Abstract
Fecal calprotectin (FC) is a highly sensitive disease activity biomarker in inflammatory bowel disease. However, there are conflicting reports on whether the diagnostic accuracy in Crohn’s disease is influenced by disease location. The aim of this study was to undertake a systematic review of the published literature. Relevant databases were searched from inception to November 8, 2016 for cohort and case control studies which had data on FC in patients with isolated small bowel (SB) and large bowel (LB) Crohn’s disease. Reference standards for disease activity were endoscopy, magnetic resonance imaging, computed tomography or a combination of these. The QUADAS-2 research tool was used to assess the risk of bias. There were 5,619 records identified at initial search. The 2,098 duplicates were removed and 3,521 records screened. Sixty-one full text articles were assessed for eligibility and 16 studies were included in the final review with sensitivities and specificities per disease location available from 8 studies. Sensitivities of FC at SB and LB locations ranged from 42.9% to 100% and 66.7% to 100% respectively while corresponding specificities were 50% to 100% and 28.6% to 100% respectively. The sensitivities and specificities of FC to accurately measure disease activity in Crohn’s disease at different disease locations are diverse and no firm conclusion can be made. Better studies need to be undertaken to categorically answer the effect of disease location on the diagnostic accuracy of FC.
Keywords: Crohn disease, Disease activity, Fecal calprotectin
INTRODUCTION
Crohn’s disease (CD) is a chronic disorder characterized by transmural inflammation and patchy distribution in the GI tract. The importance of assessing ongoing GI mucosal inflammation in this condition lies in the fact that it helps predict course of disease [1-7], response to therapy [1], advent of complications [7], need for hospitalization and surgery [4]. To this effect, various studies have shown mucosal healing to be the best predictor of positive long-term outcomes [3-6]. Endoscopy is currently regarded as the gold standard test for assessment of mucosal healing [8]. However, it is expensive, invasive, associated with patient discomfort and has an associated small risk of serious complications, thus making it an unfeasible modality for frequent monitoring. Biochemical markers like CRP are inexpensive but have moderate diagnostic accuracy with a specificity of 0.92 (95% CI, 0.72–0.96) but a sensitivity of only 0.49 (95% CI, 0.34–0.64) [9], hence limiting its use as a disease biomarker.
Since the acutely inflamed intestinal mucosa is deemed to be neutrophil–rich, fecal tests based on neutrophil-derived markers are a realistic option for assessing mucosal inflammation. Among the various fecal markers of intestinal inflammation; fecal calprotectin (FC) is the one most commonly used in clinical practice [10]. FC has a sensitivity of 87% and specificity of 67% when used to detect endoscopic activity in symptomatic CD [9]. It accurately predicts the response to therapy as well as 1-year risk of relapse [11-13]. There are though conflicting reports on whether the diagnostic accuracy in CD is influenced by disease location. FC has been shown to have a lower specificity in CD than in UC and this might be driven through the different disease locations [14-16]. Some studies report that the FC level is lower in small bowel (SB) disease location compared to large bowel (LB) location [17,18], while others did not observe any difference [14,19]. We feel this is an important matter that could potentially either change practice or serve as a basis for downstream research. We thus aimed to undertake a systematic review of published literature and discuss the effect of disease location on the sensitivity and specificity of FC to accurately measure disease activity in CD.
METHODS
1. Criteria for Inclusion and Exclusion
Case control and cohort studies that provided data on FC separately by SB and LB locations were selected. Only those studies which had clearly mentioned the use of endoscopy, MRI, CT or a combination of these modalities as reference standard to assess disease activity were included [8,20]. The subjects included both adult and pediatric patients who had been diagnosed with CD on the basis of their clinical symptoms and supporting investigations (endoscopy, biopsies, imaging, blood and stool tests). We also included studies in which healthy volunteers and subjects with IBS were recruited as controls. We excluded studies focusing only on SB-CD and studies where the reference standard for activity used was based on clinical or biochemical criteria. We also excluded studies specifically dealing with postoperative CD as it would not have been possible to define the disease location as SB or LB if the recurrence was limited to the anastomosis.
2. Search Strategy
Our search included Medline, Embase, Web of Science and Cochrane Library from inception up to November 8, 2016 with the help of a senior librarian to obtain the appropriate studies. There were no language or publication restrictions applied while searching. Details of the search strategy are provided in the Supplementary Material 1.
Conference proceedings from Digestive Diseases Week, United European Gastroenterology Week, European Crohn’s and Colitis Organisation (ECCO) and British Society of Gastroenterology annual meetings over the past 12 years (2005–2016) were also searched for relevant additional studies. We performed a manual search from references in the included studies and pertinent review articles. We also searched the Grey Literature Database OpenGrey to check for eligible studies.
3. Selection
The selected studies were initially screened for eligibility by 3 authors (E.G.S., R.W., and A.A.T.). The abstracts were reviewed and those eligible were included for full text review. The full manuscripts were independently assessed (E.G.S. and G. W.M.) as per the inclusion criteria. If there were any disagreements, these were resolved by discussion and consensus with the other authors (S.S., R.W., and A.A.T.). Studies published only in abstract format were included as long as inclusion criteria were satisfied.
4. Data Extraction
Two authors (E.G.S. and G.W.M.) independently completed the data extraction forms for studies in the final selection list. The following data was collated: general information (journal, year, author, title), publication type (full paper or abstract), location, number of centers involved, study design (prospective/cross-sectional), total number of CD subjects and stratification based on disease location, age group (adult/pediatric/both), follow up period in months, FC levels with cutoff, clinical disease activity index, relevant reference standard (with appropriate disease activity score if provided), number of true positives (TP), true negatives (TN), false positives (FP) and false negatives (FN) and miscellaneous details. If any of the selected studies had missing data or needed clarification, multiple attempts through electronic mail were made to contact the authors to furnish the same.
5. Risk of Bias
To assess the risk of bias, QUADAS-2 was used (Supplementary Material 2). This is a research tool to check the quality of systematic reviews of diagnostic accuracy studies [21]. This was assessed independently by 2 authors (E.G.S. and G.W.M.) while any disagreement was resolved by consensus with coauthors (S.S., R.W., and A.A.T.).
6. Data Synthesis
Sensitivity and specificity in the SB and LB locations were separately derived by calculation from the information provided (i.e., TP, TN, FP, and FN) or as reported in the published literature.
RESULTS
The electronic data base search on November 8, 2016 identified 5,619 results. After the removal of 2,098 duplicates, 3,521 records were screened for inclusion. From the latter, 61 studies were deemed to be relevant and subjected to full text review. Thereafter, 45 studies [12,13,15,22-63] were excluded either because the numerical data on FC at SB and LB locations were not separately available or because the reference standards used did not conform to inclusion criteria. Finally, 16 studies were included in the qualitative review (Fig. 1) involving 328 patients with SB-CD and 332 patients with LB disease location.
Fig. 1.
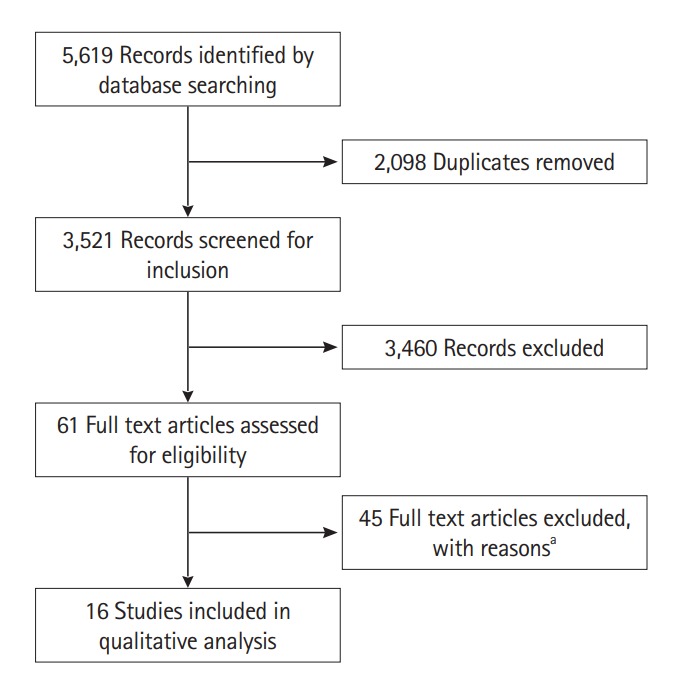
PRISMA flow diagram. aSixteen studies, numerical data not available for fecal calprotectin (FC) at large bowel and small bowel locations separately; 16 studies, reference standards for assessment of disease activity were different from those mentioned in inclusion criteria; 13 studies, both numerical data for FC at the 2 locations were not separately available and reference standards used for assessment of disease activity were different from those mentioned in inclusion criteria. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
1. Study Demographics
Fourteen relevant cross-sectional [17,19,64-75] and 2 prospective studies [14,76] were published between 2008 and 2016 (Table 1). Four of the 16 studies were published as conference abstracts [65,69,73,74] although 1 of these was subsequently published as a full text article [66]. Almost all the studies were single/dualcenter based other than the studies by Faubion et al. [66] and Lin et al. [76] which were multi-center. All the studies were performed in Europe and North America apart from a single study originating from Asia [76]. The majority of the studies involved adult subjects. The study by Jones et al. [64] included 23 subjects who were less than 16 years while the youngest subject in the study by Jensen et al. [19] was 16 years. With regard to the reference standards utilized; 11 studies used endoscopy, 2 used endoscopy and CT in combination while there was one each for MRI alone, endoscopy and MRI in combination and a composite assessment of endoscopy/capsule endoscopy/surgery (Table 1).
Table 1.
Study Characteristics
| Study | Design | Patient spectrum | SB Crohn's | LB Crohn's | Reference standard/scoring system used |
|---|---|---|---|---|---|
| af Björkesten [14] | Prospective | Anti-TNF treated luminal Crohn’s | 33 | 50 | SES-CD |
| Faubion [65] | Cross-sectional | Crohn’s cohort on follow-up | 22 | 12 | ICO-CTE score |
| Gecse [17] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 9 | 20 | SES-CD |
| Jensen [19] | Cross-sectional | Suspected Crohn’s under evaluation | 13 | 16 | Endoscopy/capsule/surgery |
| Jones [64] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 53 | 40 | SES-CD |
| Lobatón [67] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 26 | 45 | CDEIS |
| Makanyanga [68] | Cross-sectional | Crohn’s cohort on follow-up | 18 | 15 | MEGS |
| Maltz [69] | Cross-sectional | Crohn’s cohort on follow-up | 9 | 18 | Endoscopy |
| Schoepfer [70] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 35 | 20 | SES-CD |
| Sipponen [71] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 16 | 17 | SES-CD |
| Sipponen [18] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 19 | 14 | CDEIS |
| Stawczyk-Eder [72] | Cross-sectional | Hospitalized Crohn’s | 44 | 22 | SES-CD |
| Zittan [73] | Cross-sectional | Crohn’s cohort on follow-up | 14 | 23 | SES-CD, MaRIA |
| Moniuszko [74] | Cross-sectional | Hospitalized Crohn’s | NA | NA | SES-CD, CT enteroclysis |
| Goutorbe [75] | Cross-sectional | Crohn’s undergoing ileocolonoscopy | 13 | 12 | CDEIS |
| Lin [76] | Prospective | Crohn’s cohort on follow-up | 4 | 8 | CDEIS |
SB, small bowel; LB, large bowel; SES-CD, Simple Endoscopic Score for Crohn’s Disease; ICO-CTE, ileocolonoscopy and CT enterography; CDEIS, Crohn’s Disease Endoscopic Index of Severity; MEGS, MRI enterography global score; MaRIA, magnetic resonance index of activity; NA, not available.
2. Risk of Bias Assessment
With regard to QUADAS-2 risk assessments of the selected studies (Table 2), only a single study [75] scored low in all 4 domains of risk of bias and the domain of concern for applicability. There were again just 3 studies [64,71,76] by which scored low in 3 domains of risk of bias. Most studies had an unclear risk of bias in patient selection. With respect to the index test, there were 3 studies that had high risk [67,68,72] while one [69] was unclear. The studies were almost evenly distributed between low and unclear risk with regard to the reference standard. Eight studies had either high or unclear risk of bias under subject flow and selection [18,64,66-68,70,73,74]. There were just 6 studies [64,67,70,72,75,76] which had low concern for applicability under subject selection.
Table 2.
QUADAS-2 Risk Assessment for the Selected Studies
| Study | Risk of bias |
Applicability concerns |
|||||
|---|---|---|---|---|---|---|---|
| Subject selection | Index test | Reference standard | Flow and timing | Subject selection | Index test | Reference standard | |
| af Björkesten [14] | Low | Low | Low | Low | High | NC | NC |
| Faubion [66] | Unclear | Low | Unclear | Unclear | High | NC | NC |
| Gecse [17] | High | Low | Unclear | Low | Unclear | NC | NC |
| Jensen [19] | Unclear | Low | Unclear | High | High | NC | NC |
| Jones [64] | Unclear | Low | Low | Low | Low | NC | NC |
| Lobatón [67] | Low | High | Low | High | Low | NC | NC |
| Makanyanga [68] | Unclear | High | Low | Unclear | High | NC | NC |
| Maltz [69] | Unclear | Unclear | Unclear | Low | Unclear | NC | NC |
| Schoepfer [70] | High | Low | Low | Unclear | Low | NC | NC |
| Sipponen [71] | Unclear | Low | Low | Low | Unclear | NC | NC |
| Sipponen [18] | Unclear | Low | Unclear | Unclear | Unclear | NC | NC |
| Stawczyk-Eder [72] | Unclear | High | Low | Low | Low | NC | NC |
| Zittan [73] | Unclear | Low | Unclear | Unclear | Unclear | NC | NC |
| Moniuszko [74] | Unclear | Low | Unclear | Unclear | Unclear | NC | NC |
| Goutorbe [75] | Low | Low | Low | Low | Low | NC | NC |
| Lin [76] | Unclear | Low | Low | Low | Low | NC | NC |
NC, not a concern.
3. Sensitivity and Specificity of FC by Location
The data on the effect of disease location on FC is heterogeneous (Table 3). Some studies [17,18,67,69] showed that the FC was significantly higher in LB vs SB location while others [14,19,68,70,74-76] did not corroborate this finding, though absolute values have limited value.
Table 3.
Comparison of Mean or Median Levels in the 2 Locations
| Study | SB Crohn’s | LB Crohn’s | Reference standard/scoring system used | Key result | Inference |
|---|---|---|---|---|---|
| af Björkesten [14] | 33 | 50 | SES-CD | Median FC level in SB: 86 µg/g | No difference between both locations |
| LB: 158 µg/g (NS) | |||||
| Gecse [17] | 9 | 20 | SES-CD | Mean FC level in SB: 297±81 µg/g | Higher in LB location |
| LB: 1,523±97 µg/g (P<0.0001) | |||||
| Jensen [19] | 13 | 16 | Endoscopy/capsule/surgery | Median FC level in SB: 890 mg/kg | No difference between both locations |
| LB: 830 mg/kg (NS) | |||||
| Lobatón [67] | 26 | 45 | CDEIS | Median FC level in SB: 420.5 µg/g | Higher in LB location |
| LB: 1,297 µg/g (P=0.013) | |||||
| Makanyanga [68] | 18 | 15 | MEGS | Mean FC level in SB: 319.1 μg/g | No difference between both locations |
| LB: 342 μg/g (NS) | |||||
| Maltz [69] | 9 | 18 | Endoscopy | Median FC level in SB: 442 µg/g | Higher in LB location |
| LB: 66 µg/g (P<0.002) | |||||
| Schoepfer [70] | 35 | 20 | SES-CD | Mean FC level in SB: 287±279 μg/g | No difference between both locations |
| LB: 401±312 μg/g (NS) | |||||
| Sipponen [18] | 19 | 14 | CDEIS | Median FC level in SB: 180 µg/g | Higher in LB location |
| LB: 1,383 µg/g (P=0.017) | |||||
| Moniuszko [74] | NA | NA | SES-CD, CT enteroclysis | Median FC level in SB: 195 μg/g | No difference between both locations |
| LB: 401 μg/g (NS) | |||||
| Goutorbe [75] | 13 | 12 | CDEIS | Median FC level in SB: 841 µg/g | No difference between both locations |
| LB: 1,575.5 µg/g (NS) | |||||
| Lin [76] | 4 | 8 | CDEIS | Median FC level in SB: 2,693 μg/g | No difference between both locations |
| LB: 176 μg/g (NS) |
SB, small bowel; LB, large bowel; SES-CD, Simple Endoscopic Score for Crohn’s Disease; FC, fecal calprotectin; CDEIS, Crohn’s Disease Endoscopic Index of Severity; MEGS, MRI enterography global score; NA, not available.
The studies by Jones et al. [64], Sipponen et al. [71], and Zittan et al. [73] showed that FC significantly correlated with the reference standard only at the LB location but not at the SB location while the other 2 studies [67,72] showed that FC correlated with the reference standard at both the locations (Table 4). The reference standard used in these studies was endoscopy with the scoring system being either Simple Endoscopic Score for Crohn’s Disease (SES-CD) [64,71-73], Crohn’s Disease Endoscopic Index of Severity (CDEIS) [67] although in the study by Zittan et al. [73], MR enterography score (MaRIA, magnetic resonance index of activity) was also used in the SB location.
Table 4.
Correlation between Fecal Calprotectin and Reference Standard at Respective Locations
| Study | SB Crohn’s | LB Crohn’s | Reference standard/scoring system used | Key result | Comment |
|---|---|---|---|---|---|
| Jones [64] | 53 | 40 | SES-CD | Correlation in | Correlation noted only at LB location |
| SB: -0.01 (NS) | |||||
| LB: 0.8 (P<0.05) | |||||
| Lobatón [67] | 26 | 45 | CDEIS | Correlation in | - |
| SB: 0.437 (P=0.016) | |||||
| LB: 0.725 (P<0.01) | |||||
| Sipponen [71] | 16 | 17 | SES-CD | Correlation in | Correlation noted only at LB location |
| SB: 0.317 (NS) | |||||
| LB: 0.642 (P<0.01) | |||||
| Stawczyk-Eder [72] | 44 | 22 | SES-CD | Correlation with SES-CD in | - |
| SB: 0.78 (P<0.0001) | |||||
| LB: 0.78 (P<0.0002) | |||||
| Zittan [73] | 14 | 23 | SES-CD, MaRIA | Correlation in | Correlation noted only at LB location |
| SB: 0.4 (P=NS) | |||||
| LB: 0.61 (P<0.0001) |
SB, small bowel; LB, large bowel; SES-CD, Simple Endoscopic Score for Crohn’s Disease; CDEIS, Crohn’s Disease Endoscopic Index of Severity; MaRIA, magnetic resonance index of activity.
The sensitivity and specificity data were available for 8 studies in total (Table 5). Sensitivities were available in the published literature for just 2 studies [19,67] while in 1 study [73], these were retrospectively provided by the author. For the remaining 5 studies [14,17,66,74,75], the relevant authors provided the raw data on the number of TP, TN, FP and FN, from which the sensitivity and specificity values were retrospectively calculated.
Table 5.
Diagnostic Accuracy of Fecal Calprotectin in CD at SB versus LB Location
| Study | Sensitivity |
Specificity |
FC cutoff (μg/g) | ||
|---|---|---|---|---|---|
| SB (95% CI) | LB (95% CI) | SB (95% CI) | LB (95% CI) | ||
| Jensen [19]a | 92 | 94 | NA | NA | 50 |
| Lobatón [67]a | 63 | 79 | 100 | 100 | 272 |
| Zittan [79]a | 75 | 100 | 50 | 67 | 100 |
| af Björkesten [14] | 60.0 (32.9–82.5) | 78.9 (53.9–93.0) | 100.0 (62.9–100.0) | 75.0 (35.6–95.5) | 100 |
| Faubion [66] | 76.9 (46.0–93.8) | 80.0 (29.9–98.9) | 75.0 (35.6–95.5) | 28.6 (5.1–69.7) | 100 |
| Gecse [17] | 42.9 (11.8–79.8) | 100.0 (78.1–100.0) | NA | 100.0 (5.5–100.0) | 200 |
| Goutorbe [75] | 100 (51.7–100) | 100.0 (62.9–100.0) | 50.0 (13.9–86.0) | 33.3 (1.8–87.5) | 200 |
| Moniuszko [74] | 100 (31.0–100) | 66.7 (30.9–91.0) | 100.0 (31.0–100.0) | 50.0 (2.7–97.3) | 238 |
All unit of data is percent.
Raw data and associated CI are not available.
SB, small bowel; LB, large bowel; FC, fecal calprotectin; NA, not available.
Including data from all the 8 studies, the sensitivity and specificity of FC in the SB ranged from 42.9% to 100% and from 50% to 100% respectively. The sensitivity and specificity of FC in the LB ranged from 66.7% to 100% and from 28.6% to 100% respectively.
DISCUSSION
A variety of clinical studies have indicated a wide range of sensitivities and specificities for FC in CD at different disease locations [14,17-19]. We have undertaken a systematic review to objectively appraise the literature. Overall, the sensitivity and specificity of FC in the SB ranged from 42.9% to 100% and 50% to 100% while those in the LB were from 66.7% to 100% and 28.6% to 100% respectively indicating that FC may be equally useful to measure disease activity in CD at these 2 locations but no firm conclusion can be made from the published literature. The QUADAS -2 tool indicated that the quality of the selected studies was modest.
The data represented here is heterogeneous with varying gold-standards. There are only 5 studies in the published literature with the primary aim of investigating the effect of disease location on the sensitivity and specificity of FC [17,19,72-74]. In the remaining eleven studies, this information was expressed as a sub-analysis. Moreover, apart from the published data, raw data to calculate sensitivity and specificity was only available in 5 small studies. These data did not pertain to all the cohorts published but only relevant to smaller sub-groups [14,17,65,74,75]. One might speculate that LB disease location is within reach of colonoscopy and hence is more commonly validated with a gold-standard investigation. As for SB disease location, unless the disease is in the terminal ileum this might not be as accurately located though the sensitivities and specificities of MRI to measure disease activity is widely published [77]. A possible reason for the effect of disease location on the specificity of FC might be that other common disease of the colon such as diverticulitis, microscopic colitis or infectious enteritides might raise FC other than LB-CD. The same might not be said for SB inflammation in cohort studies undertaken in the Western Hemisphere where CD is the commonest cause for ileal inflammation. Effectively, this systematic analysis highlights the need of properly designed prospective studies to answer this important question.
Despite endoscopy being the gold standard for assessment of disease activity, we also included studies where radiological tests such as CT or MRI were utilized as reference standards to evaluate the SB activity as these have been supported by the ECCO guidelines [8,20]. However, the lack of a uniform gold standard was a limiting factor. This heterogeneity multiplied by the inter-observer variability for the various investigative modalities used, limited the validity of the reported sensitivities and specificities. The limitations of CT and MRI may include decreased sensitivity to detect early disease that may otherwise be detected on endoscopy. Even in those studies that have used endoscopy as the reference standard, various scoring systems such as the SES-CD and the CDEIS scores were utilized. These scoring systems themselves have limitations such as the endoscopic evaluation being confined to the terminal ileum or colon subject to the reach of the colonoscope and inter-observer variability. Capsule endoscopy is a non-invasive way to evaluate the entire SB. However, its disadvantages include lack of utility when there is a SB stricture as well as subjective nature of reporting.
There are certain limitations in the published literature that need to be highlighted. The FC cutoffs used in all the reported studies are different. The cutoff values can influence the test accuracy and there are different cutoff values for FC depending on the intent of use. The current National Institute for Health and Care Excellence (NICE) guideline [78] indicates that an FC value <50 μg/g suggest no significant GI mucosal inflammation, with a value of >250 μg/g corresponds well with endoscopic and histology activity [9,79]. The cutoff values used in the studies presented in this systematic review were not uniform. Most of the studies used cutoff of 100 μg/g with just 3 studies using a cutoff value of 50 μg/g. The diagnostic test used to determine the FC levels were not uniform. Most studies used ELISA test while some used the rapid test (Quantum Blue). Stool collection time was not standardized across the studies described in this systematic review. There was a paucity of detail regarding processing of the stool samples across the studies. These factors could also contribute to differences of FC across the studies.
Our systematic review included both pediatric and adult studies though most of the data was from the adult population and the pediatric population appeared under-represented. The specificity of FC appears to improve with patient age. van Rheenen et al. [80], in their meta-analysis of 13 studies, obtained a pooled sensitivity of 93% and specificity of 96% in adults and 92% and 76% in children respectively. The larger share of irritable bowel disease with absence of alarm symptoms was thought to overestimate the specificity in the adults subjects compared to children. Henderson et al. [54] undertook a metaanalysis of 8 studies and concluded that the sensitivity and specificity of FC in IBD in the pediatric cohort were 97.8% and 68.2%. Factors that could contribute to the difference in specificity of FC in adult versus pediatric populations include the variation in the disease prevalence and spectrum, variation in the FC threshold to trigger endoscopic evaluation, parental expectation and concerns about missed diagnosis [54]. The pediatric cohort in this systematic review was too small to be able to make any firm conclusions.
We observed that most of the studies originated from the Western Hemisphere except for the study from Taiwan [76], perhaps indicating that these findings may not be reflective of the situation in the general population worldwide. It would be difficult to get homogenous world-wide data on the accuracy of FC in SB and LB locations due to differences in incidence and prevalence of IBD across regions [81].
This systematic review has some major strengths. We had undertaken a comprehensive search including important online databases (Medline, Embase, Web of Science, and Cochrane Library). We had no language or publication restrictions. Moreover, relevant conference proceedings were searched since 2005 to ensure no publication bias was introduced within our search. We excluded studies that were merely restricted to SB-CD since we also needed information from the LB in order to compare. We excluded those studies solely describing postoperative cohorts to exclude the effect of non-IBD related anastomotic ulceration on the analysis. Moreover, since the raw figures (i.e., TP, TN, FP and FN in both SB and LB locations) of the selected studies were not provided in the original published manuscripts, electronic communication with relevant study authors was undertaken as part of our data extraction process for this systematic review.
The range of sensitivities and specificities for FC by disease location are variable and incomparable. As the gold standard comparators used in various studies are heterogeneous it has not been possible to pool the data and calculate common variables for FC. Prospective cohort studies with common comparators and similar quantification methodologies for FC are needed to answer this question; in order to better understand the right place for FC as a disease monitoring tool.
Footnotes
FINANCIAL SUPPORT
The authors received no financial support for the research, authorship, and/or publication of this article.
CONFLICT OF INTEREST
E.G.S. was supported through the NIHR Nottingham Digestive Diseases Biomedical Research Centre, Nottingham University Hospitals NHS Trust and University of Nottingham. S.S. has received consultancy fees from Falk; speaker fees from MSD and financial support for educational activities from Merck Sharp, Dohme Ltd, Abbvie and Ferring. G.W.M has received educational support from Abbvie, Janssen, NAPP, Takeda Pharmaceuticals, Merck Sharp & Dohme Ltd, Ferring and Dr Falk; speaker honoraria from Merck Sharp & Dohme Ltd, Abbvie, Janssen, Ferring and Takeda Pharmaceuticals and is on the Advisory boards for Abbvie, Takeda Pharmaceuticals, Janssen and Dr Falk. R.W., A.A.T., and J.E. have no conflicts of interest to declare.
AUTHOR CONTRIBUTION
Simon EG: conception & design of the study; data acquisition, analysis & interpretation; drafting and revising the article; final approval. Wardle R, Thi AA, Eldridge J, and Samuel S: data interpretation; revising the article; final approval. Moran GW: conception & design of the study; data interpretation; revising the article; final approval. All authors have approved the final version of the manuscript.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1.Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut. 1984;25:665–672. doi: 10.1136/gut.25.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 3.Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412–422. doi: 10.1053/j.gastro.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–1301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- 5.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:414–422. doi: 10.1016/j.cgh.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 7.Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947–953. doi: 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]
- 8.Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110:802–819. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol. 2015;8:23–36. doi: 10.1177/1756283X14553384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sipponen T, Savilahti E, Karkkainen P, et al. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn’s disease. Inflamm Bowel Dis. 2008;14:1392–1398. doi: 10.1002/ibd.20490. [DOI] [PubMed] [Google Scholar]
- 12.Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009;15:1190–1198. doi: 10.1002/ibd.20933. [DOI] [PubMed] [Google Scholar]
- 13.D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008;103:2007–2014. doi: 10.1111/j.1572-0241.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 14.af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528–537. doi: 10.3109/00365521.2012.660542. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K, Aomatsu T, Yoden A, Okuhira T, Kaji E, Tamai H. Usefulness of a novel and rapid assay system for fecal calprotectin in pediatric patients with inflammatory bowel diseases. J Gastroenterol Hepatol. 2014;29:1406–1412. doi: 10.1111/jgh.12578. [DOI] [PubMed] [Google Scholar]
- 16.D’Incà R, Dal Pont E, Di Leo V, et al. Calprotectin and lactoferrin in the assessment of intestinal inflammation and organic disease. Int J Colorectal Dis. 2007;22:429–437. doi: 10.1007/s00384-006-0159-9. [DOI] [PubMed] [Google Scholar]
- 17.Gecse KB, Brandse JF, van Wilpe S, et al. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand J Gastroenterol. 2015;50:841–847. doi: 10.3109/00365521.2015.1008035. [DOI] [PubMed] [Google Scholar]
- 18.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46:694–700. doi: 10.3109/00365521.2011.560680. [DOI] [PubMed] [Google Scholar]
- 20.Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 22.Astle VI, Lewis NR. PWE-116 Association of faecal calprotectin with extent and distribution of inflammation in IBD. Gut. 2014;63(Suppl 1):A175–A176. [Google Scholar]
- 23.Bodelier A, de Boer E, Jonkers D, Hameeteman W, Masclee A, Pierik MJ. Monitoring disease activity in IBD: correlation between clinical activity indices and biomarkers. Gastroenterology. 2011;140(5):S–423. [Google Scholar]
- 24.Bojic D, Bojic B, Protic M, Smith K. Fecal calprotectin is reliable surrogate marker of endoscopic and histologic mucosal healing in Crohn’s disease and ulcerative colitis. J Crohns Colitis. 2011;5:S34. [Google Scholar]
- 25.Canani RB, Terrin G, Rapacciuolo L, et al. Faecal calprotectin as reliable non-invasive marker to assess the severity of mucosal inflammation in children with inflammatory bowel disease. Dig Liver Dis. 2008;40:547–553. doi: 10.1016/j.dld.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 26.Cerrillo E, Beltrán B, Pous S, et al. Fecal calprotectin in ileal Crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis. 2015;21:1572–1579. doi: 10.1097/MIB.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 27.Chang J, Kennedy NA, Spurio FF, et al. Tu1132 Correlation of clinical symptoms to current biomarkers of intestinal inflammation in patients with Crohn’s disease. Gastroenterology. 2013;144(5 Suppl 1):S–770. [Google Scholar]
- 28.Chung-Faye G, Sandhu K, Logan RP, Sherwood RA. Fecal calproctectin is strongly predictive of clinical disease activity and histological severity in inflammatory bowel disease. Gastroenterology. 2011;140(5 Suppl 1):S–421. [Google Scholar]
- 29.Costa F, Mumolo MG, Bellini M, et al. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liver Dis. 2003;35:642–647. doi: 10.1016/s1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 30.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. doi: 10.1002/ibd.22917. [DOI] [PubMed] [Google Scholar]
- 31.D’Haens GR, Baert F, Vermeire S, et al. Mucosal inflammation in inflammatory bowel disease can reliably be predicted with the fecal calprotectin test. Gastroenterology. 2007;132(4 Supp 2):A174. [Google Scholar]
- 32.Dranga M, Dumitrescu G, Badea M, Blaj A, Mihai C, Prelipcean CC. The semi-quantitative calprotectin rapid test: is it useful in inflammatory bowel disease? Rev Med Chir Soc Med Nat Iasi. 2012;116:761–765. [PubMed] [Google Scholar]
- 33.Gaya DR, Duncan A, Lyon TD, et al. 343 Faecal calprotectin: a non-invasive, sensitive, and objective method in the assessment of Crohn’s disease activity. Gut. 2005;54:A90. doi: 10.1093/qjmed/hci069. [DOI] [PubMed] [Google Scholar]
- 34.Gerasimidis K, Nikolaou CK, Edwards CA, McGrogan P. Serial fecal calprotectin changes in children with Crohn’s disease on treatment with exclusive enteral nutrition: associations with disease activity, treatment response, and prediction of a clinical relapse. J Clin Gastroenterol. 2011;45:234–239. doi: 10.1097/MCG.0b013e3181f39af5. [DOI] [PubMed] [Google Scholar]
- 35.Inokuchi T, Kato J, Hiraoka S, et al. Su1248 Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn’s disease. Gastroenterology. 2015;148(4 Suppl 1):S–451. doi: 10.1097/MIB.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy NA, Clark A, Walkden A, et al. Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16-50 years. J Crohns Colitis. 2015;9:41–49. doi: 10.1016/j.crohns.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laharie D, Mesli S, El Hajbi F, et al. Prediction of Crohn’s disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. 2011;34:462–469. doi: 10.1111/j.1365-2036.2011.04743.x. [DOI] [PubMed] [Google Scholar]
- 38.Makanyanga J, Pendse D, Atkins E, et al. PWE-231 MRI is correlated to faecal calprotectin level in the evaluation of small bowel and colonic Crohn’s disease. Gut. 2012;61(Suppl 2):A392. [Google Scholar]
- 39.Minar P, Jurickova I, Haberman Y, et al. Tu1940 Neutrophil FCy receptor 1 (CD64) index as a non-invasive biomarker for clinical and mucosal disease activity in pediatric inflammatory bowel disease. Gastroenterology. 2013;144(5 Suppl 1):S–886. [Google Scholar]
- 40.Naismith GD, Smith LA, Barry SJ, et al. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn’s disease. J Crohns Colitis. 2014;8:1022–1029. doi: 10.1016/j.crohns.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Nikolaus S, Schreiber S, Nurwakagari P, Rath S, Wittig BM, Schwarz M. Su1279 Clinical epidemiology of fecal calprotectin: population data from the fire study, a prospective longitudinal study in germany to evaluate fecal calprotectin in routine monitoring of Crohn’s disease. Gastroenterology. 2014;146(5 Suppl 1):S–423. [Google Scholar]
- 42.Palmon R, Brown S, Ullman TA, Hanaway P, Mayer LF. Calprotectin and lactoferrin decrease with maintenance infliximab administration in luminal Crohn’s disease. Gastroenterology. 2006;130(4 Suppl 2):A212–A213. [Google Scholar]
- 43.Pavlidis P, Cavazza A, Siddique N, et al. PTH-059 Faecal calprotectin identifies non responders to anti-TNFalpha therapy when measured after induction in inflammatory Crohn’s disease. Gut. 2015;64(Suppl 1):A431–A432. [Google Scholar]
- 44.Scaioli E, Cardamone C, Scagliarini M, Zagari RM, Bazzoli F, Belluzzi A. Can fecal calprotectin better stratify Crohn’s disease activity index? Ann Gastroenterol. 2015;28:247–252. [PMC free article] [PubMed] [Google Scholar]
- 45.Shah R, Herrera H, Dewald R, Swaroop P. Predictors of elevated fecal calprotectin in inflammatory bowel disease patients: P-85. Inflamm Bowel Dis. 2011;17(Suppl 2):S39–S40. [Google Scholar]
- 46.Tursi A, Elisei W, Giorgetti G, Picchio M, Brandimarte G. Rapid fecal calprotectin correlates with clinical and endoscopic severity of inflammatory bowel diseases. Scand J Gastroenterol. 2013;48:1359–1360. doi: 10.3109/00365521.2013.832371. [DOI] [PubMed] [Google Scholar]
- 47.Turvill J. Mapping of Crohn’s disease outcomes to faecal calprotectin levels in patients maintained on biologic therapy. Frontline Gastroenterol. 2014;5:167–175. doi: 10.1136/flgastro-2014-100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zubin G, Peter L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and fecal calprotectin. Inflamm Bowel Dis. 2015;21:1386–1391. doi: 10.1097/MIB.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schröder O, Naumann M, Shastri Y, Povse N, Stein J. Prospective evaluation of faecal neutrophil-derived proteins in identifying intestinal inflammation: combination of parameters does not improve diagnostic accuracy of calprotectin. Aliment Pharmacol Ther. 2007;26:1035–1042. doi: 10.1111/j.1365-2036.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 50.Warner BD, Johnston EL, Ward MG, Irving PM. PTU-076 Is faecal calprotectin (FC) a reliable marker of isolated small bowel Crohn’s disease (CD) activity? Gut. 2015;64(Suppl 1):A93–A94. [Google Scholar]
- 51.Bremner A, Roked S, Robinson R, Phillips I, Beattie M. Faecal calprotectin in children with chronic gastrointestinal symptoms. Acta Paediatr. 2005;94:1855–1858. doi: 10.1111/j.1651-2227.2005.tb01870.x. [DOI] [PubMed] [Google Scholar]
- 52.Shaoul R, Sladek M, Turner D, et al. Limitations of fecal calprotectin at diagnosis in untreated pediatric Crohn’s disease. Inflamm Bowel Dis. 2012;18:1493–1497. doi: 10.1002/ibd.21875. [DOI] [PubMed] [Google Scholar]
- 53.Komraus M, Wos H, Wiecek S, Kajor M, Grzybowska-Chlebowczyk U. Usefulness of faecal calprotectin measurement in children with various types of inflammatory bowel disease. Mediators Inflamm. 2012;2012:608249. doi: 10.1155/2012/608249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henderson P, Anderson NH, Wilson DC. The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:637–645. doi: 10.1038/ajg.2013.131. [DOI] [PubMed] [Google Scholar]
- 55.Dobrzanski C, Pedersen N, Burisch J, Hansen VV, Fuglsang H, Munkholm P. P643 Faecal calprotectin: correlation with the Harvey–Bradshaw Index in patients with Crohn’s disease. J Crohns Colitis. 2013;7(Suppl 1):S268. [Google Scholar]
- 56.Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 57.García-Sánchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4:144–152. doi: 10.1016/j.crohns.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Diamanti A, Colistro F, Basso MS, et al. Clinical role of calprotectin assay in determining histological relapses in children affected by inflammatory bowel diseases. Inflamm Bowel Dis. 2008;14:1229–1235. doi: 10.1002/ibd.20472. [DOI] [PubMed] [Google Scholar]
- 59.Meunier P, Cousin F, Van Kemseke C, et al. Persisting signs of disease activity at magnetic resonance enterocolonography predict clinical relapse and disease progression in quiescent Crohn’s disease. Acta Gastroenterol Belg. 2015;78:274–281. [PubMed] [Google Scholar]
- 60.Lin WC, Wong JM, Lin CP, et al. Fecal calprotectin levels could be used as a predictor of endoscopic remission for inflammatory bowel disease patients: Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Clin Gastroenterol Hepatol. 2015;13:e101 [Google Scholar]
- 61.Bunn SK, Bisset WM, Main MJ, Gray ES, Olson S, Golden BE. Fecal calprotectin: validation as a noninvasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14–22. doi: 10.1097/00005176-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Guidi L, Marzo M, Andrisani G, et al. Faecal calprotectin assay after induction with anti-tumour necrosis factor alpha agents in inflammatory bowel disease: prediction of clinical response and mucosal healing at one year. Dig Liver Dis. 2014;46:974–979. doi: 10.1016/j.dld.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Tang J, Gao X, Zhi M, Hu P. P101 Fecal calprotectin is a valuable marker for detecting active Crohns disease with colon involvement. J Crohn Colitis. 2015;9(Suppl 1):S127. [Google Scholar]
- 64.Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2008;6:1218–1224. doi: 10.1016/j.cgh.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Faubion WA, Fletcher JG, de Villiers WJ, et al. Mo1680 Assessing Crohn’s disease inflammatory biomarker diagnostic accuracy using ileocolonoscopy or a combined ileocolonoscopy-CTE score in the Embark Study. Gastroenterology. 2012;142(Suppl 1):S–658. [Google Scholar]
- 66.Faubion WA, Jr, Fletcher JG, O’Byrne S, et al. EMerging BiomARKers in Inflammatory Bowel Disease (EMBARK) study identifies fecal calprotectin, serum MMP9, and serum IL-22 as a novel combination of biomarkers for Crohn’s disease activity: role of cross-sectional imaging. Am J Gastroenterol. 2013;108:1891–1900. doi: 10.1038/ajg.2013.354. [DOI] [PubMed] [Google Scholar]
- 67.Lobatón T, López-García A, Rodríguez-Moranta F, Ruiz A, Rodríguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J Crohns Colitis. 2013;7:e641–e651. doi: 10.1016/j.crohns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014;24:277–287. doi: 10.1007/s00330-013-3010-z. [DOI] [PubMed] [Google Scholar]
- 69.Maltz B, Milne G, Slaughter JC, Merchant N, Schwartz DA. W1153 The utility of urinary prostaglandin E-M (PGE-M) as a biomarker of Crohn’s disease activity. Gastroenterology. 2009;136(5 Suppl 1):A–665. [Google Scholar]
- 70.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s Disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 71.Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 72.Stawczyk-Eder K, Eder P, Lykowska-Szuber L, et al. Is faecal calprotectin equally useful in all Crohn’s disease locations? A prospective, comparative study. Arch Med Sci. 2015;11:353–361. doi: 10.5114/aoms.2014.43672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zittan E, Kelly O, Burns J, et al. Su1236 High fecal calprotectin correlate with active colonic disease but not with small intestinal Crohn’s disease activity. Gastroenterology. 2015;148(4 Suppl 1):S–448. doi: 10.1002/jgh3.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moniuszko A, Koziel D, Gluszek S, Rydzewska G. P244 Is prognostic utility of rapid faecal calprotectin test equal in all inflammatory bowel disease (IBD) subtypes? Retrospective analysis based on endoscopic indices. J Crohns Colitis. 2016;10(Suppl 1):S216. [Google Scholar]
- 75.Goutorbe F, Goutte M, Minet-Quinard R, et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J Crohns Colitis. 2015;9:1113–1119. doi: 10.1093/ecco-jcc/jjv150. [DOI] [PubMed] [Google Scholar]
- 76.Lin WC, Wong JM, Tung CC, et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566–13573. doi: 10.3748/wjg.v21.i48.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.García-Bosch O, Ordás I, Aceituno M, et al. Comparison of diagnostic accuracy and impact of magnetic resonance imaging and colonoscopy for the management of Crohn’s disease. J Crohns Colitis. 2016;10:663–669. doi: 10.1093/ecco-jcc/jjw015. [DOI] [PubMed] [Google Scholar]
- 78.Faecal calprotectin diagnostic tests for inflammatory diseases of the bowel 2017 National Institute for Health and Care Excellence (NICE) Web site. https://www.nice.org.uk/guidance/dg11. Accessed Aug 31,
- 79.Magro F, Lopes S, Coelho R, et al. Accuracy of faecal calprotectin and neutrophil gelatinase B-associated lipocalin in evaluating subclinical inflammation in UlceRaTIVE colitisthe ACERTIVE study. J Crohns Colitis. 2017;11:435–444. doi: 10.1093/ecco-jcc/jjw170. [DOI] [PubMed] [Google Scholar]
- 80.van Rheenen PF, Van de Vijver E, Fidler V, et al. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


