Abstract
Objective:
The objective of this study was to investigate the effectiveness and safety of interferon (IFN) α2a as an add-on treatment for refractory Behçet’s uveitis (BU).
Methods:
In this retrospective cohort study, 30 refractory BU patients who received IFNα2a treatment in Peking Union Medical College Hospital between February 2015 and June 2018 were consecutively included. IFNα2a was used mainly as an add-on treatment for BU patients who underwent relapse under corticosteroids and conventional immunosuppressive agents. The primary outcome was treatment success rate before and after initiation of IFNα2a. Changes in ocular relapse rates, disease activity, corticosteroid- and immunosuppressant-sparing effects, as well as side effects were secondary outcomes.
Results:
A total of 30 patients (27 males and 3 females) with a mean age of 30.5 ± 8.7 years were included. Twenty-one patients (70%) were treated with at least 2 immunosuppressive agents before the initiation of IFNα2a. Treatment success was achieved in 26 patients (86.7%), and the median uveitis relapse rate decreased from 7.3 (range 2–12) to 0 (range 0–6) per patient-year (p = 0.000002) during a mean follow-up of 21.7 ± 7.5 months, corticosteroids were lowered in 25 cases (83.3%) and completely withdrawn in four (13.3%). In addition, immunosuppressive agents were reduced in number and dosage in 22 (73.3%) and 29 patients (96.7%), respectively, and were completely withdrawn in 12 cases (40%). No severe adverse events were observed and serum autoantibodies remained negative during the treatment of IFNα2a.
Conclusion:
IFNα2a is effective and relatively safe in refractory BU, with significant steroid- and immunosuppressant-sparing effects.
Keywords: Behçet’s disease, interferon-alpha, uveitis
Introduction
Behçet’s disease (BD) is a multisystemic, chronic relapsing inflammatory disorder of unknown etiology. Ocular inflammation, typified by uveitis involving both the anterior and posterior segments of the eye, was reported to occur in 32.2–56.8% of BD patients,1–3 and to be one of the leading causes of morbidity that might result in irreversible vision loss.4 An increasing number of studies have contributed to our knowledge on the sophisticated cytokine networks implicated in BD onset, evolution, and organ damage.5 Genetic studies found associations of BD with SNPs of numerous cytokines including interleukin (IL) 1, tumor necrosis factor (TNF) α, interferon (IFN) γ, IL12, and IL18. Moreover, IL17 and IL18 were related to uveitis in BD, and IL8, RANTES and MIP-1α were associated with disease activity.6 Although corticosteroids in combination with conventional immunosuppressive agents such as cyclosporin A (CsA) and azathioprine (AZA) remain the mainstay of the treatment in BD patients with uveitis, a moderate proportion of patients respond inadequately to the above agents even at their maximum therapeutic doses, and some experience intolerable side effects. Therefore, there is an urgent need for additional effective treatments to combat this disease.
The development of biologics, in particular TNF inhibitors, brought hope to refractory Behçet’s uveitis (BU) with high response rates and favorable safety profiles.7–10 However, the high cost of anti-TNF agents precludes them as the preferred treatment for long-term control of refractory BU in developing countries such as China. Evidence showing their potential link with reactivation of latent tuberculosis poses another concern in tuberculosis-endemic countries.11–14 Since they were first successfully introduced by Durand et al. in 1993,15 IFNα has been shown to have comparable effectiveness and tolerance profiles as anti-TNF agents for BU in a number of studies16–19 with a much lower cost. International and domestic reports on the clinical application of IFNα in Chinese BU patients are scarce, but a large cohort from a single center in southwest China was recently reported by Yang et al.20 Whereas Yang et al.’s study included BU patients refractory to corticosteroids with only a single immunosuppressant, we herein report a series of refractory BU patients who experienced recurrence despite aggressive treatment with two or three immunosuppressants at their therapeutic doses. Annual relapse rates, side effects, and steroid- and immunosuppressant-sparing effects of IFNα2a are assessed in this study.
Patients and methods
Patients and treatments
Clinical records of BU patients who underwent IFNα2a treatment in Peking Union Medical College Hospital between February 2015 and June 2018 were reviewed retrospectively. The diagnosis of BD was made according to the 1990 International Study Group (ISG) BD criteria21 or the new International Criteria for Behçet’s Disease (ICBD),22 and the patients were evaluated and followed up collaboratively by the uveitis group of the Ophthalmology Department and the Rheumatology Department. The use of corticosteroid and immunosuppressant drugs were in accordance with international consensus.23 IFNα2a was instituted in patients who had posterior or pan-uveitis relapse under a medium-to-high dose of oral corticosteroids (no less than 15 mg/day prednisone or equivalent) and at least one of the following conventional immunosuppressive agents: CsA (200 mg/day), cyclophosphamide (CTX, 100–150 mg/day), AZA (100 mg/day), methotrexate (MTX, 10 mg/week), and tacrolimus (TAC, 2 mg/day); they were used mainly as add-on treatments with minor adjustments of the conventional drugs in some patients for safety concerns. The initial dose of IFNα2a was 3.0 million IU (MIU) subcutaneously daily for 4 weeks, followed by 3.0 MIU every other day for 3–4 months and further tapering tailored to individual needs of immunosuppression.
All patients underwent a complete set of ocular examinations including visual acuity, intraocular pressure, slit lamp examination of the anterior segment, and fundoscopy when visible at initiation and each follow-up visit. Fundus fluorescein angiography (FFA), optical coherence tomography (OCT), and B ultrasound scans of the posterior segment were obtained when necessary. Relapses of ocular inflammation were confirmed independently by two uveitis specialists, and a third senior ophthalmologist was referred when there was any disagreement. Laboratory tests including blood cell counts, routine urinalysis, liver and renal functions, erythrocyte sedimentation rate (ESR), and serum C reactive protein (CRP) were performed monthly, and autoantibodies including antinuclear antibody (ANA), antibody to double-stranded DNA (anti-dsDNA antibody), anti-neutrophil cytoplasmic antibody (ANCA), and anti-extractable nuclear antigen antibodies (anti-ENA antibodies) were monitored every six months.
This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Peking Union Medical College Hospital (approval number: S-443). Written informed consent for the collection and use of all data, all examinations, treatments, and publication was obtained from all patients in accordance with the IRB’s requirements. The patients’ records/information were anonymized and de-identified before analysis.
Primary and secondary outcomes
Intraocular inflammation was graded according to the Standardization of Uveitis Nomenclature (SUN) criteria. Treatment success was defined as a two-step decrease in the level of vitreous haze or decrease to grade 0, and/or disappearance of fundus inflammatory signs including retinal infiltrates, hemorrhage, and vascular sheathing.24,25 The primary outcome measure was success rate of IFNα2a treatment. Changes in ocular relapse rates, disease activity of BD measured mainly by ESR and CRP, corticosteroid- and immunosuppressive-agent-sparing effects, and potential side effects were secondary outcomes. Ocular relapse was defined as new-onset intraocular inflammation and/or worsening of preexisting uveitis necessitating treatment intensification.
Statistical analysis
SPSS version 22.0 (IBM Inc., Armonk, USA) was used to statistically analyze the data. Categorical variables were represented as frequencies and percentages. Quantitative variables of normal distribution were expressed as mean () ± standard deviation (SD), whereas those of abnormal distribution were shown as median and range. The significance was estimated by the Student’s t test or Wilcoxon test. A two-sided p value < 0.05 was considered statistically significant.
Results
Demographic features
A total of 30 patients (27 males and 3 females) with a mean age of 30.5 ± 8.7 years were included. The median time interval between diagnosis of BU and initiation of IFNα2a was 36 (range 4–168) months (Table 1).
Table 1.
Demographic features and general characteristics of patients*.
| Feature | Data |
|---|---|
| Age at initiation of IFN-α2a, mean±SD, years | 30.5 ± 8.7 |
| Total follow-up, mean±SD, months | 21.7 ± 7.5 |
| Gender ratio (M/F) | 27/3 |
| Ocular manifestations | |
| Affected sites | |
| Bilateral | 28 (93.3) |
| Panuveitis | 30 (100) |
| Retinal vasculitis | 7 (23.3) |
| Macular edema | 5 (16.7) |
| Cataract | 9 (30) |
| Extraocular manifestations | |
| Recurrent oral ulcers | 30 (100) |
| Genital ulcers | 18 (60) |
| Arthritis | 1 (3.3) |
| Skin lesions | 22 (73.3) |
| Pathergy test positive | 4 (13.3) |
| Epididymitis | 1 (3.3) |
| Concomitant diseases | |
| Chronic hepatitis B virus infection | 2 (6.7) |
| Pulmonary tuberculosis | 1 (3.3) |
| Ankylosing spondylitis | 1 (3.3) |
| Previous therapy | |
| Glucocorticoids | 30 (100) |
| Minimum maintenance dosage, median (range), mg/day |
20 (15–60) |
| Immunosuppressants | 30 (100) |
| Combination therapy | 21 (70) |
| Biological agents (short terms)# | 4 (13.3) |
| Adverse events of previous therapy | |
| Avascular necrosis | 2 (6.7) |
| Secondary hypertension | 4 (13.3) |
| Liver function impairment | 5 (16.7) |
| Renal function impairment | 3 (10) |
| Cyclophosphamide-induced hematuria | 2 (6.7) |
Unless otherwise indicated, data are expressed as number (percentage) of patients.
Etanercept in four cases (13.3%) and infliximab in two cases (6.7%).
Ocular clinical characteristics
The ocular disease was bilateral in 28 patients (93.3%) and unilateral in only 2 patients (6.7%). All patients had a recurrent refractory sight-threatening panuveitis. Retinal vasculitis was observed in seven patients (23.3%) and macular edema was observed in five patients (16.7%). In addition, nine patients (30%) developed cataract secondary to recurrent episodes of uveitis (Table 1).
Extraocular manifestations and concomitant diseases
Except for ocular involvement, no other major organ involvement was noted in these cases. Recurrent oral ulcers (30/30, 100%), skin lesions (22/30, 73.3%), and genital ulcers (18/30, 60%) were the most frequent extraocular manifestations in this cohort. Concomitant medical conditions included chronic hepatitis B virus infection in two patients, pulmonary tuberculosis in one patient who was treated with antitubercular agents, and ankylosing spondylitis in one patient (Table 1).
Previous treatments and associated adverse events
All patients had been treated aggressively with systemic corticosteroids and immunosuppressive agents. At the initiation of IFNα2a, 19 patients (63.3%) were on 2 immunosuppressive agents and 2 patients were on 3 immunosuppressants. Four patients had received and responded well to short term treatments of TNFα inhibitor therapy (two with etanercept and infliximab successively; and the other two with only etanercept), but stopped due to economic burden. Adverse events related to previous treatments included femoral head necrosis in two cases (6.7%), secondary hypertension in four cases (13.3%), hepatic function impairment in five cases (16.7%), renal function damage in three cases (10%), and CTX-induced constant hematuria in two cases (6.7%). The median rate of uveitis relapse was 7.3 (range 2–12) per patient-year (Table 1).
Effectiveness, outcomes, and follow up
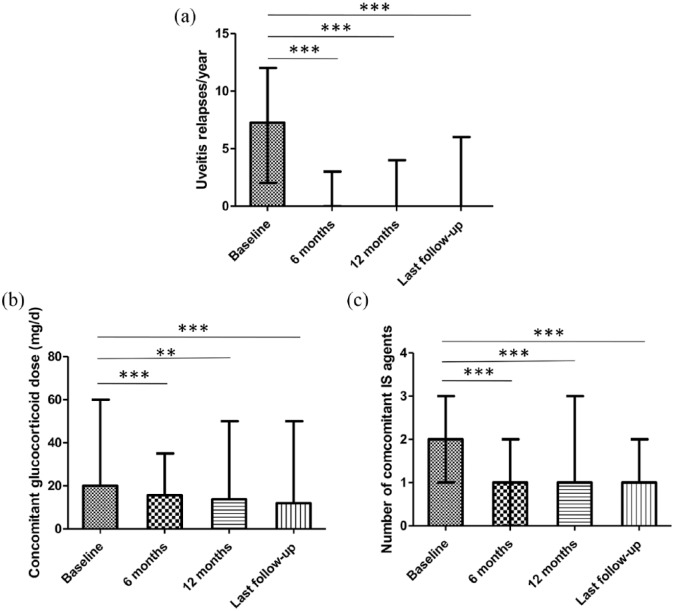
The mean follow-up length after initiation of IFNα2a treatment was 21.7 ± 7.5 months. Treatment success was achieved in a majority of the patients (26/30, 86.7%). The frequency of ocular inflammation relapse was reduced significantly in all 30 patients during the follow-up period (Figure 1), with median relapse rate decreased substantially from 7.3 (range 2–12) per patient-year before IFNα2a treatment to 0.00 (range 0–3), 0.00 (range 0–4), and 0.00 (range 0–6) per patient-year at 6 months, 12 months, and the endpoint of follow up, respectively(p = 0.000002, p = 0.000004, p = 0.000002, respectively; Figure 1(a)). In eight patients (26.7%), uveitis was successfully controlled without relapse by maintenance therapy of 3 MIU IFNα2a three times a week during the overall follow-up period. IFNα2a dosage was successfully tapered down to 3 MIU twice a week in five cases (16.7%) and to once a week in two cases (6.7%), and was completely withdrawn in six patients (20%). Notably, none of the six patients experienced uveitis attack during a mean follow up of 9.3 ± 3.3 months after discontinuation of IFN with one patient even stopping corticosteroids and immunosuppressants as well. The four patients who inadequately responded to IFNα2a at the dose of 3 MIU every other day were switched to infliximab treatment and the frequency of uveitis attack decreased afterwards in all four cases to some extent: from three relapses over an 8-month period of IFNα2a treatment to three relapses over a 20-months period of infliximab treatment in one patient, from two or three times per year to once per year in two patients, and from four times per year to none during a 5-month period of infliximab treatment in one patient. Significant improvement and worsening of visual acuity, defined as gain and loss of ⩾2 Snellen lines, respectively, were observed in six patients (7 eyes) and two patients (2 eyes), respectively, whereas visual acuity in the other 51 eyes remained largely unchanged. As for extraocular manifestations during the follow up, occasional oral ulcers were noted in four patients (13.3%), and genital pustule occurred only once in one patient. Levels of inflammatory markers such as ESR and CRP maintained normally in most of the patients (22/30, 73.3%).
Figure 1.
Outcomes of interferon α2a treatment.
Comparisons of (a) uveitis relapse rates (p = 0.000002, p = 0.000004, p = 0.000002), (b) the minimum concomitant corticosteroid dose (p = 0.000882, p = 0.001112, p = 0.000376), and (c) the number of immunosuppressive agents (p = 0.00054, p = 0.000949, p = 0.00004) at 6 months, 12 months, and last follow up with that at baseline (pretreatment Behçet’s uveitis patients), shown as median and range. The significance was determined using two-tailed Wilcoxon test. **p-value < 0.01, ***p-value < 0.001, IS, immunosuppressant.
Oral corticosteroids were lowered in 25 patients (83.3%) with median dose decreased from 20 (range 15–60) mg/day at the initiation of IFNα2a to 11.9 (range 0–50) mg/day at the last visit (p = 0.000376). At 6 months, 12 months, and endpoint of follow up, 29/30 (96.7%), 26/28 (92.9%), and 28/30 (93.3%) patients, respectively, were on less than 30 mg/day prednisone or equivalent, and 11/30 (36.7%), 14/28 (50%), and 17/30 (56.7%) patients, respectively, were on less than 15 mg/day prednisone or equivalent, with uveitis under control. Four patients discontinued corticosteroids. In addition, immunosuppressive agents were reduced in number and dosage in 22 (73.3%) and 29 patients (96.7%), respectively, and were completely withdrawn in 12 cases (40%).
Side effects
No major side effects such as severe depression were observed. A total of 24 patients (80%) experienced flu-like syndrome characterized by mild fever and headache at the initiation of the therapy but was well controlled with nonsteroidal anti-inflammatory agents. Mild/moderate reduction in leukocyte and platelet counts was observed in four cases (13.3%). However, IFNα2a was temporarily withdrawn and reinitiated in one patient due to myelosuppression. Serum creatine was elevated in two patients (6.7%). One patient developed proteinuria (1 g urinary protein in 24 hours) with a slight increase of serum creatine (range 123–130 μmol/l) during maintenance treatment and IFNα2a was stopped according to the nephrologist’s advice; the other patient experienced a transient increase of serum creatine (112 μmol/L) which returned to normal when reexamined after 20 days. Elevation of serum alanine transaminase (ALT) was detected in two patients (6.7%), and IFNα2a was discontinued in only one of them when normalization was not observed after withdrawal of CsA. The serum autoantibodies including ANA, anti-dsDNA antibody, ANCA, and anti-ENA antibodies were all negative during the treatment with IFNα2a. No depression was observed or reported by the patients or their family members during follow up.
Discussion
Evidence is accumulating that IFN might be a promising treatment, in addition to TNF inhibitors, for BU refractory to conventional immunosuppressive agents. IFN has been used either to suppress acute uveitic attack,16 or to maintain disease quiescence in the chronic phase,18 or both.26 The practical value of these studies, however, is limited by their heterogeneity in terms of ethnic and racial backgrounds of the patients, indication, dosage, and duration of IFN treatment. In addition, although IFN was commonly given only with corticosteroids,26–28 whether and (if so) how it could be used as a combinatorial agent with conventional immunosuppressants remains to be further elucidated.
The large cohort from southwest China reported recently by Yang and colleagues is worthy of particular note.20 In this study, a daily dose of 3 MIU IFNα2a and 20 mg/day prednisone was given to their patients for 3 months with complete withdrawal of conventional immunosuppressants, followed by a slow tapering of IFNα2a and corticosteroid. Their results were encouraging with an overall effective rate of over 90% and a favorable safety profile. However, one should keep in mind that this study only enrolled BU patients who were refractory to one conventional immunosuppressant in addition to corticosteroid. Our current study, on the other hand, included more refractory patients of whom over two-thirds underwent recurrence with at least two immunosuppressants at therapeutic dosages, and chose a different strategy by taking IFNα2a mainly as an add-on treatment for refractory BU patients and evaluated the immunosuppressant-sparing effect of IFNα2a in particular. In addition, the more conservative dosing strategy in the present study may explain the diminished side effects of IFNα2a observed in our study.
Encouragingly, IFNα2a was similarly effective in the current cohort of more intractable BU patients with a success rate of 86.7%. The median rate of uveitis relapse during follow up was impressively low (0.00 per patient-year), with half of the patients remained quiescent under maintenance therapy (3 MIU 3 times a week or less frequent). IFNα2a was able to be completely withdrawn in six patients without subsequent recurrence, which reflects the long-lasting effect of IFNα2a on BU. Of note is that the four patients who responded inadequately to IFNα2a in our study, later responded well after switching to infliximab. Nonetheless, considering that the cost of IFNα2a is less than 8% of that of infliximab in China, IFNα2a appeared to be a better choice than infliximab for refractory BU patients in our country. Visual acuity, however, remained largely unchanged in a majority (85%) of the eyes despite the significant improvement reported in a small percentage (11.7%). The high rate of late-stage eyes might explain the barely satisfactory visual outcome of the current study, as well as the comparable results observed in a number of previous studies.17,26,29
Although the exact pathogenesis of BD remains largely unknown, abundant evidence shows that BD is a highly complex autoimmune disease that involves a broad range of different immune cells, pathways, and molecular mechanisms.30,31 Accordingly, a combination of two or more immunosuppressive drugs targeting different components of the immune systems is usually required for long-term disease control. Whereas numerous studies have shown that high-dose IFNα, even when used alone, is highly effective at controlling acute uveitis attack in BU, its role as a long-term maintenance treatment at tolerable low dosage is inconsistent in different studies.17,18,27,32 At least in some of the highly intractable BU patients, maintenance dosage of IFNα alone is inadequate.32 It is thus meaningful to evaluate the long-term effectiveness and safety of IFNα in combination with other immunosuppressants. Favorably and notably, our current study reveals that IFNα2a can be used safely as an add-on treatment, and that IFNα2a has both satisfactory corticosteroid- and immunosuppressant-sparing effects.
The adverse effect profile of our patients is worth discussing. Interestingly, the flu-like syndrome was less frequent than most of the previous reports, and no severe side effects were observed. Although Hamuryudan et al.33 warned against the combination of IFNα with AZA due to severe myelosuppression, only slight reversible reduction in leukocyte and platelet counts was observed in four patients in our study. Intolerably elevated liver enzymes described previously19,20,27 were only observed in one of our patients. Another major concern in regard to IFNα2a is the development of autoimmune phenomena.34,35 In our study, however, serum autoantibodies such as ANA and anti-dsDNA antibodies remained negative in all patients during follow up. IFNα has been reported to induce thyroid disorders or thyroid antibody in patients with chronic hepatitis C virus (HCV) infection36 irrespective of the efficacy of IFNα treatment.37 In the clinical setting of BU, development of anti-thyroid peroxidase antibody has also been reported,38 suggesting the necessity for screening and monitoring of the thyroid condition. In addition, no depression was observed or reported by our patients or their family members, though it was noted in previous studies.25,26 In addition, renal impairment has not been noted in previous studies, but it was observed in two patients in the current study and resulted in IFN2a withdrawal in one of them. Further studies are needed to evaluate the renal toxicity of IFNα2a in the clinical setting of BU.
Our study has some limitations. First, it was a single-center retrospective study with limited participants. Second, it was designed as a self-control study, and the spontaneous remission reported in BD uveitis might potentially contribute to overestimating the effectiveness of IFNα2a therapy. Therefore, a multicenter prospective randomized controlled study evaluating the efficacy and safety between IFNα2a and conventional immunosuppressants such as CsA is warranted.
In conclusion, on the basis of corticosteroids and immunosuppressants treatment, IFNα2a is effective and relatively safe as an add-on treatment in patients with more refractory BU with satisfactory steroid- and immunosuppressant-sparing effects.
Footnotes
Authors’ contributions: All authors made substantial contributions to the conception and design of this study. JS and JZ acquired the data. JS and CZ performed the data analysis and interpretation and wrote the manuscript. WZ provided critical revisions to the manuscript. JZ, JL, LW, FG, XZ, and MZ also critically reviewed the manuscript and provided valuable input. All authors read and approved the final manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China [grant number 81571598, 81871299 and 81770917], Graduate Scientific Research Innovation Fund from Chinese Academy of Medical Sciences [grant number 1007-1002-1-19], National Key Research and Development Program: ‘Precise Medical Research’ [grant number 2016YFC0906201], CAMS Initiative for Innovative Medicine [grant number 2016-I2M-1-013], and Clinical Research Fund of Beijing Municipal Science and Technology Commission [grant number Z171100001017217].
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Wenjie Zheng  https://orcid.org/0000-0002-3165-8185
https://orcid.org/0000-0002-3165-8185
Contributor Information
Jing Shi, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing, China.
Chan Zhao, Department of Ophthalmology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
Jiaxin Zhou, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing, China.
Jinjing Liu, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing, China.
Li Wang, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing, China.
Fei Gao, Department of Ophthalmology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
Xiaofeng Zeng, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing, China.
Meifen Zhang, Department of Ophthalmology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China.
Wenjie Zheng, Key Laboratory of Rheumatology and Clinical Immunology, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Ministry of Education, Beijing 100730, China.
References
- 1. Krause L, Kohler AK, Altenburg A, et al. Ocular involvement in Adamantiades-Behçet’s disease in Berlin, Germany. Graefes Arch Clin Exp Ophthalmol 2009; 247: 661–666. [DOI] [PubMed] [Google Scholar]
- 2. Tursen U, Gurler A, Boyvat A. Evaluation of clinical findings according to sex in 2313 Turkish patients with Behçet’s disease. Int J Dermatol 2003; 42: 346–351. [DOI] [PubMed] [Google Scholar]
- 3. Davatchi F, Shahram F, Chams-Davatchi C, et al. Behçet’s disease in Iran: analysis of 6500 cases. Int J Rheum Dis 2010; 13: 367–373. [DOI] [PubMed] [Google Scholar]
- 4. Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, et al. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol 2004; 138: 373–380. [DOI] [PubMed] [Google Scholar]
- 5. Zhou ZY, Chen SL, Shen N, et al. Cytokines and Behçet’s disease. Autoimmun Rev 2012; 11: 699–704. [DOI] [PubMed] [Google Scholar]
- 6. Belguendouz H, Messaoudene D, Lahmar-Belguendouz K, et al. In vivo and in vitro IL-18 production during uveitis associated with Behçet disease: effect of glucocorticoid therapy. J Fr Ophtalmol 2015; 38: 206–212. [DOI] [PubMed] [Google Scholar]
- 7. Vitale A, Emmi G, Lopalco G, et al. Adalimumab effectiveness in Behçet’s disease: short and long-term data from a multicenter retrospective observational study. Clin Rheumatol 2017; 36: 451–455. [DOI] [PubMed] [Google Scholar]
- 8. Fabiani C, Vitale A, Emmi G, et al. Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol 2017; 36: 183–189. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi M, Kezuka T, Sugita S, et al. Evaluation of the long-term efficacy and safety of infliximab treatment for uveitis in Behçet’s disease: a multicenter study. Ophthalmology 2014; 121: 1877–1884. [DOI] [PubMed] [Google Scholar]
- 10. Calvo-Rio V, Blanco R, Beltran E, et al. Anti-TNF-alpha therapy in patients with refractory uveitis due to Behçet’s disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford) 2014; 53: 2223–2231. [DOI] [PubMed] [Google Scholar]
- 11. Nunez Martinez O, Ripoll Noiseux C, Carneros Martin JA, et al. Reactivation tuberculosis in a patient with anti-TNF-alpha treatment. Am J Gastroenterol 2001; 96: 1665–1666. [DOI] [PubMed] [Google Scholar]
- 12. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 13. Miller EA, Ernst JD. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J Clin Invest 2009; 119: 1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abreu C, Magro F, Santos-Antunes J, et al. Tuberculosis in anti-TNF-alpha treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis 2013; 7: e486–e492. [DOI] [PubMed] [Google Scholar]
- 15. Durand JM, Kaplanski G, Telle H, et al. Beneficial effects of interferon-alpha 2b in Behçet’s disease. Arthritis Rheum 1993; 36: 1025–1026. [DOI] [PubMed] [Google Scholar]
- 16. Kotter I, Eckstein AK, Stubiger N, et al. Treatment of ocular symptoms of Behçet’s disease with interferon alpha 2a: a pilot study. Br J Ophthalmol 1998; 82: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tugal-Tutkun I, Guney-Tefekli E, Urgancioglu M. Results of interferon-alfa therapy in patients with Behçet uveitis. Graefes Arch Clin Exp Ophthalmol 2006; 244: 1692–1695. [DOI] [PubMed] [Google Scholar]
- 18. Onal S, Kazokoglu H, Koc A, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory behçet uveitis. Arch Ophthalmol 2011; 129: 288–294. [DOI] [PubMed] [Google Scholar]
- 19. Hasanreisoglu M, Cubuk MO, Ozdek S, et al. Interferon alpha-2a therapy in patients with refractory Behçet uveitis. Ocul Immunol Inflamm 2017; 25: 71–75. [DOI] [PubMed] [Google Scholar]
- 20. Yang P, Huang G, Du L, et al. Long-term efficacy and safety of interferon alpha-2a in the treatment of Chinese patients with Behçet’s uveitis not responding to conventional therapy. Ocul Immunol Inflamm 2017: 1–8. [DOI] [PubMed] [Google Scholar]
- 21. Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet 1990; 335: 1078–1080. [PubMed] [Google Scholar]
- 22. International Team for the Revision of the International Criteria for Behçet’s Disease. The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 2014; 28: 338–347. [DOI] [PubMed] [Google Scholar]
- 23. Andreoli CM, Foster CS. Vogt-Koyanagi-Harada disease. Int Ophthalmol Clin 2006; 46: 111–122. [DOI] [PubMed] [Google Scholar]
- 24. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diwo E, Gueudry J, Saadoun D, et al. Long-term efficacy of interferon in severe uveitis associated with Behçet disease. Ocul Immunol Inflamm 2017; 25: 76–84. [DOI] [PubMed] [Google Scholar]
- 26. Kotter I, Zierhut M, Eckstein AK, et al. Human recombinant interferon alfa-2a for the treatment of Behçet’s disease with sight threatening posterior or panuveitis. Br J Ophthalmol 2003; 87: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gueudry J, Wechsler B, Terrada C, et al. Long-term efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behçet disease. Am J Ophthalmol 2008; 146: 837–844.e831. [DOI] [PubMed] [Google Scholar]
- 28. Sobaci G, Erdem U, Durukan AH, et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behçet’s uveitis refractory to conventional treatments. Ophthalmology 2010; 117: 1430–1435. [DOI] [PubMed] [Google Scholar]
- 29. Krause L, Altenburg A, Pleyer U, et al. Longterm visual prognosis of patients with ocular Adamantiades-Behçet’s disease treated with interferon-alpha-2a. J Rheumatol 2008; 35: 896–903. [PubMed] [Google Scholar]
- 30. Gul A. Pathogenesis of Behçet’s disease: autoinflammatory features and beyond. Semin Immunopathol 2015; 37: 413–418. [DOI] [PubMed] [Google Scholar]
- 31. Emmi G, Silvestri E, Squatrito D, et al. Behçet’s syndrome pathophysiology and potential therapeutic targets. Intern Emerg Med 2014; 9: 257–265. [DOI] [PubMed] [Google Scholar]
- 32. Kotter I, Gunaydin I, Zierhut M, et al. The use of interferon alpha in Behçet disease: review of the literature. Semin Arthritis Rheum 2004; 33: 320–335. [DOI] [PubMed] [Google Scholar]
- 33. Hamuryudan V, Ozyazgan Y, Fresko Y, et al. Interferon alfa combined with azathioprine for the uveitis of Behçet’s disease: an open study. Isr Med Assoc J 2002; 4: 928–930. [PubMed] [Google Scholar]
- 34. Ronnblom LE, Alm GV, Oberg KE. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann Intern Med 1991; 115: 178–183. [DOI] [PubMed] [Google Scholar]
- 35. Wandl UB, Nagel-Hiemke M, May D, et al. Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clin Immunol Immunopathol 1992; 65: 70–74. [DOI] [PubMed] [Google Scholar]
- 36. Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am 2007; 36: 1051–1066; x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dalgard O, Bjoro K, Hellum K, et al. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med 2002; 251: 400–406. [DOI] [PubMed] [Google Scholar]
- 38. Aydinoglu-Candan O, Araz-Ersan B, Gul A, et al. Anti-interferon alpha antibodies and autoantibodies in patients with Behçet’s disease uveitis treated with recombinant human interferon alpha-2a. Graefes Arch Clin Exp Ophthalmol 2015; 253: 457–465. [DOI] [PubMed] [Google Scholar]