Abstract
Essential genes cannot be deleted from the genome; therefore, temperature-sensitive (ts) mutants and cold-sensitive (cs) mutants are very useful to discover functions of essential genes in model organisms such as Schizosaccharomyces pombe and Saccharomyces cerevisiae. To isolate ts/cs mutants for essential genes of interest, error-prone mutagenesis (or random mutagenesis) coupled with in vitro selection has been widely used. However, this method often introduces multiple silent mutations, in addition to the mutation responsible for ts/cs, with the result that one cannot discern which mutation is responsible for the ts/cs phenotype. In addition, the location of the responsible mutation introduced is random, whereas it is preferable to isolate ts/cs mutants with single amino acid substitutions, located in a targeted motif or domain of the protein of interest. To solve these problems, we have developed a method to isolate ts/cs mutants with single amino acid substitutions in targeted regions using site-directed mutagenesis. This method takes advantage of the empirical fact that single amino acid substitutions (L/S -> P or G/A -> E/D) often cause ts or cs. Application of the method to condensin and cohesin hinge domains was successful: ∼20% of the selected single amino acid substitutions turned out to be ts or cs. This method is versatile in fission yeast and is expected to be broadly applicable to isolate ts/cs mutants with single amino acid substitutions in targeted regions of essential genes. 11 condensin hinge ts mutants were isolated using the method and their responsible mutations are broadly distributed in hinge domain. Characterization of these mutants will be very helpful to understand the function of hinge domain.
Keywords: site-directed mutagenesis, temperature-sensitive mutant, cold-sensitive mutant, condensin, hinge
Forward genetic screens of ts/cs collections for mutants exhibiting specific phenotypes identified essential genes involved in cell division or chromosome segregation (Nurse and Thuriaux 1980; Hirano et al. 1986). Reverse genetic screens that analyzed ts/cs mutants of essential genes of interest have also been popular. To isolate ts/cs mutants for targeted essential genes, error-prone mutagenesis has been commonly used (Hayashi et al. 2014; Obuse et al. 2004; Xu et al. 2015). Typically error-prone PCR is performed under conditions (usually by increasing Mg2+ concentration in the reaction) to reduce the fidelity of DNA polymerase during DNA synthesis. The number of mutations increases with the number of gene duplication events (PCR cycles) (McCullum et al. 2010). If one uses higher Mg2+ concentration, ts or cs mutants are obtained more frequently after in vitro selection, but more mutations are observed in the ts or cs mutants. On the other hand, if one uses lower Mg2+ concentration, ts or cs mutants are obtained less frequently after in vitro selection, but fewer mutations are observed in the ts or cs mutants.
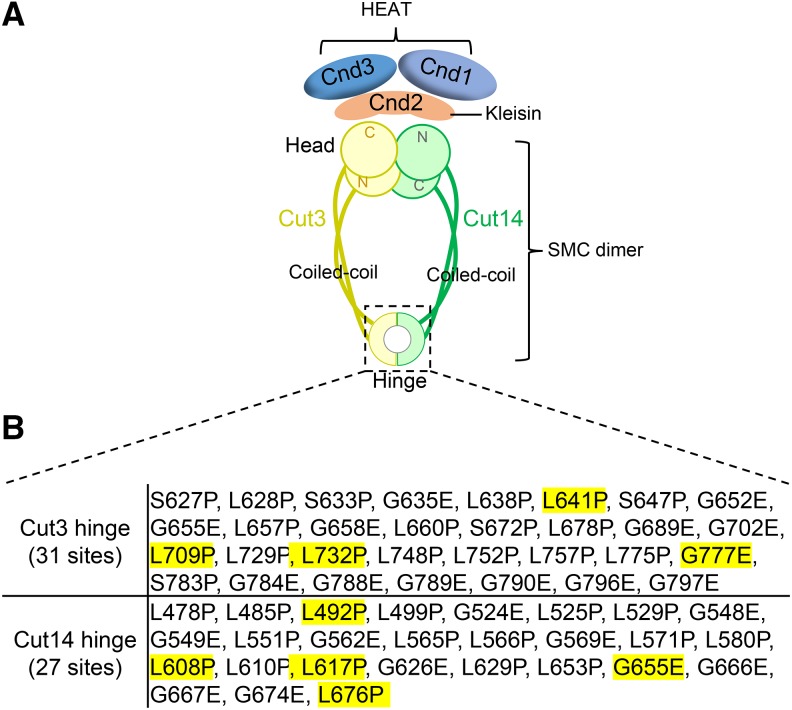
Proteins contain critical motifs or domains that execute specified functions. Condensin and cohesin are two related protein complexes essential for faithful chromosome segregation (Furuya et al. 1998; Hirano et al. 1986; Tatebayashi et al. 1998). All of their SMC (structural maintenance of chromosomes) subunits (Cut3 and Cut14 in condensin; Psm1 and Psm3 in cohesin) contain head and hinge domains that are separated by coiled coils (Hirano and Mitchison 1994; Strunnikov et al. 1993). Non-SMC subunits (Cnd1, Cnd2 and Cnd3 in condensin; Rad21, Psc3 and Mis4 in cohesin) bind to the head domains of SMC dimers. Several ts mutants are available for condensin and cohesin. For condensin, cut14-Y1 (containing a L543S substitution in the hinge) (Akai et al. 2011), cut14-aa14 (with T558L in the hinge) (Petrova et al. 2013), cut14-208 (containing S861P in the coiled coil) (Sutani and Yanagida 1997), cut3-477 (with S1147P in the coiled coil) (Sutani and Yanagida 1997) and cnd2-1 (containing A114T in the N-terminal HTH motif) (Aono et al. 2002) were identified by screening for ts mutants exhibiting chromosome segregation defects. In addition, multiple cnd (cnd1, cnd2 and cnd3) ts mutations for condensin non-SMC subunits were identified by error-prone mutagenesis (Xu et al. 2015). For cohesin, rad21-K1 (having an I67F substitution in the N-terminal HTH motif) (Tatebayashi et al. 1998; Xu et al. 2018a), psc3-407 (the responsible mutation is still unknown; Yuasa et al. 2004), and mis4-242 (with a G1326E substitution) (Furuya et al. 1998) were identified by screening for ts mutants exhibiting chromosome segregation defects. In addition, 6 ts and 6 cs cohesin hinge mutants were isolated using the method described here, proving that this technique has considerable utility (Xu et al. 2018a).
Since the method worked very well to isolate ts/cs mutants with single amino acid substitutions in the cohesin hinge, we here describe the method in detail, and then apply it to target the condensin hinge, from which few mutants were available. ∼20% of the selected mutation sites turned out to be ts mutants, therefore the success of the method is not particular and it may be applicable to any targeted region of essential genes.
Materials and Methods
Strains, plasmids, and media
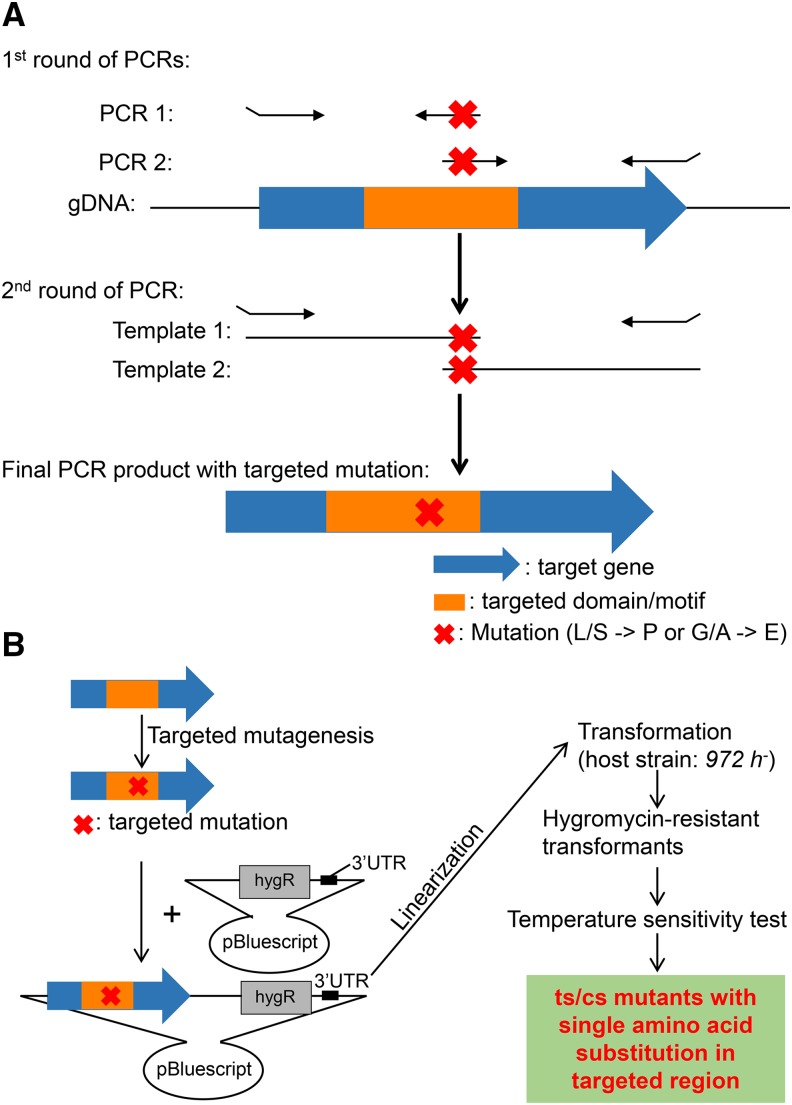
The wild-type strain 972 h- was used as the host strain for ts mutant construction. ∼500bp long sequences (3′ UTRs) after the corresponding ORFs (cut3 or cut14) were cloned and ligated into pBluescript plasmids downstream of a hygromycin-resistance antibiotic marker (hygR). Complementary pairs of synthesized DNA oligos (∼35 bp) with designed mutations were used as PCR primers (Figure 2A, Table S1 and S2), followed by two rounds of PCR (Figure S1). Mutated genes (cut3 ORF or cut14 ORF) were cloned and ligated into the pBluescript plasmids (with 3′ UTR integrated) upstream of the antibiotic marker. Then the plasmids were linearized and chromosomally integrated into corresponding endogenous loci of the aforementioned 972 h- wild-type strain using lithium acetate method described in Figure S2. Hygromycin-resistant colonies were selected on YPD plates containing 500μg/mL Hygromycin B (Wako), and then temperature-sensitive candidates, which can grow at 26° but not at 36°, were screened. ts mutations were confirmed by Sanger sequencing of mutated genes. YPD medium and plates (1% yeast extract, 2% polypeptone, 2% D-glucose) were used for culturing S. pombe strains (Forsburg and Rhind 2006).
Figure 2.
Site-directed mutagenesis. (A) Strategy to introduce targeted mutations into target genes using two-rounds of PCR. Primers used to introduce cut14 hinge mutations are presented in Table S1 and primers used to introduce cut3 hinge mutations are presented in Table S2. (B) Site-directed mutagenesis and isolation of ts/cs mutants. See ‘Materials and Methods’ for detailed description of plasmid construction and how to isolate ts/cs mutants.
Protein alignment and visualization
Cut3 and Cut14 protein sequences were downloaded from Pombase (http://pombase.org) (Wood et al. 2012). Protein sequences of Cut3 and Cut14 homologs in other organisms were downloaded from the NCBI HomoloGene Database (https://www.ncbi.nlm.nih.gov/homologene) (Wheeler et al. 2001). Protein sequences were aligned using a multiple sequence alignment program MAFFT (https://mafft.cbrc.jp/alignment/software/) (Katoh et al. 2002). Protein alignment results were visualized using a multiple sequence alignment visualization tool, Jalview (http://www.jalview.org) (Waterhouse et al. 2009).
Mutational analysis of condensin hinge ts mutations in 3D structures
An atomic model of the S. pombe condensin hinge (Akai et al. 2014) was generated from crystal structures of SMC hinges from T. maritima (PDB codes 1GXJ and 1GXL) (Haering et al. 2002) and mouse (PDB codes 2WD5 and 3L51) (Griese et al. 2010; Kurze et al. 2011) based on a sequence alignment using MODELER (Sali and Blundell 1993).
Data availability
Strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and the supplemental file. Table S1 and S2 contain lists the primers used to construct cut14 and cut3 mutants, respectively. Figure S1 describes PCR conditions used to introduce mutations into target genes. Figure S2 describes transformation protocol. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7890911.
Results
Substitution of L/S -> P or G/A -> D/E often cause ts/cs
In previous studies, ts or cs mutants with specific phenotypes were isolated by forward genetic screening of the collection of ts/cs mutants (Nurse and Thuriaux 1980; Hirano et al. 1986). We selected and used those that exhibited chromosome segregation defects at the restrictive temperature. Many of them contain substitutions from Leucine (L)/Serine (S) to Proline (P) or from Glycine (G)/Alanine (A) to Aspartic Acid (D)/Glutamic Acid (E) (L/S -> P or G/A -> D/E), as shown in Table 1. In addition, in error-prone mutagenesis for cnd mutants, 10 of 21 ts or damage-sensitive mutants with single amino acid substitutions introduced proline (Xu et al. 2015).
Table 1. ts/cs mutants with amino acid substitutions L/S -> P or G/A -> E/D.
| Gene | Allele | ts/cs | aa change | Reference |
|---|---|---|---|---|
| cnd1 | cnd1-L193P | ts | L193P | Xu et al. 2015 |
| cnd1-L331P | ts | L331P | ||
| cnd1-L685P | ts | L685P | ||
| cnd2 | cnd2-ae9 | ts | L103P | Petrova et al. 2013 |
| cnd3 | cnd3-L126P | ts | L126P | Xu et al. 2015 |
| cnd3-L269P | ts | L269P | ||
| cut3 | cut3-l23 | ts | S1116P | Petrova et al. 2013 |
| cut3-477 | ts | S1147P | Sutani and Yanagida 1997 | |
| cut14 | cut14-208 | ts | S861P | |
| mis17 | mis17-362 | ts | S353P | Hayashi et al. 2004 |
| cut8 | cut8-563 | ts | S201P | Takeda et al. 2011 |
| mis14 | mis14-271 | ts | L106P | Hayashi et al. 2004 |
| mis14-634 | ts | S130P | ||
| nuf2 | nuf2-1 | ts | S189P | Nabetani et al. 2001 |
| nuf2-3 | ts | L246P | ||
| psm3 | psm3-A561E | cs | A561E | Xu et al. 2018a |
| mis4 | mis4-242 | ts | G1326E | Furuya et al. 1998 |
| mis6 | mis6-302 | ts | G135E | Saitoh et al. 1997 |
| mis12 | mis12-537 | ts | G52E | Goshima et al. 2003 |
| nda3 | nda3-KM311 | cs | G93E | Paluh et al. 2000 |
| mis18 | mis18-262 | ts | G117D | Hayashi et al. 2004 |
| nuc2 | nuc2-663 | ts | G504D | Samejima and Yanagida 1994 |
| htb1 | htb1-72 | ts | G52D | Maruyama et al. 2006 |
| eso1 | eso1-H17 | ts | G799D | Tanaka et al. 2000 |
| cdc48 | cdc48-353 | ts | G338D | Ikai and Yanagida 2006 |
| cut14 | cut14-r8 | ts | G10D | Petrova et al. 2013 |
| clr6 | clr6-1 | ts | G269D | Grewal et al. 1998 |
Selection of conserved L/S/G/A amino acids for mutagenesis
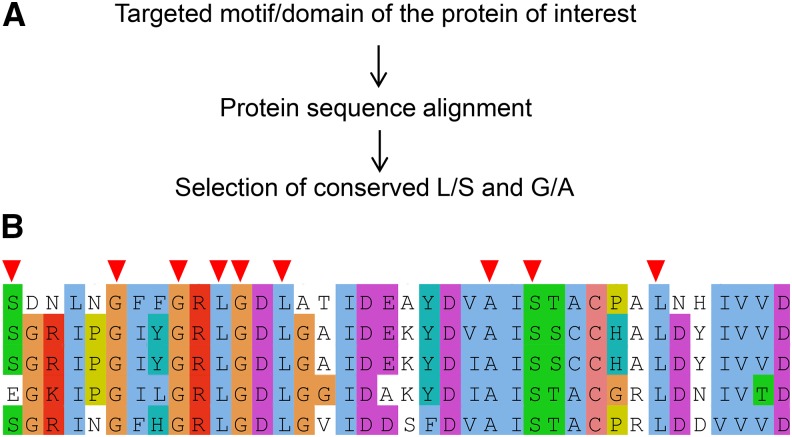
We intended to develop a ts/cs isolation method based on site-directed mutagenesis by taking advantage of the aforementioned observations (Table 1) to isolate ts/cs mutants with single amino acid substitutions in targeted motifs/domains of essential genes of interest. To perform site-directed mutagenesis, one needs to decide which protein and which domain/motif is the target based on available information (such as structural and functional annotations from a database for the fission yeast Schizosaccharomyces pombe, PomBase) (Wood et al. 2012). Then homologous protein sequences from different species are downloaded from databases, for example, the NCBI HomoloGene Database (https://www.ncbi.nlm.nih.gov/homologene) (Wheeler et al. 2001) and are aligned using MAFFT (https://mafft.cbrc.jp/alignment/software/) (Katoh et al. 2002). Based on alignment results, one needs to select conserved L/S/G/A amino acids in the target domain/motif (Figure 1A and 1B).
Figure 1.
Selection of amino acids for mutagenesis. (A) A brief procedure to select conserved L/S/G/A amino acids in the motif/domain of interest in the target protein. (B) Protein alignment result of a portion of condensin Cut3 hinge as an example. Selected amino acids (L/S/G/A) for site-directed mutagenesis are marked with red arrowheads.
Site-directed mutagenesis for mutants
Based on mutations depicted in Figure 1, PCR primers are designed to introduce mutations into the DNA sequence of the target gene (see ‘Materials and Methods’, Figure S1 and Figure S2). Two rounds of PCR are performed by following the procedure described in Figure 2A. DNA polymerase and PCR conditions are described in Figure S1. The target gene with a designed mutation was ligated into a pBluescript plasmid with its 3′ UTR (∼500 bp DNA sequences downstream of the gene) already integrated. Linearized plasmids were transformed into the 972 h- wild-type strain using lithium acetate method described in Figure S2. After hygromycin B (500μg/mL) selection for integrants, colonies were selected and streaked under three different culture conditions to measure their temperature sensitivity (36° for ts mutants, 30° as a control, and 20° for cs mutants) (Figure 2B). The number of colonies needed varies, but usually between 4 and 16 is sufficient.
Cohesin hinge ts/cs mutants isolated
Suppressors that overcome inactive separase/Cut1 or securin/Cut2 were identified and found to be located at cohesin interfaces, including the hinge (Xu et al. 2018a). To examine cut1’s suppression by cohesin hinge mutations and to further understand the underlying mechanism, more cohesin hinge mutants were conceived. Specifically, 59 conserved L/S/G/A amino acids in the cohesin hinge (26 in the Psm1 hinge and 33 in the Psm3 hinge) were selected for site-directed mutagenesis. After screening for ts/cs mutants, 12 ts/cs mutants (6 ts and 6 cs) were obtained (∼20% of the total selected amino acids).
Isolation of condensin hinge ts mutants
cut14-Y1 (Akai et al. 2011) and cut14-aa14 (Petrova et al. 2013) are the only two condensin hinge ts mutants containing L543S and T558L substitutions as their responsible mutations, respectively. No Cut3 hinge ts mutant is available yet. cut14-Y1 was important to understand the function of the hinge in DNA association and release, and also led to the discovery of the hinge-head interaction through transient phosphorylation of the hinge by the head ATPase (Akai et al. 2011; Akai et al. 2014). Application of the method described here to isolate cohesin hinge ts/cs mutants was successful (Xu et al. 2018a); therefore, to examine the versatility of the method, here we applied the method to isolate condensin hinge ts mutants (Figure 3A). Based on the homologous protein sequence alignment, conserved L/S/G/A amino acids in the Cut3 and Cut14 hinge domains were selected. 31 mutations in the Cut3 hinge domain and 27 mutations in the Cut14 hinge domain were designed for site-directed mutagenesis (Figure 3B).
Figure 3.
Mutations in the condensin hinge selected for site-directed mutagenesis. (A) Condensin is a heteropentameric complex required for faithful chromosome condensation and segregation. Cut3 and Cut14 hinges form a heterodimer containing two interfaces. (B) To understand condensin hinge’s function, conserved L/S/G/A amino acids were selected for site-directed mutagenesis. 31 mutations in Cut3 hinge and 27 mutations in Cut14 hinge were designed. Site-directed mutagenesis and isolation of condensin hinge ts mutants followed the procedure described in Figure 2B.
Condensin hinge ts mutants obtained
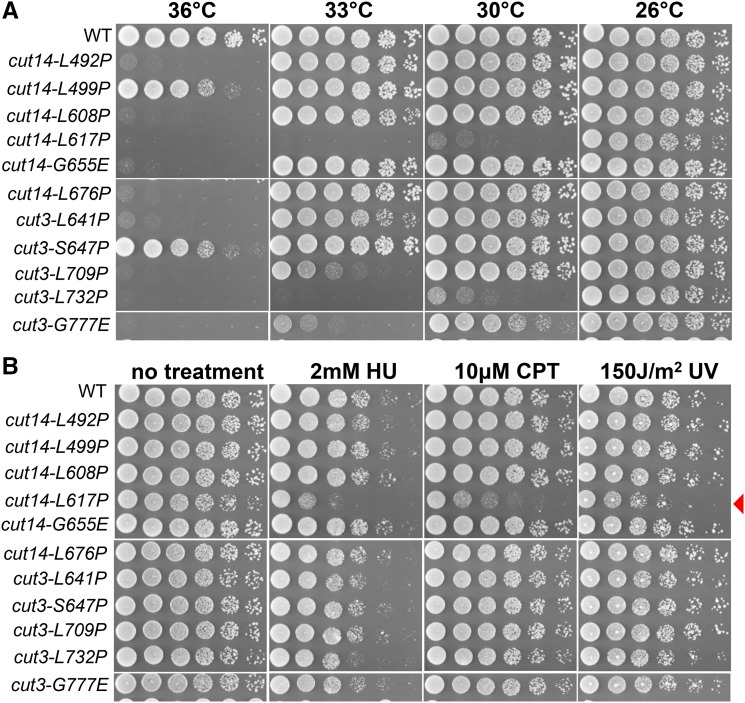
In total, 11 ts mutants with single amino acid substitutions in condensin hinge domains were obtained and 9 of them presented strong temperature sensitivity (Figure 4A). In total, 58 conserved L/S/G/A amino acids were selected (Figure 3B); therefore, ∼20% of the single amino acid substitutions caused ts. If selection for cs mutants was considered too, perhaps more than 20% of the single amino acid substitutions cause ts/cs. Among the 11 newly isolated condensin hinge ts mutants, 6 are cut14 hinge ts mutants and the other 5 are cut3 hinge ts mutants. Since the previously isolated condensin hinge ts mutant, cut14-Y1, was hyper-sensitive to DNA damaging agents at the permissive temperature (26°) (Akai et al. 2011), we examined sensitivity of these newly isolated condensin hinge ts mutants to DNA damaging agents (hydroxyurea, camptothecin and ultraviolet) at the permissive temperature (26°). Among the 11 ts mutants, only cut14-L617P was sensitive to DNA damaging agents (Figure 4B). Therefore in this study, we identified 10 condensin hinge ts mutants, which are ts, but not sensitive to DNA damaging agents (at least at 26°. It’s still possible that some of them will be sensitive to DNA damaging agents when incubated at 30° or 33°).
Figure 4.
Condensin hinge ts mutants with single amino acid substitutions. (A) Spot test results describing the temperature sensitivity of the 11 condensin hinge ts mutants newly isolated from the 58 mutations described in Figure 3B. 9 of the 11 condensin hinge ts mutants are strongly sensitive to high temperature, while the temperature sensitivity of cut14-L499P and cut3-S647P is weak. (B) Sensitivity of newly isolated condensin hinge ts mutants to DNA damaging agents at the permissive temperature (26°C). Among the 11 ts mutants, only cut14-L617P is sentive to damage. HU, CPT and UV designate hydroxyurea, camptothecin and ultraviolet respectively.
Localization of condensin hinge ts mutations
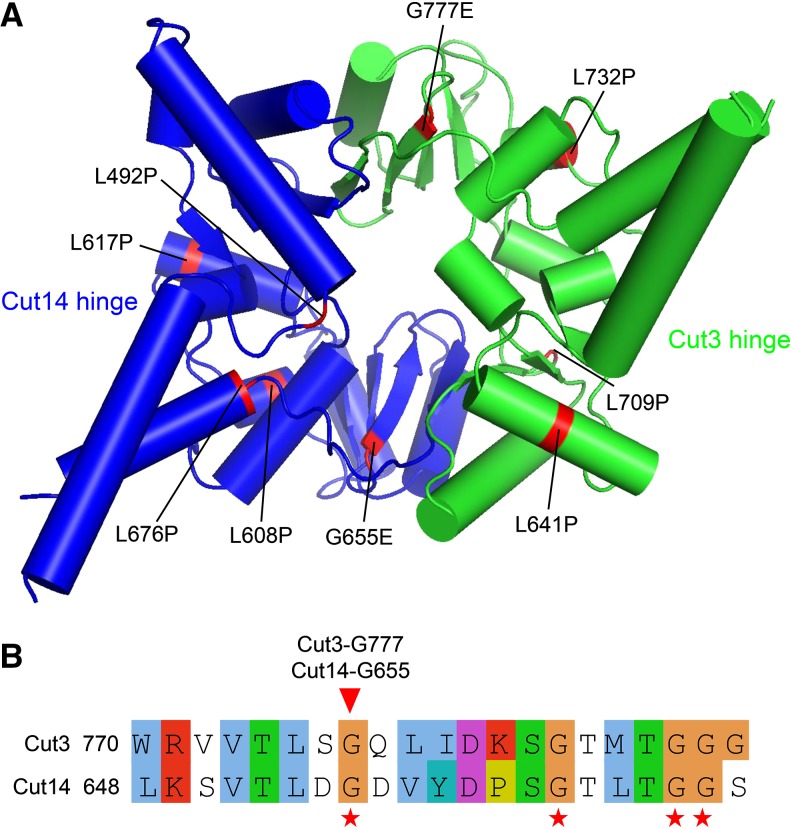
To understand these ts mutations, we mapped them onto the condensin hinge structure and found that they are broadly distributed (Figure 5A). However, Cut14-G655 and Cut3-G777 are located at the two hinge dimerization interfaces. Protein sequence alignment between the Cut14 and Cut3 hinges indicated that Cut14-G655 and Cut3-G777 are at the same position in the 3D structure, but at different interfaces (Figure 5B). A conserved arrangement of glycine residues (GX6GX3GG sequence motif) is normally found in hinge dimerization interfaces (Figure 5B), and it is required for hinge dimerization (Hirano et al. 2001; Hirano and Hirano 2002; Hirano and Hirano 2006). Cut14-G655 and Cut3-G777 are located in this GX6GX3GG sequence motif. Mutation of Cut14-G655 or Cut3-G777 to E may affect hinge dimerization. Alternatively, a ‘hold and release’ model, in which head and hinge interact to form arched coiled coils that hold chromosomal DNAs inside, has been proposed (Xu et al. 2018a), Cut14-G655E and Cut3-G777E may affect the angle formed by the coiled coils emerging from hinge and further affect hinge’s DNA binding ability.
Figure 5.
Localization of newly isolated condensin hinge ts mutations in the 3D structure. (A) Mutations of the newly isolated condensin hinge ts mutants were mapped onto the atomic structure of the condensin hinge. The Cut14 hinge is in blue and the Cut3 hinge is in green. The broadly distributed mutations are highlighted in red. (B) Alignment of the Cut3 and Cut14 hinges around the conserved arrangement of glycine residues (GX6GX3GG sequence motif), which is normally found in hinge dimerization interfaces. Conserved glycines are marked with red asterisks below the alignment. Localization of Cut14-G655 and Cut3-G777 is indicated with the red arrowhead. Cut14-G655 and Cut3-G777 are in same position, but at different interfaces.
Discussion
The method developed here can be used to isolate ts/cs mutants with single amino acid substitutions in targeted regions of essential genes in both Schizosaccharomyces pombe and Saccharomyces cerevisiae. It has great advantages in that single amino acid substitutions are introduced and mutations are located in the domain/motif of interest. ∼20% of conserved L/S/G/A amino acids selected for site-directed mutagenesis caused ts/cs; therefore, the chance to obtain ts/cs mutants is high using the method described here.
Error-prone mutagenesis (or random mutagenesis) was frequently used to isolate ts or cs mutants for essential genes. In a previous study using error-prone mutagenesis for ts mutants of condensin non-SMC genes (cnd1, cnd2 and cnd3) (Xu et al. 2015), in total 59 ts mutants were isolated from ∼18,000 transformant colonies (∼0.3%), and among these 59 ts mutants only 16 of them contain a single amino acid substitution (∼0.1%). In another study using error-prone mutagenesis for ts mutants of sam1 gene (which encodes a S-adenosylmethionine synthetase; Hayashi et al. 2018), 5 ts mutants were obtained from ∼3000 colonies screened (∼0.2%) and 2 of them contain a single amino acid substitution (∼0.07%). Therefore the frequency to get ts mutants with single amino acid substitution is low. By using the method described here, ∼20% of the designed mutations caused ts or cs, therefore there’s no need to screen many colonies. In addition, responsible mutations of the ts mutants, isolated using error-prone mutagenesis, is random (Hayashi et al. 2018; Xu et al. 2015), they may not locate in the domain or motif of interest. The method described here designs mutations, by taking advantage of the empirical fact that single amino acid substitutions (L/S -> P or G/A -> E/D) often cause ts or cs, in the target domain or motif of interest, therefore all the responsible mutations of the ts or cs mutants isolated are located in the target domain or motif. However, not much information about the genes of interest are required before error-prone mutagenesis, protein features of the genes of interest are required in this method and more plasmid construction works are required prior to screening.
The 11 ts mutants isolated for condensin hinge is valuable to understand hinge’s function. Among the 11 ts mutants, cut14-L617P and cut3-L732P are the strongest, they can’t grow even at 30° (Figure 4A). However, we still don’t know why their temperature sensitivities are the strongest only from the mutations’ locations in the hinge structure (Figure 5A). In cohesin, it is proposed in a ‘hold and release’ model (Xu et al. 2018a) that hinge mutations affect coiled coils that are connected to hinge. Orientation of coiled coils, which hold and release chromosomal DNAs in between, was largely changed in a cohesin hinge cs mutant, psm3-A561E. Whether these condensin ts mutants affect coiled coils connected to the hinge is still unknown yet.
An efficient and cost-effective suppressor mutation identification method using next-generation sequencing of genomic DNA Mixture was developed (Xu et al. 2018b). Therefore, ts/cs mutants isolated can be further applied for suppressor screening. The combination provides a complete pipeline to understand the function of essential genes and further identifies pathways that regulate the gene’s function.
ts/cs mutations often disorder protein structure or protein-protein interactions in a protein complex, and suppressors of the original ts/cs mutation often occur close to the original ts/cs mutation in protein/protein complex structure. Suppressors restore structural defects caused by the original ts/cs mutation. The current method identifies ts/cs mutants with single amino acid substitutions in targeted motifs/domains, and in combination with suppressor screening, identifies amino acid sequences (either in the same protein or in different proteins in the same complex) interacting with the original ts/cs mutation in the 3D structure. In cohesin, the N-terminal HTH motif (Helix-Turn-Helix) of Rad21 interacts with the Psm3 head-coiled coil junction (Gligoris et al. 2014; Huis in ’t Veld et al. 2014). Suppressors of rad21-K1 (containing an I67F substitution in the Rad21 N-terminal HTH motif) were identified in the Psm3 head-coiled coil junction (Xu et al. 2018a) that interacts with the N-terminal HTH motif of Rad21. Most intragenic suppressors of mis4-242 (containing a G1326E substitution at its C-terminus) were located in two regions (AA: 642∼878 in the Mis4 middle region and AA: 1316∼1415 at the Mis4 C-terminus) (Xu et al. 2018a). The Mis4 C-terminal region (AA: 1316∼1415) is near the original ts mutation G1326E, while the Mis4 middle region (AA: 642∼878) in far from the original ts mutation G1326E in the protein sequence. Mis4 represents a hooked structure, in which the two regions are brought close in the 3D structure (Chao et al. 2015; Chao et al. 2017; Kikuchi et al. 2016). Overall, suppressors of ts/cs mutants reflect the 3D structure of the protein or organization of the protein complex.
The condensin (and cohesin) hinge ts mutants will be very useful to identify proteins or domains in condensin complex itself that may directly interact with hinge domain. Head and hinge of cohesin, which are far in planar, were proposed to interact in a ‘hold and release’ model (Xu et al. 2018a), if suppressor mutations of these condensin and cohesin ts/cs mutants can be identified and mapped in head domain or head-associating non-SMC subunits, it will be a strong evidence to support the ‘hold and release’ model.
Acknowledgments
We thank Dr. Norihiko Nakazawa for his valuable comments, Dr. Steven D. Aird for technical editing. Generous support from the Okinawa Institute of Science and Technology Graduate University is gratefully acknowledged.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.7890911.
Communicating editor: B. Andrews
Literature Cited
- Akai Y., Kanai R., Nakazawa N., Ebe M., Toyoshima C., et al. , 2014. ATPase-dependent auto-phosphorylation of the open condensin hinge diminishes DNA binding. Open Biol. 4: 140193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akai Y., Kurokawa Y., Nakazawa N., Tonami-Murakami Y., Suzuki Y., et al. , 2011. Opposing role of condensin hinge against replication protein A in mitosis and interphase through promoting DNA annealing. Open Biol. 1: 110023 10.1098/rsob.110023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono N., Sutani T., Tomonaga T., Mochida S., Yanagida M., 2002. Cnd2 has dual roles in mitotic condensation and interphase. Nature 417: 197–202. 10.1038/417197a [DOI] [PubMed] [Google Scholar]
- Chao W. C., Murayama Y., Munoz S., Costa A., Uhlmann F., et al. , 2015. Structural Studies Reveal the Functional Modularity of the Scc2-Scc4 Cohesin Loader. Cell Reports 12: 719–725. 10.1016/j.celrep.2015.06.071 [DOI] [PubMed] [Google Scholar]
- Chao W. C., Murayama Y., Munoz S., Jones A. W., Wade B. O., et al. , 2017. Structure of the cohesin loader Scc2. Nat. Commun. 8: 13952 10.1038/ncomms13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Rhind N., 2006. Basic methods for fission yeast. Yeast 23: 173–183. 10.1002/yea.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K., Takahashi K., Yanagida M., 1998. Faithful anaphase is ensured by Mis4, a sister chromatid cohesion molecule required in S phase and not destroyed in G1 phase. Genes Dev. 12: 3408–3418. 10.1101/gad.12.21.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gligoris T. G., Scheinost J. C., Burmann F., Petela N., Chan K. L., et al. , 2014. Closing the cohesin ring: structure and function of its Smc3-kleisin interface. Science 346: 963–967. 10.1126/science.1256917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Iwasaki O., Obuse C., Yanagida M., 2003. The role of Ppe1/PP6 phosphatase for equal chromosome segregation in fission yeast kinetochore. EMBO J. 22: 2752–2763. 10.1093/emboj/cdg266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S. I., Bonaduce M. J., Klar A. J., 1998. Histone deacetylase homologs regulate epigenetic inheritance of transcriptional silencing and chromosome segregation in fission yeast. Genetics 150: 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griese J. J., Witte G., Hopfner K. P., 2010. Structure and DNA binding activity of the mouse condensin hinge domain highlight common and diverse features of SMC proteins. Nucleic Acids Res. 38: 3454–3465. 10.1093/nar/gkq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering C. H., Lowe J., Hochwagen A., Nasmyth K., 2002. Molecular architecture of SMC proteins and the yeast cohesin complex. Mol. Cell 9: 773–788. 10.1016/S1097-2765(02)00515-4 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Ebe M., Nagao K., Kokubu A., Sajiki K., et al. , 2014. Schizosaccharomyces pombe centromere protein Mis19 links Mis16 and Mis18 to recruit CENP-A through interacting with NMD factors and the SWI/SNF complex. Genes Cells 19: 541–554. 10.1111/gtc.12152 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., et al. , 2004. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell 118: 715–729. 10.1016/j.cell.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Hayashi, T., T. Teruya, R. Chaleckis, S. Morigasaki and M. Yanagida, 2018 S-Adenosylmethionine Synthetase Is Required for Cell Growth, Maintenance of G0 Phase, and Termination of Quiescence in Fission Yeast. iScience 5: 38–51. [DOI] [PMC free article] [PubMed]
- Hirano M., Anderson D. E., Erickson H. P., Hirano T., 2001. Bimodal activation of SMC ATPase by intra- and inter-molecular interactions. EMBO J. 20: 3238–3250. 10.1093/emboj/20.12.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Hirano T., 2002. Hinge-mediated dimerization of SMC protein is essential for its dynamic interaction with DNA. EMBO J. 21: 5733–5744. 10.1093/emboj/cdf575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M., Hirano T., 2006. Opening closed arms: long-distance activation of SMC ATPase by hinge-DNA interactions. Mol. Cell 21: 175–186. 10.1016/j.molcel.2005.11.026 [DOI] [PubMed] [Google Scholar]
- Hirano T., Funahashi S., Uemura T., Yanagida M., 1986. Isolation and characterization of Schizosaccharomyces pombe cutmutants that block nuclear division but not cytokinesis. EMBO J. 5: 2973–2979. 10.1002/j.1460-2075.1986.tb04594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Mitchison T. J., 1994. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell 79: 449–458. 10.1016/0092-8674(94)90254-2 [DOI] [PubMed] [Google Scholar]
- Huis in ’t Veld P. J., Herzog F., Ladurner R., Davidson I. F., Piric S., et al. , 2014. Characterization of a DNA exit gate in the human cohesin ring. Science 346: 968–972. 10.1126/science.1256904 [DOI] [PubMed] [Google Scholar]
- Ikai N., Yanagida M., 2006. Cdc48 is required for the stability of Cut1/separase in mitotic anaphase. J. Struct. Biol. 156: 50–61. 10.1016/j.jsb.2006.04.003 [DOI] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T., 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30: 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S., Borek D. M., Otwinowski Z., Tomchick D. R., Yu H., 2016. Crystal structure of the cohesin loader Scc2 and insight into cohesinopathy. Proc. Natl. Acad. Sci. USA 113: 12444–12449. 10.1073/pnas.1611333113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurze A., Michie K. A., Dixon S. E., Mishra A., Itoh T., et al. , 2011. A positively charged channel within the Smc1/Smc3 hinge required for sister chromatid cohesion. EMBO J. 30: 364–378. 10.1038/emboj.2010.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Nakamura T., Hayashi T., Yanagida M., 2006. Histone H2B mutations in inner region affect ubiquitination, centromere function, silencing and chromosome segregation. EMBO J. 25: 2420–2431. 10.1038/sj.emboj.7601110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullum E. O., Williams B. A., Zhang J., Chaput J. C., 2010. Random mutagenesis by error-prone PCR. Methods Mol. Biol. 634: 103–109. 10.1007/978-1-60761-652-8_7 [DOI] [PubMed] [Google Scholar]
- Nabetani A., Koujin T., Tsutsumi C., Haraguchi T., Hiraoka Y., 2001. A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma 110: 322–334. 10.1007/s004120100153 [DOI] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., 1980. Regulatory genes controlling mitosis in the fission yeast Schizosaccharomyces pombe. Genetics 96: 627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse C., Iwasaki O., Kiyomitsu T., Goshima G., Toyoda Y., et al. , 2004. A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6: 1135–1141. 10.1038/ncb1187 [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Nogales E., Oakley B. R., McDonald K., Pidoux A. L., et al. , 2000. A mutation in gamma-tubulin alters microtubule dynamics and organization and is synthetically lethal with the kinesin-like protein pkl1p. Mol. Biol. Cell 11: 1225–1239. 10.1091/mbc.11.4.1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova B., Dehler S., Kruitwagen T., Heriche J. K., Miura K., et al. , 2013. Quantitative analysis of chromosome condensation in fission yeast. Mol. Cell. Biol. 33: 984–998. 10.1128/MCB.01400-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S., Takahashi K., Yanagida M., 1997. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell 90: 131–143. 10.1016/S0092-8674(00)80320-7 [DOI] [PubMed] [Google Scholar]
- Sali A., Blundell T. L., 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234: 779–815. 10.1006/jmbi.1993.1626 [DOI] [PubMed] [Google Scholar]
- Samejima I., Yanagida M., 1994. Bypassing anaphase by fission yeast cut9 mutation: requirement of cut9+ to initiate anaphase. J. Cell Biol. 127: 1655–1670. 10.1083/jcb.127.6.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunnikov A. V., Larionov V. L., Koshland D., 1993. SMC1: an essential yeast gene encoding a putative head-rod-tail protein is required for nuclear division and defines a new ubiquitous protein family. J. Cell Biol. 123: 1635–1648. 10.1083/jcb.123.6.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutani T., Yanagida M., 1997. DNA renaturation activity of the SMC complex implicated in chromosome condensation. Nature 388: 798–801. 10.1038/42062 [DOI] [PubMed] [Google Scholar]
- Takeda K., Tonthat N. K., Glover T., Xu W., Koonin E. V., et al. , 2011. Implications for proteasome nuclear localization revealed by the structure of the nuclear proteasome tether protein Cut8. Proc. Natl. Acad. Sci. USA 108: 16950–16955. 10.1073/pnas.1103617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Yonekawa T., Kawasaki Y., Kai M., Furuya K., et al. , 2000. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol. Cell. Biol. 20: 3459–3469. 10.1128/MCB.20.10.3459-3469.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi K., Kato J., Ikeda H., 1998. Isolation of a Schizosaccharomyces pombe rad21ts mutant that is aberrant in chromosome segregation, microtubule function, DNA repair and sensitive to hydroxyurea: possible involvement of Rad21 in ubiquitin-mediated proteolysis. Genetics 148: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A. M., Procter J. B., Martin D. M., Clamp M., Barton G. J., 2009. Jalview Version 2–a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. 10.1093/bioinformatics/btp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. L., Church D. M., Lash A. E., Leipe D. D., Madden T. L., et al. , 2001. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 29: 11–16. 10.1093/nar/29.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood V., Harris M. A., McDowall M. D., Rutherford K., Vaughan B. W., et al. , 2012. PomBase: a comprehensive online resource for fission yeast. Nucleic Acids Res. 40: D695–D699. 10.1093/nar/gkr853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Kanai R., Nakazawa N., Wang L., Toyoshima C., et al. , 2018a Suppressor mutation analysis combined with 3D modeling explains cohesin’s capacity to hold and release DNA. Proc. Natl. Acad. Sci. USA 115: E4833–E4842. 10.1073/pnas.1803564115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Nakazawa N., Yanagida M., 2015. Condensin HEAT subunits required for DNA repair, kinetochore/centromere function and ploidy maintenance in fission yeast. PLoS One 10: e0119347 10.1371/journal.pone.0119347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang L., Yanagida M., 2018b Whole-Genome Sequencing of Suppressor DNA Mixtures Identifies Pathways That Compensate for Chromosome Segregation Defects in Schizosaccharomyces pombe. G3 (Bethesda) 8: 1031–1038. 10.1534/g3.118.200048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa T., Hayashi T., Ikai N., Katayama T., Aoki K., et al. , 2004. An interactive gene network for securin-separase, condensin, cohesin, Dis1/Mtc1 and histones constructed by mass transformation. Genes Cells 9: 1069–1082. 10.1111/j.1365-2443.2004.00790.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and the supplemental file. Table S1 and S2 contain lists the primers used to construct cut14 and cut3 mutants, respectively. Figure S1 describes PCR conditions used to introduce mutations into target genes. Figure S2 describes transformation protocol. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7890911.