011). The sperm concentration and total mobility was negatively correlated with serum estradiol (r = − 0.507, P = 0.001; r = − 0.404, P = 0.030), respectively (Supplemental figure 1).
We found that 65% of the obese patients presented an altered morphology of spermatozoa by staining with May Grünwald-Giemsa. The most frequent morphological changes observed were the following: large, double, amorphous or piriform head; thick and irregular neck; coiled or short tail or spermatozoa without tail; predominantly head alterations versus combined alterations.
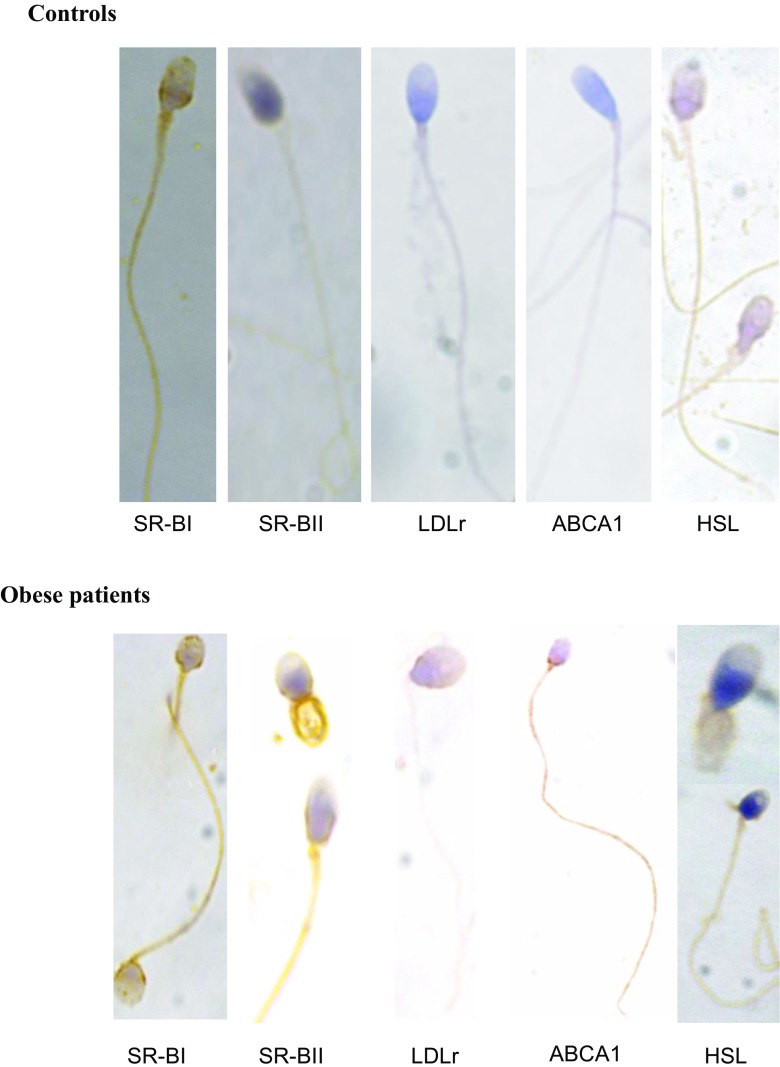
We analyzed by immunocytochemical techniques the cellular location of the different proteins in spermatozoa. We observed positive staining for class B scavenger receptors (SR-BI/CLA-1 and SR-BII), LDLr, and ABCA1 on the cell membrane and specifically in the lower part of the head, neck, and tail of the spermatozoa of obese patients and controls (Fig. 1). These results indicate that the localization of membrane receptors does not change between obese patients and controls. The spermatozoa of the controls showed a more intense positive staining for SR-BI/CLA-1, and more tenuous for the rest of the receptors analyzed, which would indicate a greater expression of this receptor in the control spermatozoa. On the contrary, in the spermatozoa of obese patients, we observed a trend toward a more intense positive staining for SR-BII, LDLr, and ABCA1 than in the controls.
Fig. 1.
Immunolocalization of SR-BI/CLA-1, SR-BII, LDLr, ABCA1, and HSL in controls and obese patient’s spermatozoa. Semen samples were incubated with the primary antibodies and counterstained with Carazzi’s hematoxylin for the visualization of cellular nucleus. The color of the stain is brown for the receptors and blue for the nucleus. We present a representative image of a total of 10 control samples and 20 samples of obese patients analyzed
We also analyzed the location of the hormone-sensitive lipase (HSL), an enzyme with cholesterol ester hydrolase activity, important in the hydrolysis of cholesterol esters captured by SR-BI/CLA-1, SR-BII, and LDLr, and stored in the spermatozoa. As shown in Fig. 1, positive HSL immunostaining appears in the cytoplasm of the head of the spermatozoa and in the tail of the control samples. In the spermatozoa of obese patients, we clearly observed positive HSL staining in the tail; while a less-intense staining of HSL in the head was observed in the spermatozoa of obese patients when compared to controls (Fig. 1).
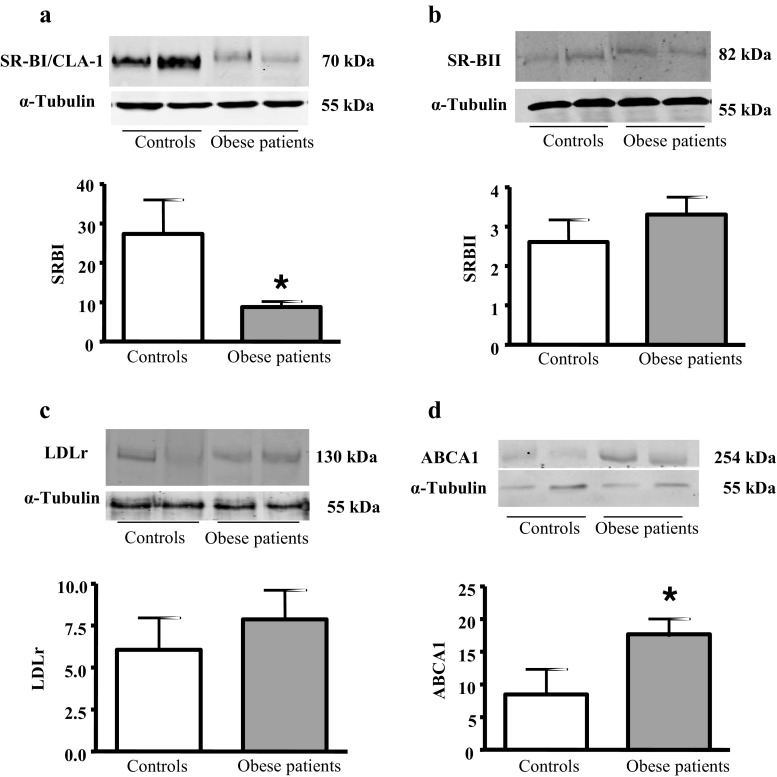
We analyzed the protein expression of the different SR-Bs on the spermatozoa because of the relevance for cholesterol depletion in the spermatozoid capacitation. We determined SR-BI/CLA-1 protein expression by western blotting in total lysates of human sperm (Fig. 2 a). Human sperm expresses a SR-BI/CLA-1 immunoreactive protein of 70 kDa. This isoform of the SR-BI/CLA-1 receptor protein is expressed in the cell lysates of control and obese patient’s spermatozoa. The spermatozoa of obese patients expressed less SR-BI/CLA-1 than controls, and these differences were statistically significant (Fig. 2 a).
Fig. 2.
SR-BI/CLA-1, SR-BII, LDLr, and ABCA1 expression in controls and obese patient’s spermatozoa. a Western blotting analysis of SR-BI/CLA-1 in lysates of sperm samples. The results correspond to an experiment representative of two independent experiments; in total, six control and six obese patient samples were analyzed by densitometry. Results are mean ± SEM of six control and six obese patient samples. b Western blotting analysis of SR-BII in lysates of sperm samples. The results correspond to an experiment representative of two independent experiments; in total, eight control and 13 obese patient samples were analyzed by densitometry. Results are mean ± SEM of eight control and 13 obese patient samples. c Western blotting analysis of LDL receptor in lysates of sperm samples. The results correspond to an experiment representative of two independent experiments; in total, six control and 16 obese patient samples were analyzed by densitometry. Results are mean ± SEM of six control and 16 obese patient samples. Results are mean ± SEM of seven control and 16 obese patient samples. d Western blotting analysis of ABCA1 in lysates of sperm samples. The results correspond to an experiment representative of two independent experiments; in total, six control and six obese patient samples were analyzed by densitometry. Results are mean ± SEM of six control and six obese patient samples. Statistical comparisons show the differences between controls and obese patients (*P < 0.05)
When analyzing the expression of SR-BII in sperm lysates, we observed a band at 82 kDa, corresponding to the mature isoform of SR-BII receptor, and we did not find significant statistically differences in their expression between obese patients and controls (Fig. 2 b).
We analyzed the expression of LDL receptor on human sperm because of the relevance of this receptor in the cholesterol uptake from the plasma lipoproteins for steroidogenesis and spermatogenesis. When analyzing the expression of LDL receptor in sperm lysates, we observed a band at 130 kDa in controls and obese patients (Fig. 2 c). The LDL receptor has a low sperm expression in the semen samples, and although we observed a tendency for a higher LDLr expression in obese patients than in controls, these differences are not statistically significant (Fig. 2 c).
We analyzed the expression of ABCA1 receptor on human sperm because of the relevance of this transporter in the cholesterol efflux from the cells. We observed that ABCA1 254-kDa protein is expressed in the sperm of controls and obese patients (Fig. 2 d). We found that obese patients overexpress ABCA1 compared to the controls.
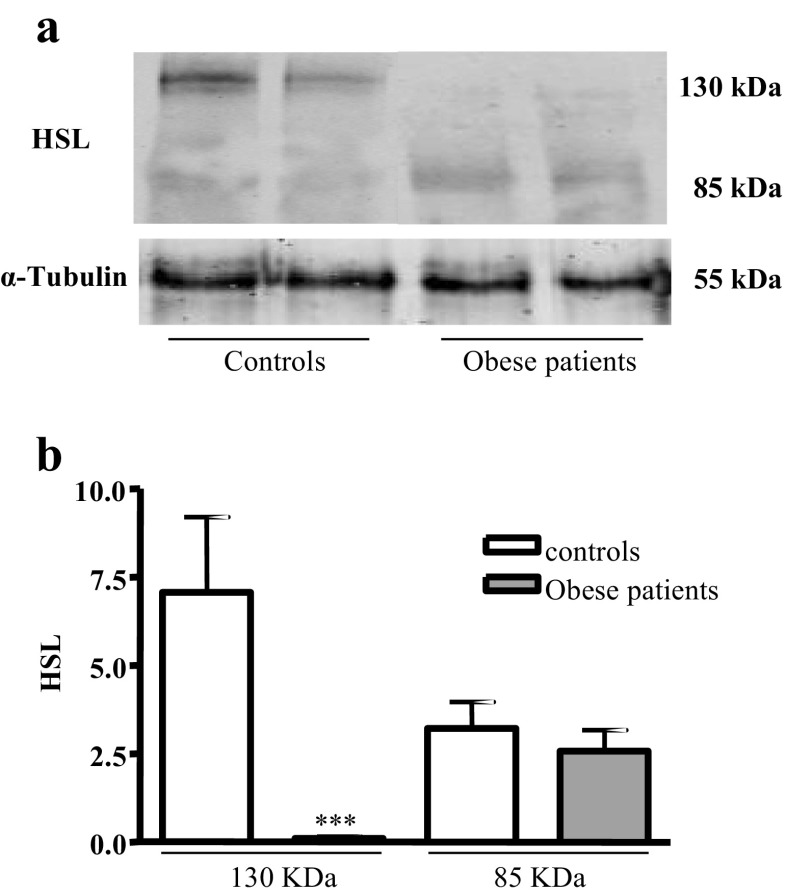
Finally, human sperm expresses the 130- and 82-kDa HSL isoforms, the two HSL isoforms already described in the testis (Fig. 3 a). The 130-kDa HSL isoform is expressed in the control sperm, and it is practically not expressed in the sperm of obese patients, P < 0.001. The expression of the 82-kDa HSL isoform is lower than that of the 130-kDa HSL isoform in the control sperm, and yet the expression of this isoform is greater than that of 130-kDa HSL in obese patients. We did not observe statistically significant differences in the expression of the 82-kDa HSL isoform between controls and obese patients (Fig. 3 b).
Fig. 3.
HSL expression in controls and obese patient’s spermatozoa. a Western blotting analysis of HSL in lysates of sperm samples. The results correspond to an experiment representative of two independent experiments; in total, six control and 16 obese patient samples were analyzed. b Densitometric analysis of HSL western blots of sperm samples from controls and obese patients. Results are mean ± SEM of six control and 16 obese patient samples. Statistical comparisons show the differences between controls and obese patients (***P < 0.001)
Discussion
Dyslipidemia caused by obesity is associated with infertility, and there is a close relationship between fertility, lipid accumulation, and changes in cholesterol and lipid metabolism during spermatogenesis in the testis [31].
In the present work, we have described in human spermatozoa the expression and cellular localization of the LDL receptor, responsible for the uptake of cholesterol from lipoproteins; the ABCA1 transporter, responsible for cholesterol efflux; SR-BI/CLA-1 and SR-BII, which are involved in the uptake of cholesterol esters of lipoproteins (HDL) and in the efflux of cellular cholesterol. All of them are located in the cell membrane of the spermatozoa. The localization of these cholesterol receptors/transporters does not change between obese patients and controls. The presence of these receptors in human spermatozoa allows us to suggest their role in the process of maturation/capacitation.
SR-BI/CLA-1, as we have already mentioned, may play a role both in the uptake of cholesterol esters of lipoproteins (HDLs) and in efflux or reverse cholesterol transport [32]. SR-BI/CLA-1 is more efficient than SR-II in the uptake of cholesterol esters from HDLs and reverse cholesterol transport (shown in other human cells, not sperm cells) [9]. The results of the present work also indicate that SR-BI/CLA-1 is one of the most widely expressed scavenger receptors on the cell membrane of human spermatozoa. SR-BI/CLA-1 expression in spermatozoa was higher in controls than in obese patients, which indicates a higher uptake or efflux of cholesterol esters in controls than in obese patients, and this result leads us to hypothesize that these differences may alter the process of capacitation of the spermatozoa in obese patients.
Other receptors with a role in the uptake of lipoproteins and lipids such as LDL receptor and SR-BII appear to have a tendency to overexpress in the spermatozoa of obese patients, which could probably reflect a compensatory effort to maintain an adequate lipid composition of the plasma membrane (this should be checked in future studies both in vivo and in vitro). This compensation theory could be supported by the ABCA1 overexpression in obese patients, which together with the SR-BI/CLA-1 participates in the reverse transport of cholesterol from the spermatozoid to an external acceptor.
Since the presence of some of these receptors was not previously described in human spermatozoa, there are few studies on whether their absence or overexpression in humans produces infertility. In vivo and in vitro models demonstrate that experimental SR-BI deficiency causes abnormalities in the development of female gametes, resulting in sterility in female mice lacking SR-BI [33].
It is also interesting to note that disturbances in cholesterol homeostasis in Sertoli cells produce infertility or subfertility caused by the excessive accumulation of cholesterol esters. This has been demonstrated in a model of ABCA1 knockout mice [34] and mice lacking nuclear retinoid beta receptor (RXRβ, Rxrb gene) or hepatic X receptor (LXR) (Nr1h gene) [35, 36]. In the case of the LXR double knockout (Lxrα; β−/−), the accumulation of cholesterol esters is due to a decrease in the ABCA1-dependent cholesterol efflux of Sertoli cells, since ABCA1 is a target gene of LXR [35]. Previous studies in LXR-deficient mice fed with high-cholesterol diets report that at 8–9 months they become infertile, since LXR is one of the transcription factors that regulate cholesterol homeostasis. These mice store cholesterol esters in the head of the epididymis and the spermatozoa recovered from the head of the epididymis show structural fragility between the head and the intermediate piece resulting in a large amount of broken spermatozoa [37]. Therefore, these mice are a good model to address the question of how an excess of dietary lipid affects reproductive functions in dyslipidemic animals.
Men with Tangier disease (caused by the mutation in homozygosis or compound heterozygosity of the ABCA1 gene) are fertile, but they may have a higher incidence of age-related reproductive difficulties than normolipidemic subjects without this mutation [34]. Therefore, we leave open this line of research for future studies to answer questions still unresolved about the role of each of these receptors/transporters in maturation and capacitation, which ultimately translates into fertile sperm capacity.
Finally, HSL is the main enzyme in the testis that hydrolyzes the cholesterol esters internalized by the different receptors (SR-BI/CLA-1, SR-BII, LDLr) and stored in the lipid droplets. Previous studies have described that genetically deficient HSL mice show an increase of 2 to 4 times in the accumulation of cholesterol esters in testes and are infertile [38]. This infertility is reversed in transgenic mice expressing the testicular form of HSL in germ cells post meiosis [39, 40], which demonstrates that this enzyme is essential in spermatogenesis.
In the present work, we have shown that the 130-kDa isoform of HSL is highly expressed in spermatozoa from controls and very little expressed in obese patients, which could indicate lower hydrolysis of cholesterol esters in spermatozoa of obese patients, favoring their accumulation, also facilitated by decreased SR-BI cholesterol excretion in the spermatozoa of obese patients. This alteration in lipid homeostasis in obese patients’ spermatozoa could induce lipotoxicity and infertility.
Our previous studies in HSL knockout mice [30, 41] showed that the lack of HSL in mouse testis disturbs lipid composition of the plasma membrane, disrupts lipid raft microdomains, and alters the localization and expression of SR-BI. These results support the hypothesis that alterations at a critical point for correct homeostasis of cholesterol in the testis try to be compensated through overexpression of other receptors, such as SR-BI and SR-BII, which favor the uptake of cholesterol esters; however, the lack of HSL prevents hydrolysis of the cholesterol esters internalized by these receptors and favors their accumulation in the cytoplasm contributing to the testicular lipotoxicity and infertility observed in these mice.
A good example that establishes the association between the defects of cholesterol homeostasis and defects in post-testicular maturation is Niemann–Pick disease, which is characterized by accumulation of sphingomyelin and cholesterol in cells and tissues, by deficit of acid sphingomyelinase (catalyzes sphingomyelin to ceramide and phosphorylcholine). In murine models of this disease, in the head and tail of the epididymis, epithelial cells have lipid vacuoles and the spermatozoa recovered in the tail have high levels of sphingomyelin and cholesterol, which allows disruption of the membrane and acrosome with defects in morphology and motility, which alter the maturation process [42].
All these studies, together with the results we provide, continue to reinforce the hypothesis of the important role of lipids in post-testicular maturation and sperm capacitation, and raise the need for new studies that can answer unresolved questions about spermatozoa lipid homeostasis.
In summary, these findings highlight an important and previously unrecognized role of the lipid receptors/transporters in sperm development. Further studies into the molecular pathways may yield significant new insights into the concept of sperm maturation and the cause of infertility in obese humans.
In conclusion, the presence of lipid receptors/transporters and HSL in human spermatozoa suggests their role in the process of maturation/capacitation. The changes in the expression of lipid receptors/transporters and the lack of the 130-kDa HSL isoform in morbid obese patients prevent the hydrolysis of cholesterol esters internalized by these receptors, and favor their accumulation in the cytoplasm of the spermatozoa that could contribute to lipotoxicity and infertility.
Electronic supplementary material
Linear correlations between sperm concentration and BMI; EBW and serum estradiol; and sperm mobility and BMI, EBW and serum estradiol. Sperm concentration was inversely correlated with BMI (r = −0,428, p = 0.006), EBW (r = −0.419, p = 0.007) and serum estradiol (r = −0.507, P = 0.001). Total sperm motility was inversely correlated with BMI (r = −0.433, P = 0.005), EBW (r = −0.397, P = 0.011) and serum estradiol (r = −0.404, P = 0.030). (PPT 573 kb)
Primary and secondary antibodies used in the studies of immunolocalization of proteins by immunohistochemistry in the human semen samples. The dilution and antibody source for each antibody is shown. (DOC 36.5 kb)
Primary and secondary antibodies used in the studies of protein expression by western blot technique in human semen samples. The dilution and antibody source for each antibody is shown. (DOCX 14.1 kb)
Acknowledgments
We thank the members of Department of Biochemistry-Research for their help.
Author contribution
B.C. participated in the sample collection and data analysis and revised the manuscript.
L.H. participated in the sample collection and conducted the experiments.
M.E.C. participated in the data analysis.
J.M.G.C. participated in sample collection.
J.I.B.C. participated in the study design and revised the manuscript critically for important intellectual content.
A.M.H. participated in the study design and coordination, data analysis, and interpretation and wrote the manuscript.
Funding
Supported by Grants PI1501686 and PI1600154 from Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness. Supported in part by Fondo Europeo de Desarrollo Regional (FEDER) from the European Union. CIBERobn is also an initiative of Instituto de Salud Carlos III. Dr. Calderón received a grant from MESCYT, Dominican Republic Ministry of Superior Education.
Compliance with ethical standards
We included 10 healthy controls and 20 male patients with moderate to severe obesity who gave written informed consent to participate in a substudy and the study was approved by the Institutional Review Board of our hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Issue Section: Reproductive endocrinology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molecular mechanisms in spermatogenesis | PDF Free Download Ebook.
- 2.Keber R, Rozman D, Horvat S. Sterols in spermatogenesis and sperm maturation. J Lipid Res. 2013;54(1):20–33. doi: 10.1194/jlr.R032326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper TG. Sperm maturation in the epididymis: a new look at an old problem. Asian J Androl. 2007;9(4):533–539. doi: 10.1111/j.1745-7262.2007.00285.x. [DOI] [PubMed] [Google Scholar]
- 4.Feki NC, Thérond P, Couturier M, Liméa G, Legrand A, Jouannet P, et al. Human sperm lipid content is modified after migration into human cervical mucus. Mol Hum Reprod. 2004;10(2):137–142. doi: 10.1093/molehr/gah018. [DOI] [PubMed] [Google Scholar]
- 5.Nimmo MR, Cross NL. Structural features of sterols required to inhibit human sperm capacitation. Biol Reprod. 2003;68(4):1308–1317. doi: 10.1095/biolreprod.102.008607. [DOI] [PubMed] [Google Scholar]
- 6.Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47(7):1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, et al. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272(20):13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- 9.Webb NR, Connell PM, Graf GA, Smart EJ, de Villiers WJ, de Beer FC, et al. SR-BII, an isoform of the scavenger receptor BI containing an alternate cytoplasmic tail, mediates lipid transfer between high density lipoprotein and cells. J Biol Chem. 1998;273(24):15241–15248. doi: 10.1074/jbc.273.24.15241. [DOI] [PubMed] [Google Scholar]
- 10.Brown MS, Kovanen PT, Goldstein JL. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JL, Brown MS. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 12.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94(23):12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gwynne JT, Strauss JF. The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3(3):299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 14.Thuahnai ST, Lund-Katz S, Anantharamaiah GM, Williams DL, Phillips MC. A quantitative analysis of apolipoprotein binding to SR-BI: multiple binding sites for lipid-free and lipid-associated apolipoproteins. J Lipid Res. 2003;44(6):1132–1142. doi: 10.1194/jlr.M200429-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 16.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30(2):139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolnerciks JK, Andress EJ, Nicolaou M, Linton KJ. Structure of ABC transporters. Essays Biochem. 2011;50(1):43–61. doi: 10.1042/bse0500043. [DOI] [PubMed] [Google Scholar]
- 18.Wellington CL, Walker EKY, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Investig. 2002;82(3):273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 19.Palme N, Becher AC, Merkl M, Glösmann M, Aurich C, Schäfer-Somi S. Immunolocalization of the cholesterol transporters ABCA1 and ABCG1 in canine reproductive tract tissues and spermatozoa. Reprod Domest Anim. 2014;49(3):441–447. doi: 10.1111/rda.12294. [DOI] [PubMed] [Google Scholar]
- 20.Sugkraroek P, Kates M, Leader A, Tanphaichitr N. Levels of cholesterol and phospholipids in freshly ejaculated sperm and Percoll-gradient-pelletted sperm from fertile and unexplained infertile men. Fertil Steril. 1991;55(4):820–827. [PubMed] [Google Scholar]
- 21.Calderón B, Gómez-Martín JM, Vega-Piñero B, Martín-Hidalgo A, Galindo J, Luque-Ramírez M, Escobar-Morreale HF, Botella-Carretero JI. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology. 2016;4(1):62–67. doi: 10.1111/andr.12135. [DOI] [PubMed] [Google Scholar]
- 22.Campbell JM, Lane M, Owens JA, Bakos HW. Paternal obesity negatively affects male fertility and assisted reproduction outcomes: a systematic review and meta-analysis. Reprod BioMed Online. 2015;31(5):593–604. doi: 10.1016/j.rbmo.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Montero PN, Stefanidis D, Norton HJ, Gersin K, Kuwada T. Reported excess weight loss after bariatric surgery could vary significantly depending on calculation method: a plea for standardization. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2011;7(4):531–534. doi: 10.1016/j.soard.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Shah B, Sucher K, Hollenbeck CB. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2006;21(3):312–319. doi: 10.1177/0115426506021003312. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 27.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 28.Alegria-Schaffer A. Western blotting using chemiluminescent substrates. Methods Enzymol. 2014;541:251–259. doi: 10.1016/B978-0-12-420119-4.00019-7. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SC, Posch A. The design of a quantitative western blot experiment. Biomed Res Int. 2014;2014:361590. doi: 10.1155/2014/361590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casado ME, Huerta L, Ortiz AI, Pérez-Crespo M, Gutiérrez-Adán A, Kraemer FB, Lasunción MÁ, Busto R, Martín-Hidalgo A. HSL-knockout mouse testis exhibits class B scavenger receptor upregulation and disrupted lipid raft microdomains. J Lipid Res. 2012;53(12):2586–2597. doi: 10.1194/jlr.M028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasquali R, Patton L, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):482–487. doi: 10.1097/MED.0b013e3282f1d6cb. [DOI] [PubMed] [Google Scholar]
- 32.Leahy T, Gadella BM. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J Androl. 2015;17(4):561–567. doi: 10.4103/1008-682X.153309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trigatti B, Rayburn H, Viñals M, Braun A, Miettinen H, Penman M, et al. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci U S A. 1999;96(16):9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selva DM, Hirsch-Reinshagen V, Burgess B, Zhou S, Chan J, McIsaac S, Hayden MR, Hammond GL, Vogl AW, Wellington CL. The ATP-binding cassette transporter 1 mediates lipid efflux from Sertoli cells and influences male fertility. J Lipid Res. 2004;45(6):1040–1050. doi: 10.1194/jlr.M400007-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Volle DH, Mouzat K, Duggavathi R, Siddeek B, Déchelotte P, Sion B, et al. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol. 2007;21(5):1014–1027. doi: 10.1210/me.2006-0277. [DOI] [PubMed] [Google Scholar]
- 36.Robertson KM, Schuster GU, Steffensen KR, Hovatta O, Meaney S, Hultenby K, Johansson LC, Svechnikov K, Söder O, Gustafsson JÅ. The liver X receptor-{beta} is essential for maintaining cholesterol homeostasis in the testis. Endocrinology. 2005;146(6):2519–2530. doi: 10.1210/en.2004-1413. [DOI] [PubMed] [Google Scholar]
- 37.Ouvrier A, Alves G, Damon-Soubeyrand C, Marceau G, Cadet R, Janny L, Brugnon F, Kocer A, Pommier A, Lobaccaro JMA, Drevet JR, Saez F. Dietary cholesterol-induced post-testicular infertility. PLoS One. 2011;6(11):e26966. doi: 10.1371/journal.pone.0026966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osuga J, Ishibashi S, Oka T, Yagyu H, Tozawa R, Fujimoto A, Shionoiri F, Yahagi N, Kraemer FB, Tsutsumi O, Yamada N. Targeted disruption of hormone-sensitive lipase results in male sterility and adipocyte hypertrophy, but not in obesity. Proc Natl Acad Sci U S A. 2000;97(2):787–792. doi: 10.1073/pnas.97.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SP, Chung S, Soni K, Bourdages H, Hermo L, Trasler J, Mitchell GA. Expression of human hormone-sensitive lipase (HSL) in postmeiotic germ cells confers normal fertility to HSL-deficient mice. Endocrinology. 2004;145(12):5688–5693. doi: 10.1210/en.2004-0919. [DOI] [PubMed] [Google Scholar]
- 40.Vallet-Erdtmann V, Tavernier G, Contreras JA, Mairal A, Rieu C, Touzalin A-M, Holm C, Jégou B, Langin D. The testicular form of hormone-sensitive lipase HSLtes confers rescue of male infertility in HSL-deficient mice. J Biol Chem. 2004;279(41):42875–42880. doi: 10.1074/jbc.M403495200. [DOI] [PubMed] [Google Scholar]
- 41.Casado ME, Pastor O, García-Seisdedos D, Huerta L, Kraemer FB, Lasunción MA, Martín-Hidalgo A, Busto R. Hormone-sensitive lipase deficiency disturbs lipid composition of plasma membrane microdomains from mouse testis. Biochim Biophys Acta. 2016;1861(9 Pt A):1142–1150. doi: 10.1016/j.bbalip.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Butler A, He X, Gordon RE, Wu H-S, Gatt S, Schuchman EH. Reproductive pathology and sperm physiology in acid sphingomyelinase-deficient mice. Am J Pathol. 2002;161(3):1061–1075. doi: 10.1016/S0002-9440(10)64267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Linear correlations between sperm concentration and BMI; EBW and serum estradiol; and sperm mobility and BMI, EBW and serum estradiol. Sperm concentration was inversely correlated with BMI (r = −0,428, p = 0.006), EBW (r = −0.419, p = 0.007) and serum estradiol (r = −0.507, P = 0.001). Total sperm motility was inversely correlated with BMI (r = −0.433, P = 0.005), EBW (r = −0.397, P = 0.011) and serum estradiol (r = −0.404, P = 0.030). (PPT 573 kb)
Primary and secondary antibodies used in the studies of immunolocalization of proteins by immunohistochemistry in the human semen samples. The dilution and antibody source for each antibody is shown. (DOC 36.5 kb)
Primary and secondary antibodies used in the studies of protein expression by western blot technique in human semen samples. The dilution and antibody source for each antibody is shown. (DOCX 14.1 kb)