Graphical abstract
Keywords: Periprosthetic joint infection, Mycobacterium abscessus, Total knee replacement
Abstract
Periprosthetic joint infection (PJI) can be protracted, incapacitating, needing multiple interventions and could even lead to mortality. Early post-operative PJI has been ascribed to peri-operative introduction of highly virulent bacteria, while delayed post-operative to low-virulence bacteria. Non-tuberculous mycobacteria (NTM) do not figure in the usual list of etiological agents. We report a case of difficult diagnosis of bilateral PJI caused by Mycobacterium abscessus, following bilateral total knee arthroplasty in an elderly male, but treated successfully despite prolonged infection. M. abscessus complex comprises a group of rapidly growing, multidrug-resistant NTM, capable of forming biofilms on prostheses, responsible for wide spectrum of hospital acquired infections. M. abscessus as a cause of PJI is not reported widely. There are a few cases described in literature worldwide. There are no policy guidelines available for treating such cases. High clinical suspicion, with a concerted effort to grow and identify the causal pathogen is important. Standard anti-tubercular therapy is not recommended for treatment due to inherent resistance. Complete excision of infected tissues and removal of prosthesis along with prolonged combination antimicrobial regimen is the treatment of choice.
Introduction
Prosthetic joint infections (PJI) are one of the most challenging complications of joint replacement surgery [1]. Most early onset post-operative cases are ascribed to virulent bacteria such as Staphylococcus aureus, while late-onset cases are ascribed to low virulence bacteria. Of late, NTM are being reported from several device related infections but PJI due to non-tubercular mycobacteria (NTM) are relatively under-reported [[2], [3], [4], [5], [6], [7]]. NTM, especially Mycobacterium abscessus, are capable of forming bio-films on prosthetic material and hence are difficult to treat using antibacterials alone. Removal of prosthetic material with prolonged antimicrobial combination regimen is the treatment of choice. The present case describes delay in diagnosis and arduous but successful management of bilateral PJI after total knee replacement (TKR) caused by Mycobacterium abscessus.
Case report
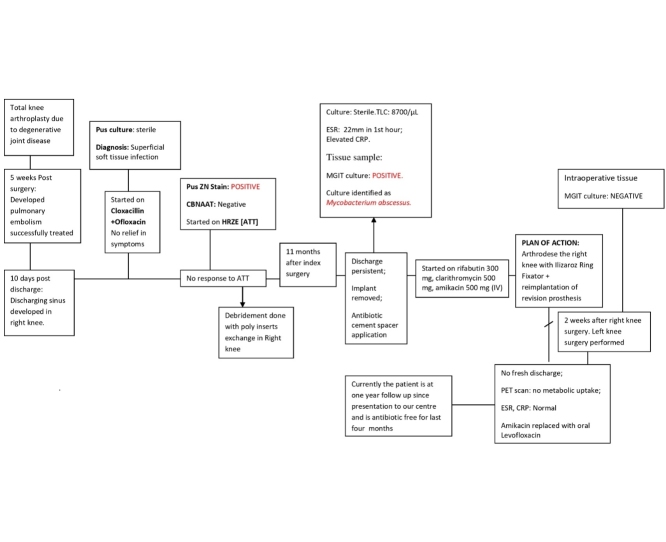
A 78-year-old diabetic and hypertensive male patient, presented with pain, swelling and discharging sinuses in both knees, 5 weeks after sequential bilateral TKR at a peripheral hospital, with no response to cloxacillin and ofloxacin. Discharge revealed acid-fast bacilli (AFB), but was negative for cartridge-based-nucleic-acid-amplification test (Xpert MTB/Rif). Empirical anti-tubercular therapy (ATT) was initiated, (rifampin, isoniazid, pyrazinamide and ethambutol) with minimal response, stopped after seven months, followed by bilateral knee debridement and poly-exchange but the discharge continued.
Eleven months after index surgery, he underwent implant removal and articulating antimicrobial cement spacer application in both knees (Fig. 1a) and was referred to our institute. He presented with difficulty in mobilization due to displaced cement spacer in both knees, thick purulent discharge from left knee and copious sero-purulent discharge from right knee. Laboratory investigations revealed Erythrocyte Sedimentation Rate (ESR) of 22 mm and a C-reactive protein (CRP) at 27 mg/L. All antibacterials were stopped, tissue samples excised from sinus edge and discharge from both knees sent for microbiological analysis.
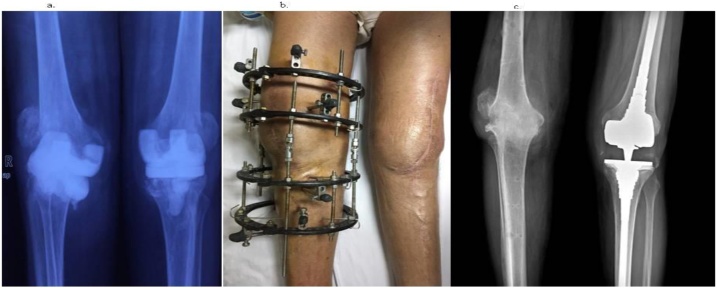
Fig. 1.
a: Radiograph of both knees in Antero-Posterior view showing cement spacer in situ. b: Clinical photograph showing Ilizarov ring fixator in right knee and clean, healed skin on left side following thorough debridement on both sides. c: Radiograph showing arthrodesed right knee and revision knee prosthesis in the left knee.
Bacteriology and mycology laboratory reports were inconclusive. In the mycobacteriology laboratory, the Ziehl Neelsen stain and Gene-Xpert MTB/RIF from the material was negative. Some growth was observed on MacConkey agar on day 3. Colonies were confirmed as AFB and grew on Lowenstein Jensen’s media with 5% NaCl. Nitrate reduction test was negative. MGIT automated liquid culture system (Becton Dickinson, NJ, USA) revealed the growth of the same phenotype. As per the growth pattern and biochemical properties the isolate was identified as Mycobacterium abscessus. The isolate was sequenced for 16S ribosomal gene and confirmed as M. abscessus, The sequence was submitted to Genbank and granted Accession No. MH720215. Drug susceptibility test was done by broth micro-dilution assay as per CLSI guidelines [8]. The isolate was sensitive to amikacin, clarithromycin, linezolid and rifampin but resistant to imipenem.
The patient was started on rifabutin 300 mg, clarithromycin 1000 mg and injection amikacin 500 mg daily. Baseline audiometry, renal function tests, ECG and liver function tests were within normal limits. Serial QTc monitoring, renal function, electrolytes and liver enzymes were closely monitored. After 3 weeks of antibacterial, thorough debridement of right knee with sinus excision, and distal femoral canal curettage followed by arthrodesis using Ilizarov ring fixator was done. After two weeks, the left knee was debrided and left with antimicrobial impregnated cement spacer in-situ. After another 8 weeks of antibacterials, the left knee was re-implanted with revision knee prosthesis and the patient was mobilized with full weight bearing on both knees. The intraoperative tissue samples on both knees revealed negative culture for mycobacteria.
Post-intervention, there was no fresh discharge from both knee joints (Fig. 1b). PET scan at end of four months revealed no metabolic uptake. Amikacin was replaced with oral levofloxacin due to bilateral sensory-neural hearing loss for higher frequencies after 6 months. By end of 7 months, his wounds were clean and dry, ESR, CRP were within normal limits. Ilizarov ring fixator was removed and knee brace used as the X-ray showed signs of trabeculae across the distal femur and proximal tibia. Currently the patient is on one-and-half year follow-up and is antimicrobial free for last four months. He is mobile without braces, is able to bear weight on both his legs for last two months, and a normal for age renal and liver functions. His latest X-rays showed bony ankylosis of right knee with stable revision knee arthroplasty implants in situ in his left knee (Fig. 1c).
Discussion
Rapidly growing mycobacteria (RGM) are ubiquitous in environment and reported worldwide. They are implicated in nosocomial infections following medical or surgical interventions leading to wound infections and sepsis. M. fortuitum, M. chelonae and M. abscessus are potentially pathogenic RGM responsible for 90% of the clinical cases [2,9]. M. abscessus complex demonstrates inducible macrolide resistance due to a novel erm gene (erm41) [10].
Mycobacterium abscessus is resistant to disinfectants and therefore can cause post-surgical and post-procedural infections. It is associated with a wide range of skin and subcutaneous tissue, respiratory tract, central nervous system, pulmonary and ocular infections, bacteremia and disseminated infections [[11], [12], [13], [14], [15], [16]]. Possible sources for nosocomial outbreaks include contaminated saline, disinfectants or surgical equipments and contact transmission between patients [14].
Incidence of PJI ranges from 2.05% to 2.18% after total knee replacement (TKR) with primary arthroplasties [17]. There are 43 cases of PJI reported by RGM, including 17 cases of knee-PJI caused by RGM, including Mycobacterium chelonae, M. smegmatis, M. fortuitum, M. wolinskyi and M. abscessus [1,2,10]. M. abscessus is reported in eight cases, one from India [2,3,[5], [6], [7],9]. (Table 1). Diagnosis is frequently delayed due to lack of clinical suspicion or lack of appropriate laboratory support.
Table 1.
Summary of all the cases of prosthetic knee joint infections due to Mycobacterium abscessus reported in the literature.
| S.no | Author’s Reference | Age/sex | Underlying Disease | Region | Arthroplasty & period of prosthetic Joint Infection | Organism Cultured | Antibiotic Regimen | Surgical Intervention | Final Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Eid AJ et al. | 71/F | Rheumatoid arthritis | Not known | Knee (652 days) | M. abscessus | CFX & CLR (2 weeks) | Resection arthroplasty | Palliative care (3 weeks) |
| 2 | Ryu SW et al. | 58/F | Degenerative joint disease | 2001, Korea | Left Knee arthroplasty (21 days) | M. abscessus | Kanamycin+Clarithromycin+ Pyrazinamide(9months) | Debridement | Palliative care (not known) |
| Amk (2 mnts Perioperatively) | |||||||||
| 3 | Wang SX et al | 72 /F | Intra articular steroid injection& osteoarthritis | 2011, Taiwan | Right Knee arthroplasty (5 months) | M. abscessus & M. fortuitum | Doxycycline+Ciprofloxacin+ Clarithromycin(5 months) | Reimplantation at 4 months | No relapse (43 weeks) |
| Amk (2 mnts Perioperatively) | |||||||||
| 4 | Amit P et al | 71/F | Degenerative joint disease | 2010, New Delhi, India | Right Knee arthroplasty (2 years) | M. abscessus | CLR+LEV+AMK(3 WEEKS) | Resection arthroplasty | No Relapse |
| CLR+LEV+IMP(6 WEEKS) | Debridement at 4.5 months | Follow up 104 weeks | |||||||
| CLR+LEV (13 WEEKS) | Reimplantation at 6.5 months | ||||||||
| 6 | Kim et al | 83/F | Degenerative joint disease | 2017, South Korea | Right Knee arthroplasty (18 Days) | M. abscessus | Cefoxitin (IV)Clarithromycin+ Amikacin (IV)Moxifloxacin (6months) | Resection arthroplasty | No Relapse |
| Debridement at 7 months | Follow up 4 years and 3months | ||||||||
| Reinfection after 10 months of surgery(Open debridement and polyethylene insert exchange) | |||||||||
| 71/F | Degenerative joint disease | 2017, South Korea | Right Knee arthroplasty (13 month) | M. abscessus | ATT (6weeks)Cefoxitin (IV) | open debridement, open debridement with removal of prosthesesand insertion of antibiotic cement spacers, Revision TKA, open debridement (7Months) | No relapse | ||
| Clarithromycin+Amikacin (IV) | Follow up 2 years | ||||||||
| (Cefoxitin replaced by tigecycline) (6mnts+10 months) | Reimplantation (15 months after revision TKA) | ||||||||
| 7 | Spanyer et al | 61/F | Knee arthritis | 2018, Boston, USA | Left Knee arthroplasty (9 days) | M. abscessus | (IV) cefoxitin, oral clarithromycin, and thrice-weekly intravenous amikacin. (15 weeks) | Resection arthroplasty | No relapse |
| Debridement at 4.5 months | Follow up 4 years | ||||||||
| Reimplantation at 3.5 months | |||||||||
| 8 | Present Case | 78/M | Degenerative Joint disease | New Delhi, India 2018 | Bilateral Total Knee arthroplasty | M. abscessus | Rifabutin + Clarithromycin+ Amikacin (IV) | Debridement at 4 months | No relapse |
| Prosthesis removal and antibiotic spacer insertion 11 months | Follow up 1 year | ||||||||
| Arthrodesis of the right knee | |||||||||
| Reimplantation left knee after 2 months |
This case reported discharge from surgical wounds, without fever since the TKR. Mycobacterial infection was suspected considering his prolonged illness and repeated negative bacterial cultures. Clear temporal association of symptoms with surgery and involvement of both knees, with a typical presentation suggestive of RGM, suggests that infection was likely acquired from the hospital setting during or after initial intervention.
RGM infections are resistant to several groups of antibacterials and combination therapy of oral macrolides with parenteral medications (amikacin, cefoxitin or imipenem) is advocated for serious bone and soft tissue infections by M. abscessus. Isolate in this case was resistant to imipenem and there is limited availability of cefoxitin in India. Rifabutin is active against M. abscessus and inhibits clarithromycin resistant strains [18], hence regimen was designed with rifabutin, clarithromycin and intravenous amikacin. There are no guidelines for the optimal empiric therapy or duration for PJI caused by RGMs.
PJI due to Mycobacterium abscessus have been infrequently reported and no specific guidelines exist to inform treatment. M. abscessus is resistant to disinfectants, intrinsically resistant to first line ATT and increasingly reported resistant to macrolides; are known to form biofilms on prosthetic material and hence difficult to eradicate. High clinical suspicion, accurate laboratory diagnosis, combination of surgical and carefully designed medical regimen can eradicate non-responding PJI even after delayed diagnosis and a long course of infection.
Conflict of interest
None.
Research funding
None.
Consent
Written informed consent has been taken from the patient regarding publication of the case report and is available for review as per the request of Editor-in-chief.
References
- 1.Kurtz S.M., Lau E., Watson H., Schmier J.K., Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8):e1. doi: 10.1016/j.arth.2012.02.022. Suppl: 61–65. [DOI] [PubMed] [Google Scholar]
- 2.Eid A.J., Berbari E.F., Sia I.G., Wengenack N.L., Osmon D.R., Razonable R.R. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;15;45(September (6)):687–694. doi: 10.1086/520982. [DOI] [PubMed] [Google Scholar]
- 3.Ryu S.W., Lee C.K., Heo J., Shin K.S., Kim J.S., Bae S.Y. A case of knee joint infection by Mycobacterium abscessus. Korean J Clin Pathol. 2001;21:371–376. [Google Scholar]
- 4.Petrosoniak A., Kim P., Desjardins M., Lee B.C. Successful treatment of a prosthetic joint infection due to Mycobacterium abscessus. Can J Infect Dis Med Microbiol. 2009;20:e94–6. doi: 10.1155/2009/968052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S.X., Yang C.J., Chen Y.C., Lay C.J., Tsai C.C. Septic arthritis caused by Mycobacterium fortuitum and Mycobacterium abscessus in a prosthetic knee joint: case report and review of literature. Intern Med. 2011;50:2227–2232. doi: 10.2169/internalmedicine.50.5610. [DOI] [PubMed] [Google Scholar]
- 6.Amit P., Rastogi S., Marya S. Prosthetic knee joint infection due to Mycobacterium abscessus. Indian J Orthopaedics. 2017;51(3):337–342. doi: 10.4103/0019-5413.205685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M., Ha C.W., Jang J.W., Park Y.B. Rapidly growing non-tuberculous mycobacteria infection of prosthetic knee joints: a report of two cases. Knee. 2017;24(4):869–875. doi: 10.1016/j.knee.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards . NCCLS; Wayne, PA: 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved Standard. Document No. M24-A. [PubMed] [Google Scholar]
- 9.Spanyer J.M., Foster S., Thum-DiCesare J.A., Kwon Y.M., Burke D.W., Nelson S.B. Mycobacterium abscessus: a rare cause of periprosthetic knee joint infection. Am J Orthop (Belle Mead NJ) 2018;47(9) doi: 10.12788/ajo.2018.0077. [DOI] [PubMed] [Google Scholar]
- 10.Lee M.-R., Sheng W.-H., Hung C.-C., Yu C-J Lee L.-N., Hsueh P.-R. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis. 2015;21(9):1638–1646. doi: 10.3201/2109.141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tande A.J., Patel R. Prosthetic Joint Infection. Clin Microbiol Rev. 2014;27(2):302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jitmuang A., Yuenyongviwat V., Charoencholvanich K., Chayakulkeeree M. Rapidly-growing mycobacterial infection: a recognized cause of early-onset prosthetic joint infection. BMC Infect Dis. 2017;28;17(December(1)):802. doi: 10.1186/s12879-017-2926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kothavade R.J., Dhurat R.S., Mishra S.N., Kothavade U.R. Clinical and laboratory aspects of the diagnosis and management of cutaneous and subcutaneous infections caused by rapidly growing mycobacteria. Eur J Clin Microbiol Infect Dis. 2013;32:161–188. doi: 10.1007/s10096-012-1766-8. [DOI] [PubMed] [Google Scholar]
- 14.Viana-Niero C., Lima K.V., Lopes M.L., Rabello M.C., Marsola L.R., Brilhante V.C. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J Clin Microbiol. 2008;46:850–855. doi: 10.1128/JCM.02052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 16.Girgis D.O., Karp C.L., Miller D. Ocular infections caused by non-tuberculous mycobacteria: update on epidemiology and management. Clin Experiment Ophthalmol. 2012;40:467–475. doi: 10.1111/j.1442-9071.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Renz N., Trampuz A. Management of periprosthetic joint infection. Hip Pelvis. 2018;30(September (3)):138–146. doi: 10.5371/hp.2018.30.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz D.B., Low J.L., Wu M.-L., Gengenbacher M., Teo J.W.P., Dartois V., Dick T. Rifabutin is active against Mycobacterium abscessus complex. Antimicrob Agents Chemother. 2017;61:e00155–17. doi: 10.1128/AAC.00155-17. [DOI] [PMC free article] [PubMed] [Google Scholar]