Abstract
Background
The legume Medicago truncatula has emerged as a model plant for the molecular and genetic dissection of various plant processes involved in rhizobial, mycorrhizal and pathogenic plant-microbe interactions. Aiming to develop essential tools for such genetic approaches, we have established the first genetic map of this species. Two parental homozygous lines were selected from the cultivar Jemalong and from the Algerian natural population (DZA315) on the basis of their molecular and phenotypic polymorphism.
Results
An F2 segregating population of 124 individuals between these two lines was obtained using an efficient manual crossing technique established for M. truncatula and was used to construct a genetic map. This map spans 1225 cM (average 470 kb/cM) and comprises 289 markers including RAPD, AFLP, known genes and isoenzymes arranged in 8 linkage groups (2n = 16). Markers are uniformly distributed throughout the map and segregation distortion is limited to only 3 linkage groups. By mapping a number of common markers, the eight linkage groups are shown to be homologous to those of diploid alfalfa (M. sativa), implying a good level of macrosynteny between the two genomes. Using this M. truncatula map and the derived F3 populations, we were able to map the Mtsym6 symbiotic gene on linkage group 8 and the SPC gene, responsible for the direction of pod coiling, on linkage group 7.
Conclusions
These results demonstrate that Medicago truncatula is amenable to diploid genetic analysis and they open the way to map-based cloning of symbiotic or other agronomically-important genes using this model plant.
Background
The need for a more sustainable and environmentally safe agriculture has reinforced interest in the cultivation of legumes which include some of the most important agricultural species such as alfalfa, clover, pea, soybean, bean and peanut. These species have the capacity to establish atmospheric dinitrogen fixing symbiose with soil bacteria collectively named rhizobia, and to form symbiotic root mycorrhizae with soil fungi, thus facilitating their uptake of phosphate, water and other soil nutrients [1,2]. However, genetic analysis of these processes remains difficult in the major crop legumes due to features such as tetraploidy, large genomes and/or the lack of efficient methods for transgenesis. Since the model plant Arabidopsis thaliana, as indeed for other Cruciferacae, is unable to establish either rhizobial or mycorhizal symbioses, the need to establish a model legume has been recognized for over a decade. Furthermore, studying a model legume offers the opportunity to compare in the same plant both symbiotic and plant-pathogen interactions and also to analyse plant physiological processes which cannot be satisfactorily studied in A. thaliana[3].
The alfalfa relative, Medicago truncatula, was originally proposed as a model plant for legume biology because it possesses a number of interesting characteristics for both molecular and classical genetics [4-6]. Key attributes of M. truncatula include diploidy and autogamous fertilization, a small genome (500–600 Mbp/1C, [7]), a rapid reproductive cycle, a high level of biodiversity, a number of available cultivars [8-10] and a well characterized nitrogen-fixing symbiont, Sinorhizobium meliloti[6,11]. M. truncatula is also being used as a model plant for studying mycorrhizal interactions [12,13]. Several tools for molecular genetics and genomics have been recently developped including: mutant collections [12,14], cDNA libraries [15-17], a large-insert BAC library [18] and efficient methods of transformation [19-21].
Furthermore, the genus Medicago is part of the Galegoid phylum and is therefore related to a number of important crop legumes in addition to alfalfa such as pea, faba bean, chickpea, lentil and clover [22]. Members of this phylum are expected to show a high level of nucleotide sequence conservation and similar genetic organization, and hence the potential for retively easy transfer of genome information between member species.
The diploid Lotus japonicus has also been proposed as a model legume [5,23,24]. This plant has similar attributes to those of M. truncatula but it is phylogenetically distant from the Galegoid phylum and other legumes such as soybean or bean.
Up to now, five genetic linkage maps have been constructed for either diploid M. sativa[25-29] or tetraploid M. sativa[30]. In this paper, we describe the first genetic map of the diploid model legume M. truncatula, an essential tool for genetics and genomics in this species. We report on the selection of two polymorphic M. truncatula genotypes providing the basis for classical and molecular genetics. An improved protocol was used to cross these two lines, leading to the construction of a genetic map of M. truncatula. Based on an F2 population of 124 plants, this map comprises 289 molecular markers and shows a low level of distortion in segregation and no clustering of markers. We took advantage of the existing high density genetic map of the diploid allogamous species Medicago sativa[29] to map a number of orthologous genes and to identify homologous linkage groups. These have been similarly numbered for the two Medicago species. Finally, we illustrate the use of these new tools to precisely map two genes of M. truncatula: the Mtsym6 gene [31] involved in strain x cultivar specificity of nitrogen fixation and the SPC gene, determining the direction of pod coiling [32].
Results
Selection of parental genotypes
Since genetic analysis is based on the study of the segregation pattern of traits which differ between the two parents, two M. truncatula lines were selected on the basis of their phenotypic and molecular differences. M. truncatula is a self-fertilizing plant whose natural populations are composed of a variable number of different homozygous genotypes [33]. Cultivars can be genetically heterogeneous due either to a multiline selection process or to genetic pollution from other plant species during multiplication. In addition, in some natural populations, M. truncatula flowers occasionally cross-pollinate (<1%) [34,35]. For these reasons, single seeds from the cultivar Jemalong and from DZA315, an Algerian natural population (JMP, unpublished results) were selfed at least twice in order to ensure maximal homozygosity of the M. truncatula line. These two parental lines (Jemalong 6 and DZA315.16) were named after their respective population or cultivar, followed by a number which identifies the particular line.
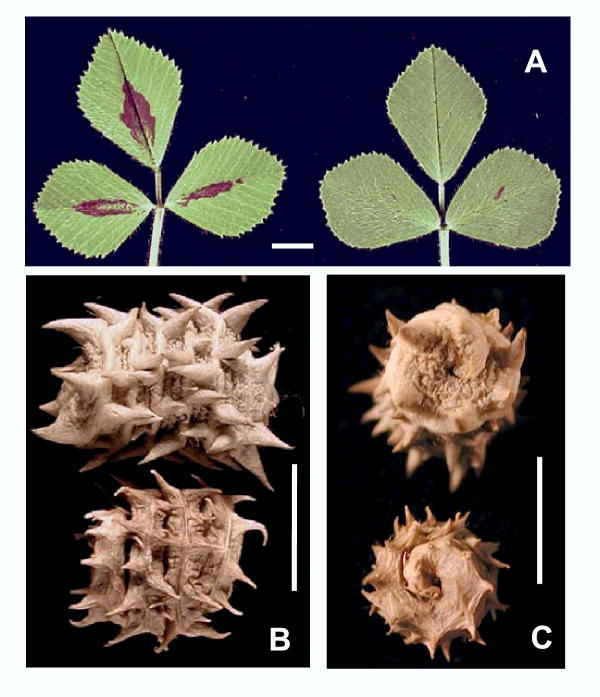
As stated above our choice of line DZA315.16 was based on the large number of morphological, developmental or symbiotic traits which are polymorphic with Jemalong (JMP and TH, unpuplished results). For instance, the two lines can be easily distinguished from each other by leaf pigmentation pattern: Jemalong 6 shows a typical pattern on the adaxial leaf surface whereas DZA315.16 leaves have only infrequent and randomly distributed spots (Figure 1A). Another striking difference lies in pod shape: Jemalong 6 pods have a spiny barrel shape characteristic of the M. truncatula ssp truncatula pods described by Lesins and Lesins [36], whereas DZA315.16 forms shorter and smaller pods and spines (Figure 1B). Finally, the two lines show opposite coiling: anticlockwise for Jemalong 6 and clockwise for DZA315.16 (Figure 1C).
Figure 1.
Phenotypic patterns which distinguish Jemalong 6 and DZA315.16 lines. Bars = 5 mm. (A) Typical leaf pigmentation found on adaxial leaf surface of Jemalong 6 (left) and DZA315.16 (right). (B) Pod shape of Jemalong 6 (above) and DZA315.16 (below). (C) Anticlockwise pod coiling of Jemalong 6 (above). Clockwise pod coiling of DZA315.16 (below).
The molecular diversity between the two lines was estimated by using anonymous AFLP banding patterns. We observed that 32% of AFLP bands are polymorphic between Jemalong 6 and DZA315.16 (not shown).
Another criteria for choosing these two lines was their identical DNA content since Blondon et al[7] have shown that M. truncatula genotypes can differ by up to 20% in their DNA content. The DNA content of the two parental lines was measured by flow cytometry and shown to be identical (1.16 pg/2C), corresponding approximately to a haploid genome size of 580 Mb. According to Arumuganathan and Earle [37], as well as Bennett et al[38], this value is similar to Lotus japonicus (1.0 pg/2C, [23]) and about 4 times greater than the 145 Mb/1 C of Arabidopsis thaliana (0.30 pg/2 C). It is worth noting that the diploid M. sativa (800 Mb/1 C, [7]) possesses a significantly higher DNA content than M. truncatula.
In studies carried out over the last decade, a number of other single-seed descent lines of M. truncatula have been described. Three of these are derived from the Jemalong cultivar. The Jemalong line A17 (TH, unpublished results) has been used for the construction of most cDNA and BAC libraries as well as for mutagenesis [39] and the Jemalong line J5 was used in a γ-ray mutagenesis program [12]. So far we have failed to observe any difference between the Jemalong lines A17, J5 and J6 despite the use of more than 4000 AFLP markers (TH and JMA, data not shown). Furthermore, we have never been able to identify any phenotypic (morphological or symbiotic) difference between these three Jemalong lines. Our conclusion is that lines, A17, J5 and J6 can be considered as having an identical genotype, even if very limited differences in nucleotide sequence cannot be ruled out. On the other hand, Rose et al[40] have isolated, after in vitro selection, a derivative, 2HA, from Jemalong with high regeneration capacity. In addition to Jemalong, Penmetsa and Cook [41] proposed A20, a line selected from natural populations (TH, unpublished results), for genetic studies. Similarly, the R108-1 (c3) line, isolated from cell culture selection and possessing a higher capacity for regeneration has also been proposed as a model genotype [17,20,42]. Since all these four M. truncatula genotypes (Jemalong, DZA315.16, A20 and R108-1 (c3) possess distinctive traits, it is clear that there is considerable potential in exploiting the natural genetic variability of M. truncatula for revealing plant developmental processes.
Construction of the genetic map
The 124 individuals of the mapping population were genotyped for 313 markers comprising, 292 dominant anonymous markers (72 RAPD and 220 AFLP), 19 genes with known functions (Table 3) and 2 codominant isoenzyme markers (Table 3). The 72 polymorphic RAPD bands were generated using a set of 18 10-mer primers (Table 1, 4 bands generated per primer on average). All the 13 AFLP primer combinations (Table 2) were shown to be polymorphic and generated an average of 16.9 markers/primer combination.
Table 3.
List of genes and isoenzymes used
| Markers | Nature | Function | Primer sequences (5'-3') | RE | LG | References |
| ENBP1 | SSR | Chloroplastic protein | L: CACTTCCCACTGTCCTAGTCCTAC R: GACTCGTCATCACCAGTTTCATCC | 1 | [65]* | |
| ENOD8 | CAPS | Nodulin | L: ATGGTGCAAACTTCGCATCAGGAGG R: ACAACCCTTTGGTCCTGTGCCGTG | SspI | 1 | [66] |
| MtPT-1 | CAPS | Phosphate transporter | L: TTGCTAAGAACCCGAAACAAGCTGC R: ATGTTCATGATCCTTCTTGCTCAAGC | RsaI | 1 | [13] |
| PEPC | CAPS | PhosphoEnolPyruvate Carboxylase | L: CTCATCCTACTCAGTCGGTTCGTCG R: ACACGTAGCTCATCGTTGCAACGCC | RsaI | 2 | [67] |
| Z-F1 | RFLP | Zn-Finger1 | EcoRI | 3 | [68] | |
| GS.B | PCR | Glutamine synthetase | L: AGAAGTAGCGTGGTGGC R: GCTGCCTATGGAGAAGG | 3 | [69] | |
| Mtlec2 | SSR | Lectine 2 | L: AAAGAATTCCATCTTCCAAGGCGA R: GAAGTCTAGAAACCAATCCTTACCC | 3 | [70] | |
| ENOD12 | PCR | Nodulin | L: CCTGCTTATAGGCCACCAC R: CTTCTGCTGGAGGATGCC | 3 | [71] | |
| ATPase | SSR | Vacuolar ATPase | L: GGGTTTTTGATCCAGATCTT R: AAGGTGGTCATACGAGCTCC | 4 | [15]** | |
| MtST-1 | PCR | Sucrose transporter | L: ATCAAAGGTGGATGGGGATGGAG R: TAGACCAGAAGGGATGTGATTTCC | 4 | [72] | |
| PGM | Isoenz. | PhosphoGlucoMutase | 4 | [34] | ||
| Gs.C | CAPS | Glutamine synthetase | L: GCACAAGGAGCACATTG R: GCCCATAATTAAACATCATG | VspI | 5 | [69] |
| Lb I | CAPS | Leghemoglobin I | L: GATAGTCCTCAACTCCAAGC R: CCTGTTGCTCGAAGTTGAGA | SspI | 5 | [73] |
| rDNA | CAPS | 45s Ribosomal DNA | L: ATGGTCCGGTGAAGTGTTCG R: CCCGGTTCGCTCGCCGTTAC | XbaI | 5 | This paper |
| ENOD40 | CAPS | Nodulin | L: TTAGTAGGATCTTCTCTTTCACTAGC R: GCCTCCGATTATCAAAGGTCAAG | AluI | 5 | [74] |
| GS.A | PCR | Glutamine synthetase | L: AAAGACAAGTGGTTGTGAC R: GCTGCCTATGGAGAAGG | 6 | [69] | |
| MtN1 | PCR | Nodulin | L: ATCTATCTTCTTCGCTC R: TGACTGTTGAACATCTC | 6 | [75] | |
| GSHS | SSR | Glutathione synthetase | L: AGCAATAGGCAATGGCTGCTCCTGC R: AATGGTGAGATGCTTATGATGAGAC | 7 | [76] | |
| VR | CAPS | Vestitone reductase | L: GAGTGTGTGTAACTGGAGGTACAGG R: ATGCCTAATGCGCCATCGACGGTTC | DdeI | 7 | [77] |
| PGD | Isoenz. | PhosphoGlucoDeshydrogenase | 7 | [34] | ||
| ENOD16 | RFLP | Nodulin | L: ATCGGGACACTCTTTAGCAAGACCC R: TGGGCGGTGGAATTGGACCTCTTG | EcoRV | 8 | [78] |
Underlined markers have been used for synteny studies RE : Restriction Enzyme LG : M. truncatula Linkage Group SSR : Simple Sequence Repeat: polymorphism is revealed by the length of the amplified fragment CAPS : Cleaved Amplified Polymorphic Sequence : polymorphism is revealed after digestion of the amplified fragment with restriction enzymes PCR : Polymerase Chain Reaction : polymorphism is revealed by the length of the amplified fragment L : Left primer R : Right primer *: Accession n° AJ002479 ** : Accession n° AA660456
Table 1.
RAPD primers sequences used to generate fingerprints
| Code | Sequence(5'-3') |
| B07 | GGTGACGCAG |
| G03 | GAGCCCTCCA |
| G04 | AGCGTGTCTG |
| G06 | GTGCCTAACC |
| G10 | AGGGCCGTCT |
| G16 | AGCGTCCTCC |
| G17 | ACGACCGACA |
| G18 | GGCTCATGTG |
| G19 | GTCAGGGCAA |
| L02 | TGGGCGTCAA |
| L03 | CCAGCAGCTT |
| L07 | AGGCGGGAAC |
| L12 | GGGCGGTACT |
| L13 | ACCGCCTGCT |
| L14 | GTGACAGGCT |
| L17 | AGCCTGAGCC |
| M07 | CCGTGACTCA |
| M10 | TCTGGCGCAC |
Table 2.
Amplified Fragment Length Polymorphism (AFLP) primers used to generate fingerprints
| Code | Primer 1 | Primer 2 |
| PA | Eco+AG | Mse+CAC |
| PB | Eco+AG | Mse+CAA |
| PC | Eco+AG | Mse+CAT |
| PD | Eco+AT | Mse+CAC |
| PE | Eco+AT | Mse+CAG |
| PF | Eco+AGA | Mse+CGT |
| PG | Eco+AGA | Mse+CCA |
| PH | Eco+AC | Mse+CTG |
| PI | Eco+AC | Mse+CAC |
| PJ | Eco+AC | Mse+CTT |
| PK | Eco+AC | Mse+CAT |
| PL | Eco+AG | Mse+CTA |
| PM | Eco+AG | Mse+CGG |
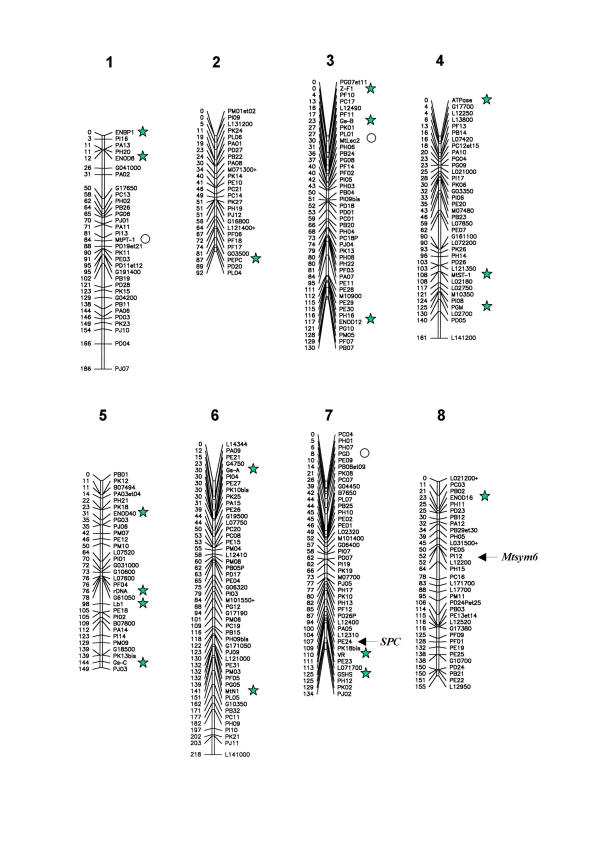
The M. truncatula genetic map was built using the MAPMAKER software [43] with a minimum LOD score value of 5. Three hundred and four markers were organized in 8 linkage groups, corresponding to the haploid chromosome set of M. truncatula (2n = 16). Only 9 markers (2 RAPD and 7 AFLP markers) remained unlinked. Five pairs of RAPD and ten pairs of AFLP dominant markers were transformed into, respectively, 5 and 10 codominant markers when the following conditions were observed: the two bands should be in repulsing phase, have a similar size, and no situation of band absence should be observed in the progeny. Following this, a new genetic map was recalculated (Figure 2). Apart from higher LOD scores values, no significant modifications were found in marker order or in map distances, and the number of unlinked markers was unchanged.
Figure 2.
Global F2 genetic map of Medicago truncatula. The number above the linkage groups refers to the homologous linkage group in M. sativa [29]. The code to the right of the linkage groups refers to the marker name. The numbers to the left of the linkage groups refers to the genetic distances (Kosambi cM) from the top and have been rounded up for clarity. The sign + indicates that two RAPD markers have been transformed into a codominant marker. In the case of codominant AFLP markers, the codes of the two bands are given together. Stars refer to known genes used for synteny studies. Circles refer to known genes not used for synteny studies.
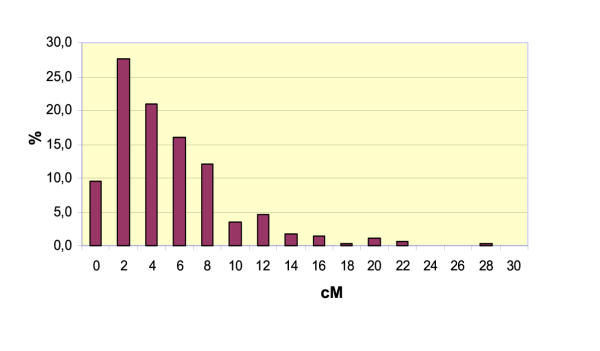
The resulting M. truncatula genetic map spans 1225 Kosambi cM, with an average of 470 kb/cM. The 8 linkage groups have different genetic sizes ranging from 92 to 219 cM (Table 4). Figure 3 shows the distribution diagram of interval distance between two adjacent markers with an average value of 4.4 cM and a standard deviation of 4.3 cM; 90% of the markers are located within an interval of less than 10 cM. According to the number, origin and nature of the markers their distribution is approximately similar between the linkage groups (Table 5).
Table 4.
Distribution of markers according to the linkage group
| LG | Number of markers | cM | Average distance (cM) | SD(cM) |
| 1 | 32 | 186 | 5.6 | 5.1 |
| 2 | 26 | 92 | 3.7 | 2.5 |
| 3 | 42 | 130 | 3.2 | 3.4 |
| 4 | 36 | 161 | 4.6 | 5.7 |
| 5 | 31 | 149 | 4.6 | 4.4 |
| 6 | 48 | 218 | 4.6 | 3.9 |
| 7 | 41 | 134 | 3.3 | 2.7 |
| 8 | 33 | 155 | 5.1 | 4.2 |
| 289 | 1225 | 4.4 | 4.3 |
LG = M. truncatula Linkage Group cM = Kosambi centimorgan SD = Standard Deviation
Figure 3.
Distribution of the 281 intervals between adjacent markers on the F2 genetic map of M. truncatula. X-axis: genetic distance in Kosambi cM. Y-axis: frequency of intervals (%). The average distance between two markers is 4.4 cM with a standard deviation of 4.3 cM. 90% of the markers are closer than 10 cM.
Table 5.
Distribution of markers according to their origin and their dominant/codominant nature.
| LG | Number of markers | Dominant markers | Codominant markers | |||||||
| AFLP | RAPD | RFLP | PCR | AFLP | RAPD | RFLP | PCR | Isoenz | ||
| 1 | 32 | 23 | 4 | 0 | 0 | 2 | 0 | 0 | 3 | 0 |
| 2 | 26 | 19 | 3 | 0 | 0 | 1 | 2 | 0 | 1 | 0 |
| 3 | 42 | 35 | 2 | 1 | 0 | 1 | 0 | 0 | 3 | 0 |
| 4 | 36 | 16 | 16 | 0 | 0 | 1 | 0 | 0 | 2 | 1 |
| 5 | 31 | 18 | 8 | 0 | 0 | 1 | 0 | 0 | 4 | 0 |
| 6 | 48 | 34 | 11 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| 7 | 41 | 28 | 9 | 0 | 0 | 1 | 0 | 0 | 2 | 1 |
| 8 | 33 | 20 | 7 | 0 | 0 | 3 | 2 | 1 | 0 | 0 |
| Total | 289 | 193 | 60 | 1 | 2 | 10 | 5 | 1 | 15 | 2 |
LG = M. truncatula Linkage Group
A serious problem in creating F2 genetic maps comes from the presence of alternating markers in coupling and repulsion phase. To circumvent this problem, we separated the markers which are dominant into two sets, grouping separately either male (DZA315.16) or female (Jemalong 6) dominant markers with codominant markers (Table 6). A dominant marker is considered as a male (or female) marker if the recessive allelic form (absence) is male (or female). Male and female genetic maps were built that span 888 and 979 Kosambi cM respectively (Table 7). Since the order of codominant markers is not modified between the two maps (data not shown), we present here the easier-to-read global F2 representation (Figure 2) with 289 markers.
Table 6.
Distribution of markers on linkage groups according to their sexual origin
| LG | Number of markers | Distorted markers | Female markers | distorted Female markers | Male markers | distorted Male markers | Codominant markers | distorted Codominant markers |
| 1 | 32 | 19 | 10 | 2 | 17 | 13 | 5 | 4 |
| 2 | 26 | 12 | 10 | 8 | 11 | 0 | 5 | 4 |
| 3 | 42 | 37 | 21 | 21 | 17 | 13 | 4 | 3 |
| 4 | 36 | 1 | 17 | 0 | 13 | 1 | 6 | 0 |
| 5 | 31 | 0 | 14 | 0 | 12 | 0 | 5 | 0 |
| 6 | 48 | 2 | 22 | 1 | 24 | 0 | 1 | 1 |
| 7 | 41 | 2 | 11 | 1 | 27 | 1 | 4 | 0 |
| 8 | 33 | 3 | 10 | 0 | 17 | 0 | 6 | 3 |
| 289 | 79 (27%) | 115 | 33 (29%) | 138 | 31 (22%) | 36 | 15 (42%) |
LG = M. truncatula Linkage Group A dominant marker is considered as a male marker if the recessive allelic form is male (and the same for female). χ2 for equality of number of male and female dominant markers = 1.729 χ2 for equality of number of male and female distorted markers = 0.077 Tabulated χ2 for degrees of freedom = 1 is 3.84 at P = 0.95 level of significance.
Table 7.
Male and Female genetic map size.
| LG | cM | Female map (cM) | Average distance | SD | Male map (cM) | Average distance | SD |
| 1 | 186 | 107 | 7.6 | 6 | 117 | 6.5 | 6 |
| 2 | 92 | 92 | 6.6 | 2.8 | 80 | 5.7 | 4 |
| 3 | 130 | 150 | 6 | 6.7 | 103 | 5.1 | 4.8 |
| 4 | 161 | 137 | 6.2 | 7.1 | 55 | 5.4 | 4.1 |
| 5 | 149 | 120 | 7.1 | 5.7 | 105 | 7 | 5.9 |
| 6 | 218 | 193 | 7.7 | 6.5 | 135 | 5.6 | 3.5 |
| 7 | 134 | 97 | 6.9 | 4.7 | 148 | 4.8 | 4.6 |
| 8 | 155 | 83 | 6.4 | 6.1 | 145 | 6.9 | 5 |
| 1225 | 979 | 6.8 | 6.1 | 888 | 5.8 | 4.8 |
The average genetic distances between adjacent markers for male and female linkage groups are not significantly different (P = 0.95). cM = Kosambi Centimorgan SD = Standard Deviation
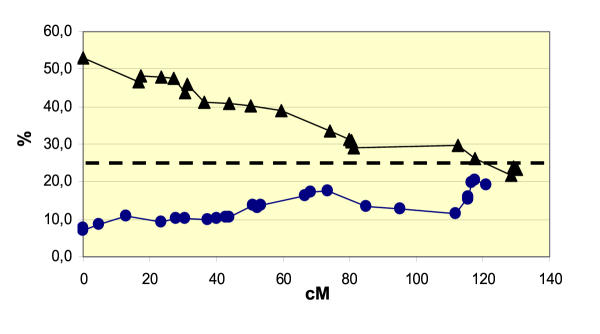
As shown in Table 6, about 27% of the markers do not show a typical Mendelian segregation ratio at α = 0.05. It is noteworthy that there is a high level of deviation from the theoretical ratio only in the case of 3 linkage groups. 59, 46 and 88% of the markers in linkage groups 1, 2 and 3, respectively, are distorted, together representing 86% of all the distorted markers with high χ2 values for 1:3 segregation. On the other hand, each of the 5 other linkage groups has less than 3 moderately distorted markers (data not shown). Furthermore these distorted markers are not randomly distributed throughout chromosomes: for example, a nearly linear gradient of distortion can be observed on linkage group 3 (Figure 4) ranging from Mendelian equality of male and female markers to a 5-fold frequency in favor of male alleles. On a whole genome basis, the frequency of distorted male markers compared to female markers appears to be similar: 22% and 29% respectively (Table 6).
Figure 4.
Segregation distortion of the female and male markers along linkage group 3 of the overall F2 genetic map of M. truncatula. Circles and triangles refer to female and male alleles respectively. X-axis: genetic distance from the top of the linkage group in Kosambi cM. Y-axis: frequency (%) of segregation of female and male alleles in the mapping population. A dominant marker is considered to be a male marker if the recessive allelic form is male (and the same for the female markers). If no distortion occurs, the segregation value should be close to 25% for both male and female markers (dashed line).
Alignment of M. sativa and M. truncatula genetic maps
The agronomically important legume Medicago sativa (alfalfa) is taxonomically very close to M. truncatula. However, the colinearity of the two genomes has not been evaluated. For this reason, we mapped 18 gene or isoenzyme markers (Table 3, Figure 2) which tag the 8 linkage groups of the diploid M. sativa genetic map [29]. All these markers were similarly linked in the two species with the exception of the rDNA which was found on linkage group 5 of M. truncatula, compared to linkage group 6 of M. sativa. To facilitate comparison, we gave the same numbering to homologous linkage groups. The relative orientation of linkage groups 1, 3, 4, 5, 6 and 7 between M. sativa and M. truncatula could be determined because at least 2 common markers had been mapped. On the other hand, the orientations of linkage groups 2 and 8 were deduced from the distal genetic position of the PEPC and ENOD16 markers respectively.
Mapping of symbiotic (Mtsym6) and developmental (SPC) genes
Many polymorphisms exist between Jemalong 6 and DZA315.16. One symbiotic and one developmental monogenic trait were selected and genetically mapped to illustrate the use of our genetic map.
Tirichine et al[31] showed that a Fix-/Fix+ polymorphism is present between the Jemalong 6 and DZA315.16 parents respectively following inoculation with the wild type Sinorhizobium meliloti A 145 strain. This polymorphism is controlled by a single recessive gene, named Mtsym6: the Fix+ trait being dominant. In order to precisely map this gene, we determined the nitrogen-fixing genotype of 73 F2 plants of the mapping population by inoculating their F3 progeny. In total, 15 F3 families were Fix-, 19 were Fix+, and 39 displayed both Fix+ and Fix-, thus indicating that their F2 parent was heterozygous. The χ2 value for 1:2:1 segregation is 0.768 in agreement with the monogenic segregation data of Tirichine et al[31]. Symbiotic typing data together with molecular marker segregation allowed us to map the Mtsym6 gene at less than 1 cM (LOD > 10) from the cosegregating PI 12 and L12200 markers on linkage group 8 (Figure 2).
The clockwise (DZA315.16) or anticlockwise (Jemalong 6) polymorphism observed on parental pods (Figure 1) was treated as a morphological marker. Pods obtained after selfing of F1 plants have clockwise coiling. We inferred the genotype of F2 plants in the mapping population by examining the pods on F3 plants. From 110 F3 families, we observed that 28 (25.5%) had anticlockwise pod coiling and 82 had clockwise pod coiling. The χ2 value for the 1:3 segregation is 0.012 which is in agreement with a monogenic determinism with the DZA315.16 clockwise trait being dominant. We named this gene SPC (for Sense of Pod Coiling) and were able to map it on linkage group 7 by cosegregation with the PE24 marker.
Discussion
Selection of parental lines
In order to construct a genetic map for M. truncatula, we selected two polymorphic lines, one from an Australian cultivar and the other from a natural Algerian population. These two lines are very polymorphic and have traits which allow them to be readily distinguished. For instance, Jemalong leaf markings are almost absent in the DZA315.16 line. Penmetsa and Cook [41] reported the existence of a single gene responsible for the leaf spots between Jemalong and A20 lines.
The cultivar Jemalong was the first M. truncatula cultivar to be released [44] and is widely used in agricultural ley-farming [45]. The cultivar Jemalong was initially selected for molecular studies because of its transformation/regeneration characteristics [4,19,46]. So far, most of the Medicago programs including mutagenesis, the construction of large scale cDNA and genomic libraries, DNA sequencing and genetic resources [39] have made use of Jemalong lines. A major interest of the Jemalong line is the fact that it can be nodulated by the Sinorhizobium meliloti strain 1021, whose genome has been recently sequenced [11]http://sequence.toulouse.inra.fr/meliloti.html.
The percentage of AFLP bands which are polymorphic between Jemalong and DZA315.16 (32%), is similar to the 30 to 34% observed between 3 ecotypes of Arabidopsis thaliana[47]. In addition to the limited level of marker distortion, this high % of polymorphism is convenient for generating genetic maps in the model legume M. truncatula.
The genetic map
We have built a genetic map using a combination of anonymous dominant markers and known genes of co-dominant evaluation. The reliability and value of this genetic map is demonstrated in the following ways: i) identical number of linkage groups and chromosomes as well as the small number of unlinked markers (2.8%) at a minimal LOD 5 ; ii) no discrepancies between the global F2 map and both male and female maps; iii) only a limited clustering of markers on a few linkage groups. The excellent distribution of markers is illustrated by the fact that 90% of the markers are located within an interval of less than 10 cM from adjacent markers. This M. truncatula genetic map is composed of 289 markers forming 8 linkage groups which correspond to the haploid chromosome set of the species and spans 1225 Kosambi cM, which gives an average of 470 kb/cM. Despite the fact that genetic data has been obtained with a variety of segregating populations (F2, F3, RILs), it is now well known that the genetic size of genomes is not proportional to haploid DNA content. This leads to different values for the average ratio of physical to genetic distance (kb/cM): 263 kb/cM for Arabidopsis thaliana[47], 3000 kb/cM for Pisum sativum[48] 1900 kb/cM for Helianthus annuus[49] and 1000 kb/cM for diploid Medicago sativa[29].
An F2 genetic map is difficult to construct using dominant markers because recombination between markers can be estimated with confidence only when the markers are in coupling phase. For this reason, we established two additional maps containing the codominant markers, as anchor markers, combined with either male or female markers in coupling phase. The comparison of these two maps to the global F2 map gives a reasonable estimate of the quality of the genetic map. Except for a shorter genetic length due to an underestimation of crossing overs, the estimated distances between codominant markers and their relative order is very similar in the two maps.
The 8 linkage groups of M. truncatula differ in genetic size by a factor of 2.4 from 92 to 219 cM, whereas M. truncatula chromosome length differs by a factor of 1.5 (3 to 4.5 μm) or 2.3 (29 to 68 μm) for metaphasic [50] or pachytene [51] chromosomes respectively. No obvious correlation could be found between genetic size of linkage groups and pachytene chromosome length measurements [51]. Finally, the range of genetic size of the M. truncatula linkage groups suggests that, on average, 1 to 2 crossing-over occur per chromosome per meiosis.
Segregation distortion of markers and quality of the genetic map
About 27% of the markers do not have the expected Mendelian ratios (at α = 0.05). This can be compared with the 40% value observed by Jenczewski et al[52] in M. truncatula × M. tornata intraspecific crosses and to the 15 to 50% value for loci having distorted segregation in diploid alfalfas [26,27,29]. High frequencies of segregation distortion in the M. sativa genome can be explained by the exposure of deleterious recessive alleles during inbreeding which caused gametic and/or zygotic selection as hypothesized by Brouwer and Osborn [30]. Interestingly, the distorted markers are not scattered over the M. truncatula genome but concentrated on linkage groups I, II and III suggesting structural reasons for this distortion.
From the analysis carried out by Jenczewski et al[52], it is clear that variations from expected Mendelian ratios are common within both inter- and intraspecific crosses. Distorting factors can be deleterious recessive alleles [53], self-incompatibility alleles [54], structural rearrangements [55] or differences in DNA content [52]. In the case of Jemalong 6 and DZA315.16, the two genotypes have a similar overall DNA content. However, we do not know if DNA content is identical for homologous chromosomes of the two lines. However, the gradient in distortion in favor of male alleles along linkage group 3 suggests the presence of specific gene(s) interfering with meiosis. Whatever the cause, a consequence of segregation distortion is that chromosomes, or at least parts of chromosomes, are not transmitted equally to the progeny. It would be of interest to know if the segregation distortion frequency depends on the lines involved in the cross and if it is always limited to the same 3 linkage groups. Cytogenetics experiments are in progress to identify the linkage between meiotic pairing and segregation distortion.
Macrosynteny between Medicago species
Despite different genome sizes and evolutionary divergence, a remarkable degree of genome conservation has already been revealed by comparative genetic mapping experiments, as for instance in the Poaceae family [56]. We took advantage of the availability of a dense genetic map with more than 850 markers in diploid M. sativa[29], including many known genes, to address the question of macrosynteny between M. truncatula and alfalfa. At least two distant markers per chromosome were selected on the alfalfa map and mapped onto the M. truncatula segregation population. These results allowed identification of homologous linkage groups between the two species and consequently to name the M. truncatula linkage groups following the M. sativa nomenclature, as already stated in Kalo et al[29]. This nomenclature has been further extended to chromosome nomenclature [51]. The only observed discrepancy was the different localization of rDNA in the two species, on linkage group 5 for M. truncatula instead of group 6 for M. sativa[29]. The localisation of rDNA on linkage group 5 is ascertained from the LOD score for linkage between ENOD40 and rDNA and Lb1 and rDNA of, respectively, 7 and 10. Moreover, FISH experiments show that rDNA is localized on the same chromosome than ENOD40 [51]. The estimation of the degree of genome colinearity between these two closely related legume species (as well as with more distant legumes) is presently in progress. This should open the way to use M. truncatula as a nodal plant for a identifying legume genes of interest, characterized either after mutagenesis or within natural diversity. Unfortunately, due to its heterozygous character, alfalfa cannot be easily used either in mutagenesis or in genomic programs, thus reinforcing the interest in a closely related model legume. However, several questions remain open: i) what is the extent of macro- and microsynteny between M. truncatula and diploid alfalfa? ii) are diploid and tetraploid alfalfa syntenic? and iii) is synteny general within the Medicago genus and more generally within legumes?
Gene mapping
Both parental lines, Jemalong 6 and DZA315.16, are efficiently nodulated by the reference Sinorhizobium meliloti strains 1021 and 2011 (data not shown). However, we have observed that inoculation with a variety of other S. meliloti and S. medicae strains, all of which efficiently nodulate alfalfa reveals the existence of a high level of strain × cultivar symbiotic specificity in M. truncatula (TH, unpublished results). In this way, one particular gene (Mtsym6) implicated in the inefficient symbiosis between Jemalong and the wild type S. meliloti strain A145 was recently identified in our laboratory [31]. Here, we precisely map this gene to linkage group 8. This is the first report of the genetic mapping of a symbiotic gene in the model legume M. truncatula. The map-based cloning of Mtsym6 is currently under progress in the laboratory.
Anticlockwise pod coiling is a characteristic of most Medicago species [36]. Bena et al[57] have shown that only a single monophyletic clade contains species (including M. truncatula), where pods can be coiled in either orientation, suggesting that a single change must have led to the clockwise coiling character. Lilienfeld and Kihara [32] have demonstrated that, in Medicago littoralis (a close relative of M. truncatula), the clockwise direction is dominant and under monogenic control. We took advantage of the fact that Jemalong 6 and DZA315.16 are polymorphic for this character, having anticlockwise and clockwise pods respectively, to make a genetic analysis of this trait within our mapping population. In agreement with Lilienfeld and Kihara [32], we reached the conclusion that pod coiling sense has a monogenic determinism with clockwise turning dominant. We named this gene SPC and mapped it to linkage group 7. The map-based cloning of SPC is currently under progress in the laboratory.
Conclusions
The identification of polymorphic Jemalong 6 and DZA315.16 lines together with the making of a F2 genetic map and the identification of synteny with alfalfa, described in this report are likely to provide a powerful tool for both fundamental and applied approaches, such as studying the conservation of synteny between genomes of legumes and other model species, and the positional cloning of agriculturally important genes.
Materials and Methods
Plant material
Individual seeds of the M. truncatula cultivar Jemalong and the Algerian natural population DZA315 (JMP, unpublished results) were selfed at least twice and then crossed manually with Jemalong being the female parent. A population of 124 F2 plants, derived from a single F1 plant, was used for genetic mapping and is designated thereafter as the "mapping population". The hybrid nature of the F1 plant was confirmed with molecular markers. More than 95% of F2 seeds germinated, out of which about 6% gave albino plantlets that were discarded. One plant from each parental line, one F1 plant and the F2 individuals of the mapping population were cultivated axenically in Magenta boxes on SHb10 medium [58] and maintained by cuttings every two months.
Crossing procedure
An efficient manual crossing procedure has been developped based on a microscopic study of pollination and fertilization kinetics in relation to the intact flower morphology of M. truncatula (E.-P. Journet, unpublished). This method is similar to method 3 described in Pathipanawat et al[59] and is outlined here. Parental plants are vernalized at 4°C for 15 days after germination and cultivated in a growth chamber (25°C, 16 h light photoperiod, 100 μE.m-2.sec-1). For pollen sampling, flowers at the optimal stage (just past anthesis) are selected on the male parent and dissected under a stereomicroscope using curved extra fine forceps. At this stage, released pollen appears turgescent, moist and sticky and is packed around the stigma. On the female parent, flowers are selected at a developmental stage just prior to anther bursting and pollen release. The standard petal is longitudinally incised ~0.5 mm below its central line using a scalpel. The 10 non-open anther bags are then carefully removed, and the tip of a freshly harvested sexual column applied to the stigma in order to saturate its sticky surface with exogenous pollen. The pollinated pistil is then gently placed back under the standard petal. The branch tip bearing the cross-pollinated flower is labelled, inserted into a ~25-ml clear plastic vial containing 1 ml water and gently secured with a cotton wool plug. Female plants are kept under indirect light until the protecting vial is removed 24–48 h later. The success of crosses is indicated by the development of small coiling pods that become visible 2–4 days after pollination. The efficiency of this method is close to 80 % on average with 5 viable seeds per pod. Significant variations in the optimal stage for crossing and in the rate of success have been observed for different M. truncatula genotypes and appear to depend on the characteristics of the maternal genotype and genetic distance between parental lines. A more detailed crossing protocol is available from EPJ upon request.
DNA content measurements
DNA content was determined using an EPICS V flow cytometer at the Service de Cytometrie, Institut des Sciences Végétales, CNRS, Gif-sur-Yvette, France. Measurements were assessed as described by Blondon et al[7] using ethidium bromide fluorescence and Petunia hybrida PxPC6 (2C = 2.85 pg) as the routine internal standard. Each genome size estimation resulted from five independent measurements for each single plant.
DNA isolation and marker typing
DNA isolation and RAPD amplification were as described by Ghérardi et al[60]. RAPD markers were named as follows: the letter and the first two digits refer to the identification of the 10-mer primer (Table 1) from Operon Kits (Operon Technologies, Alameda, Calif, USA) and the last digits correspond to the molecular weight of the polymorphic bands.
AFLP markers were produced essentially as described by Vos et al[61] as modified by Moreau et al[62] with the EcoRI and MseI restriction enzymes and using, respectively, a +2/+3 combination. Polymorphic bands were identified by the code of the Eco/Mse primer combinations (Table 2) followed by a number corresponding to the band number in the parental profile. RAPD and AFLP markers were considered as dominant markers on a presence/absence alternative. The intensity of the bands was not taken into account.
Among the 19 genes used for mapping (Table 3) two genes were typed as RFLP markers. RFLP analysis was performed as follows: from each individual of the mapping population, DNA was isolated from a pool of more than 10 F3 plants, digested with restriction enzymes (Table 3) and Southern blotted. DNA probes (Table 3) were obtained from the respective authors, and labelled and hybridized as described in Kalo et al[29]. The polymorphism of the 17 other genes was identified after PCR amplification with primers anchored either on both sides of a microsatellite motif (SSR) or within exons and crossing introns (data not shown). When a length polymorphism could not be observed, the amplification products were digested with restriction enzymes (CAPS, [63]). RFLP, CAPS and PCR markers were scored as codominant.
Isozyme markers
Fresh pieces of actively growing young leaf tissue were ground in a cold 0.1 M Tris-HCl (pH = 7.2) extraction buffer. The supernatant was absorbed onto filter paper wicks, kept frozen at -80°C overnight, and then loaded onto 13% starch gels. The 6-phosphogluco dehydrogenase (PGD) and the phosphogluco-mutase (PGM) enzyme isoforms were resolved with a Tris-citrate (pH = 7.0) buffer system as described by Chaulet [34]. Banding intensity was used to identify heterozygotes. Isozymes were scored as codominant markers.
Map construction
The genetic map was constructed using the MapMaker/Exp V3.0 software [43] with the Kosambi map function and was drawn with the Drawmap package [64]. The segregation data were tested for deviation from the expected Mendelian ratio using a Chi-square test. The markers showing significant deviation at α = 0.05 were nevertheless used to build the linkage map.
Gene mapping
For the Mtsym6 gene, each F2 plant of the mapping population was scored for its symbiotic phenotype by testing a minimum of 10 F3 plants inoculated with Sinorhizobium meliloti strain A145 as described by Tirichine et al[31]. The nitrogen fixation phenotype (Fix) of F3 individuals was scored 30 days after inoculation and treated as a codominant marker. Thus the heterozygous F2 were identified by the presence of both Fix+ and Fix- in their progeny. For the SPC gene, each F2 plant of the mapping population was scored for pod coiling (clockwise or anticlockwise) and the marker was treated as dominant.
Acknowledgments
Acknowledgements
This work was supported in part by grants from INRA/CNRS for the Medicago truncatula genome project (1998–2000). We thank E. Jenczewski (INRA, Rennes, France), S. Santoni (INRA, Montpellier, France) and P. Frendo (CNRS, Nice, France) for their help with designing microsatellites markers for MtLec2, ATPase and GSHS, respectively. We thank S. Brown (CNRS, Gif s/Yvette, France) for his help with flow cytometry measurements. We are grateful to GB. Kiss and P. Kalo for providing alfalfa mapping data before publication. We thank DG. Barker and J. Dénarié for their critical reading of the manuscript. PT and AK were recipients of postdoctoral fellowships from INRA (Santé des Plantes et Environnement Department). JMA was a recipient of a grant from the Ministère de la Recherche.
Contributor Information
Philippe Thoquet, Email: thoquet@toulouse.inra.fr.
Michele Ghérardi, Email: gherardi@toulouse.inra.fr.
Etienne-Pascal Journet, Email: journet@toulouse.inra.fr.
Attila Kereszt, Email: Kereszta@nucleus.szbk.u-szeged.hu.
Jean-Michel Ané, Email: jmane@touluse.inra.fr.
Jean-Marie Prosperi, Email: prosperi@ensam.inra.fr.
Thierry Huguet, Email: thuguet@toulouse.inra.fr.
References
- Gianinazzi-Pearson V. Plant cell responses to arbuscular mycorrhizal fungi: getting to the roots of the symbiosis. The Plant Cell. 1996;8:1871–1883. doi: 10.1105/tpc.8.10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht C, Geurts R, Bisseling T. Legume nodulation and mycorrhizae formation: two extremes in host specificity meet. EMBO J. 1999;18:281–288. doi: 10.1093/emboj/18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Dénarié J. Progress in the genomics of Medicago truncatula and the promise of its application to grain legume crops. Grain Legumes. 2000;28:12–13. [Google Scholar]
- Barker DG, Bianchi S, Blondon F, Dattée Y, Duc G, Flament P, Gallusci P, Génier G, Guy P, Muel X, Tourneur J, Dénarié J, Huguet T. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-iegume symbiosis. Plant Mol Biol Rep. 1990;8:40–49. [Google Scholar]
- Cook D, Vandenbosch K, DeBruijn F, Huguet T. Model legumes get the Nod. Plant Cell. 1997;9:275–281. doi: 10.1105/tpc.9.3.275. [DOI] [Google Scholar]
- Cook D. Medicago truncatula: A model in the making ! Current Opinion in Plant Biology. 1999;2:301–304. doi: 10.1016/S1369-5266(99)80053-3. [DOI] [PubMed] [Google Scholar]
- Blondon F, Marie D, Brown S, Kondorosi A. Genome size and base composition in Medicago sativa and M. truncatula species. Genome. 1994;37:264–270. doi: 10.1139/g94-037. [DOI] [PubMed] [Google Scholar]
- Trumble HC. Barrel medic (Medicago tribuloides, Desr.) as a pasture legume. J Agric S Austr. 1939;42:953. [Google Scholar]
- Prosperi JM. Selection of annual medics for French Mediterranean regions. Workshop on Introducing the Ley Farming System in the Mediterranean Basin (edited by S. Christiansen, L. Materon, M. Falcinelli and P. Cocks) International Center for Agricultural Research in the Dry Areas, P.O. Box 5466, Aleppo, Syria. 1993. pp. 173–191.
- Prosperi JM, Auricht G, Génier G, Johnson R. Medics (Medicago L.). Plant Genetic Resources of Legume in the Mediterranean (eds N. Maxted and S.J. Bennett), Kluwer Academic Publishers. 2001. pp. 99–114.
- Galibert F, Finan TM, Long SR, Puhler A, Abola P, Ampe F, Barloy-Hubler F, Barnett MJ, Becker A, Boistard P, et al. The composite genome of the legume symbiont Sinorhizobium meliloti. Science. 2001;293:668–672. doi: 10.1126/science.1060966. [DOI] [PubMed] [Google Scholar]
- Sagan M, Morandi D, Tarenghi E, Duc G. Selection of nodulation and mycorhizal mutants in the model plant Medicago truncatula Gaertn after gamma rays mutagenesis. Plant Science. 1995;111:63–71. doi: 10.1016/0168-9452(95)04229-N. [DOI] [Google Scholar]
- Liu H, Trieu AT, Blaylock LA, Harrison MJ. Cloning and characterization of two phosphate transporters from Medicago truncatula roots: regulation in response to phosphate and to colonization by arbuscular mycorrhizal (AM) fungi. Mol Plant Microbe Interact. 1998;11:14–22. doi: 10.1094/MPMI.1998.11.1.14. [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- Cowitz PA, Smith LS, Long SR. Expressed sequence tags from a root-hair enriched Medicago truncatula cDNA library. Plant Physiology. 1998;117:1325–1332. doi: 10.1104/pp.117.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamas P, Niebel FD, Lescure N, Cullimore JV. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol Plant Microbe Interact. 1996;9:233–242. doi: 10.1094/mpmi-9-0233. [DOI] [PubMed] [Google Scholar]
- Györgyey J, Vaubert D, Jiménez-Zurdo JI, Charon C, Troussard L, Kondorosi A, Kondorosi E. Analysis of Medicago truncatula nodule expressed tags. Mol Plant Microbe Interact. 2000;13:62–71. doi: 10.1094/MPMI.2000.13.1.62. [DOI] [PubMed] [Google Scholar]
- Nam YW, Penmetsa RV, Endre G, Uribe P, Kim DJ, Cook DR. Construction of a bacterial artificial chromosome library of Medicago truncatula and identification of clones containing ethylene-response genes. Theor Appl Genet. 1999;98:638–646. doi: 10.1007/s001220051115. [DOI] [Google Scholar]
- Chabaud M, Larsonnaud C, Marmouget C, Huguet T. Transformation of Barrel Medic (Medicago truncatula Gaertn.) by Agrobacterium tumefaciens and regeneration via somatic embryogenesis of transgenic plants with the MtENOD12 nodulin promoter fused to the GUS gene. Plant Cell Report. 1996;15:305–310. doi: 10.1007/s002990050022. [DOI] [PubMed] [Google Scholar]
- Trinh TH, Ratet P, Kondorosi E, Durand P, Kamate K, Bauer P, Kondorosi A. Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp falcata lines improved in somatic embryogenesis. Plant Cell Report. 1998;17:345–355. doi: 10.1007/s002990050405. [DOI] [PubMed] [Google Scholar]
- Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, Weigel D, Harrison MJ. Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J. 2000;22:531–541. doi: 10.1046/j.1365-313X.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL, Ballenger JA, Palmer JD. The distribution and phylogenetic significance of a 50 kb chloroplast DNA inversion in the flowering plant family Leguminosae. Mol Phylogenet Evol. 1996;5:429–438. doi: 10.1006/mpev.1996.0038. [DOI] [PubMed] [Google Scholar]
- Handberg K, Stougaard J. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. Plant J. 1992;2:487–496. [Google Scholar]
- Jiang Q, Gresshoff PM. Classical and molecular genetics of the model legume Lotus japonicus. Mol Plant Microbe Interact. 1997;10:59–68. doi: 10.1094/MPMI.1997.10.1.59. [DOI] [PubMed] [Google Scholar]
- Kiss GB, Csanadi G, Kalman K, Kalo P, Ökrész L. Construction of a basic genetic map of Medicago using RFLP, RAPD, Isozyme and morphological markers. Mol Gen Genet. 1993;238:129–137. doi: 10.1007/BF00279539. [DOI] [PubMed] [Google Scholar]
- Brummer EC, Bouton JH, Kochert G. Development of an RFLP map in diploid alfalfa. Theor Appl Genet. 1993;86:329–332. doi: 10.1007/BF00222097. [DOI] [PubMed] [Google Scholar]
- Echt CS, Kidwell KK, Knapp SJ, Osborn TC, McCoy TJ. Linkage mapping in diploid alfalfa (Medicago sativa). Genome. 1994;37:61–71. doi: 10.1139/g94-008. [DOI] [PubMed] [Google Scholar]
- Tavoletti S, Veronesi F, Osborn TC. RFLP linkage map of an alfalfa meiotic mutant based on an F1 population. J Hered. 1996;87:167–170. [Google Scholar]
- Kalo P, Endre G, Zimanyi L, Csanadi G, Kiss GB. Construction of an improved linkage map of diploid alfalfa (Medicago sativa). Theor Appl Genet. 2000;100:641–657. doi: 10.1007/s001220051335. [DOI] [Google Scholar]
- Brouwer DJ, Osborn TC. A molecular marker linkage map of tetraploid alfalfa (Medicago sativa L.). Theor Appl Genet. 1999;99:1194–1200. doi: 10.1007/s001220051324. [DOI] [Google Scholar]
- Tirichine L, De Billy F, Huguet T. Mtsym6, a gene conditioning Sinorhizobium strain-specific nitrogen fixation in Medicago truncatula. Plant Physiol. 2000;123:845–851. doi: 10.1104/pp.123.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienfeld FA, Kihara H. Dextrality and Sinistrality in plants. Proc Japan Acad. 1956;32:620–632. [Google Scholar]
- Bonnin I, Huguet T, Ghérardi M, Prosperi JM, Olivieri I. High level of polymorphism and spatial structure in a selfing plant species Medicago truncatula Gaertn. using RAPDs markers. American J of Botany. 1996;83:843–855. [Google Scholar]
- Chaulet E. Diversité génétique de populations naturelles de luzernes annuelles (Medicago sp.) d'Algérie. Thèse Ecole Nationale Supérieure Agronomique de Montpellier 1995, Montpellier (France)
- Bonnin I, Ronfort J, Wozniak F, Olivieri I. Spatial effects and rare outcrossing events in Medicago truncatula (Fabaceae). Molecular Ecology. 2001;10:1371–1384. doi: 10.1046/j.1365-294X.2001.01278.x. [DOI] [PubMed] [Google Scholar]
- Lesins KA, Lesins L. Genus Medicago (Leguminosae). A taxogenetic study. Dr W Junk Publishers 1979, The Hague.
- Arumuganathan K, Earle ED. Nuclear DNA content of some important plant species. Plant Mol Biol Rep. 1991;9:208–219. [Google Scholar]
- Bennett MD, Bhandol P, Leitch IJ. Nuclear DNA amounts in Angiosperms and their modern uses- 807 new estimates. Annals of Botany. 2000;86:859–909. doi: 10.1006/anbo.2000.1253. [DOI] [Google Scholar]
- Frugoli J, Harris JM. Medicago on the move. The Plant Cell. 2001;13:458–63. doi: 10.1105/tpc.13.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Nolan KE, Bicego L. The development of the highly regenerable seed line Jemalong 2HA for transformation of Medicago truncatula – implications for regenerability via somatic embryogenesis. J Plant Physiol. 1999;155:788–791. [Google Scholar]
- Penmetsa RV, Cook DR. Production and characterization of diverse developmental mutants of Medicago truncatula. Plant Physiol. 2000;123:1387–1397. doi: 10.1104/pp.123.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Trinh TH, Leung J, Kondorosi A, Kondorosi E. A new Medicago truncatula line with superior in vitro regeneration, transformation and symbiotic properties isolated through cell culture selection. Mol Plant Microbe Interact. 1997;10:307–315. [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Andrew WD, Hudson WJ. A superior strain of medic- Barrel medic 173. The Agricultural Gazette. 1954. pp. 76–80.
- Oram RN. Register of Australian Herbage Plant Cultivars, 3rd edition. CSIRO (Australia) 1990.
- Nolan KE, Rose RJ, Gorst JR. Regeneration of Medicago truncatula from tissue culture: Increase somatic embryogenesis using explants from regenerated plants. Plant Cell Rep. 1989;8:278–281. doi: 10.1007/BF00274129. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Peeters AJM, Koorneef M, Lister C, Dean C, van den Bosch N, Pot J, Kuiper MTR. Development of an AFLP based linkage map of Ler, Col and Cvi Arabidopsis thaliana ecotypes and construction of a Ler /Cvi recombinant inbred line population. Plant. 1998;14:259–271. doi: 10.1046/j.1365-313X.1998.00115.x. [DOI] [PubMed] [Google Scholar]
- Gilpin BJ, McCallum JA, Frew TJ, Timmerman-Vaughan GM. A linkage map of the pea (Pisum sativum L.) genome containing cloned sequences of known function and expressed sequence tags. (ESTs) Theor Appl Genet. 1997;95:1289–1299. doi: 10.1007/s001220050695. [DOI] [Google Scholar]
- Gentzbittel L, Mestries E, Mouzeyar S, Mazeyrat F, Badaoui S, Vear F, Tourvieille de Labrouhe D, Nicolas P. A composite map of expressed sequences and phenotypic traits of the sunflower (Helianthus annuus L.) genome. Theor Appl Genet. 1999;99:218–234. doi: 10.1007/s001220051228. [DOI] [Google Scholar]
- Cerbah M, Kevei Z, Siljak-Yakovlev S, Kondorosi E, Kondorosi A, Trinh TH. FISH chromosome mapping allowing karyotype analysis in Medicago truncatula lines Jemalong J5 and R-108-1. Mol Plant Microbe Interact. 1999;12:947–950. [Google Scholar]
- Kulikova O, Gualtieri G, Geurts R, Kim D-J, Cook D, Huguet T, de Jong H, Fransz P, Bisseling T. Integration of the FISH-pachytene and genetic maps of Medicago truncatula. Plant J. 2001;27:49–58. doi: 10.1046/j.1365-313X.2001.01057.x. [DOI] [PubMed] [Google Scholar]
- Jenczewski E, Ghérardi M, Bonnin I, Prosperi JM, Olivieri I, Huguet T. Insight on segregation distortions in two intraspecific crosses between annual species of Medicago (Leguminosae). Theor Appl Genet. 1997;94:682–691. doi: 10.1007/s001220050466. [DOI] [Google Scholar]
- Berry ST, Leon AJ, Hanfrey CC, Challis P, Burkholz A, Barnes SJ, Rufener GK, Lee M, Caligari PDS. Molecular marker analysis of Helianthus annuus L. 2. Construction of an RFLP linkage map for cultivated sunflower. Theor Appl Genet. 1995;91:195–199. doi: 10.1007/BF00220877. [DOI] [PubMed] [Google Scholar]
- Barzen E, Mechelke W, Ritter E, Schulte-Kappert E, Salamini F. An extended map of the sugar beet genome containing RFLP and RFLP loci. Theor Appl Genet. 1995;90:189–193. doi: 10.1007/BF00222201. [DOI] [PubMed] [Google Scholar]
- Quillet MC, Madjidian N, Griveau Y, Serieys H, Tersac M, Lorieux M, Bervillé A. Mapping genetic factors controlling pollen viability in an interspecific cross in Helianthus sect. Helianthus Theor Appl Genet. 1995;91:1195–1202. doi: 10.1007/BF00220929. [DOI] [PubMed] [Google Scholar]
- Schmidt R. Synteny: recent advances and future prospects. Current opinion in Plant Biology. 2000;3:97–102. doi: 10.1016/S1369-5266(99)00048-5. [DOI] [PubMed] [Google Scholar]
- Bena G, Prosperi JM, Lejeune B, Olivieri I. Evolution of annual species of the genus Medicago: A molecular phylogenetic approach. Molecular Phylogenetics and Evolution. 1998;9:552–559. doi: 10.1006/mpev.1998.0493. [DOI] [PubMed] [Google Scholar]
- Stavarek SJ, Croughan TP, Rains DW. Regeneration of plants from long-term cultures of alfalfa cells. Plant Sci Let. 1980;19:253–261. [Google Scholar]
- Pathipanawat W, Jones RAC, Sivasithamparam K. An improved method for artificial hybridization in annual Medicago species. Austr J Agric Res. 1994;45:1329–1335. [Google Scholar]
- Ghérardi M, Mangin B, Goffinet B, Bonnet D, Huguet T. A method to measure genetic distance between allogamous populations of alfalfa (Medicago sativa) using RAPD molecular markers. Theor Appl Genet. 1998;96:406–412. doi: 10.1007/s001220050756. [DOI] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Thoquet P, Olivier J, Latterot H, Grimsley N. Genetic mapping of Ph-2, a single locus controlling partial resistance to Phytophtora infestans in tomato. Mol Plant Microbe Interact. 1998;11:259–269. [Google Scholar]
- Koniecny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using coDom ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313X.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Ooijen van JW. Drawmap: a computer program for drawing genetic linkage maps. J Hered. 1994;85:66. [PubMed] [Google Scholar]
- Christiansen H, Barker DG. Unpublished results. 1998.
- Liu C, Yeung AT, Dickstein R. The cDNA sequence of Medicago truncatula cv. Jemalong ENOD8, a gene associated with nitrogen fixing root nodule organogenesis. Plant Physiol. 1998;117:1125. doi: 10.1104/pp.116.3.1125. [DOI] [Google Scholar]
- Pathirana SM, Vance CP, Miller SS, Gantt JS. Alfalfa root nodule phosphoenolpyruvate carboxylase – Characterization of the cDNA and expression in effective and plant controlled ineffective nodules. Plant Mol Biology. 1992;20:437–450. doi: 10.1007/BF00040603. [DOI] [PubMed] [Google Scholar]
- Frugier F, Kondorosi A, Crespi M. Identification of novel putative regulatory genes induced during alfalfa nodule development with a cold-plaque screening procedure. Mol Plant Microbe Interact. 1998;11:358–366. doi: 10.1094/MPMI.1998.11.5.358. [DOI] [PubMed] [Google Scholar]
- Stanford AC, Larsen K, Barker DG, Cullimore JV. Differential expression within the glutamine synthetase gene family of the model legume Medicago truncatula. Plant Physiol. 1993;103:73–81. doi: 10.1104/pp.103.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchrowitz MA, Barker DG, Nadaud I, Rouge P, Lescure B. Lectin genes from the legume Medicago truncatula. Plant Mol Biology. 1992;19:1011–1017. doi: 10.1007/BF00040532. [DOI] [PubMed] [Google Scholar]
- Pichon M, Journet EP, Dedieu A, De Billy F, Truchet G, Barker DG. Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. The Plant Cell. 1992;4:1199–1121. doi: 10.1105/tpc.4.10.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison MJ. A sugar transporter from Medicago truncatula: altered expression pattern in roots during vesicular-arbuscular (VA) mycorrhizal associations. Plant J. 1996;9:491–503. doi: 10.1046/j.1365-313X.1996.09040491.x. [DOI] [PubMed] [Google Scholar]
- Barker DG, Gallusci P, Lullien V, Kahn H, Gherardi M, Huguet T. Identification of two groups of leghemoglobin genes in alfalfa (Medicago sativa) and a study of their expression during root nodule development. Plant Mol Biology. 1988;11:761–772. doi: 10.1007/BF00019516. [DOI] [PubMed] [Google Scholar]
- Crespi MD, Jurkevitch E, Poiret M, d'Aubenton-Carafa Y, Petrovics G, Kondorosi E, Kondorosi A. enod40, a gene expressed during nodule organogenesis codes for a non-translatable RNA involved in plant growth. Embo J. 1994;13:5099–5112. doi: 10.1002/j.1460-2075.1994.tb06839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamas P, De Billy F, Truchet G. Symbiosis-specific expression of two Medicago truncatula nodulin genes, MtN1 and MtN13, encoding products homologous to plant defence proteins. Mol Plant Microbe Interact. 1998;11:393–403. doi: 10.1094/MPMI.1998.11.5.393. [DOI] [PubMed] [Google Scholar]
- Frendo P, Hernandez Jiménez MJ, Mathieu C, Duret L, Gallesi D, Van de Sype G, Hérouart D, Puppo A. A Medicago truncatula homogluthatione synthetase is derived from glutathione synthetase by gene duplication. Plant Physiol. 2001;126:1706–1715. doi: 10.1104/pp.126.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Paiva NL. Molecular cloning and expression of alfalfa (Medicago sativa L.) vestitone reductase, the penultimate enzyme in medicarpin biosynthesis. Arch Biochem Biophys. 1995;320:353–360. doi: 10.1016/0003-9861(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Greene EA, Erard M, Dedieu A, Barker DG. MtENOD16 and 20 are members of a family of phytocyanin-related early nodulins. Plant Mol Biology. 1998;36:775–783. doi: 10.1023/A:1005916821224. [DOI] [PubMed] [Google Scholar]