Abstract
Patients with familial adenomatous polyposis (FAP) depend on a lifelong endoscopic surveillance programme and prophylactic surgery, and usually suffer nutritional problems. Intestinal inflammation has been linked to both FAP and colorectal cancer. Epidemiological studies show a relationship between diet and inflammation. Preventive dietary recommendations for FAP patients are so far lacking. We have designed a nonrandomized prospective pilot study on FAP patients to assess whether a low-inflammatory diet based on the Mediterranean diet principles and recipes, by interacting with the microbiota, reduces gastrointestinal markers of inflammation and improves quality of life. This report describes the scientific protocol of the study and reports the participants’ adherence to the proposed dietary recommendations. Thirty-four FAP patients older than 18 years, bearing the APC pathogenic variant, who underwent prophylactic total colectomy with ileo-rectal anastomosis were eligible into the study. During the 3-month dietary intervention, they reported improvements in their consumption of Mediterranean foods (vegetables, fruits, fish, and legumes), and a reduction in pro-inflammatory foods (red/processed meat and sweets); this led to a significant increase in their adherence to the Mediterranean diet. The improvement was accompanied by a decrease in the number of diarrhoeal discharges. These preliminary results are encouraging with regard to feasibility, dietary outcome measures, and safety.
Keywords: FAP, hereditary tumors, low-inflammatory diet, biomarkers, microbiota, quality of life, adenomas, prevention
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited colorectal cancer (CRC) predisposition syndrome characterized by hundreds to thousands of adenomas in the colon; the duodenum may also be affected. FAP is caused by germline inactivating mutations in the adenomatous polyposis coli (APC) gene. Mutation carriers have theoretically a 100% risk of developing CRC and a 4% to 12% risk of duodenal cancer if they are not adequately treated.1,2 Therefore endoscopic screening should start from the age of 10 to 14 years and prophylactic surgery is recommended no later than the second decade.3,4 There are 2 options for preventive surgery: total colectomy with ileo-rectal anastomosis (TC/IRA) and proctocolectomy with ileal pouch anal anastomosis (PC/IPAA). Generally, TC/IRA guarantees patients a better quality of life, but intensive lifelong endoscopic surveillance is mandatory to limit the risk of rectal stump cancer.5
The FAP phenotype is closely correlated to the genotype. However, there is epidemiologic evidence that chronic intestinal inflammation drives the formation and growth of adenomas and CRC.6 Chronic inflammation, mediated by loss of IL10-secreting regulatory T cells (Treg) and increases in Th17cells producing IL17A, promotes IL17A-dependent tumor growth, helping amplify and sustain colonic tumorigenesis in the APCMin/+ mouse model, which recapitulates FAP in most respects.7 In the same model, overexpression of interleukin-6 (IL-6) can increase the polyp burden in the mice. Pro-inflammatory cytokines (IL-6, IL-10) were involved in the progression of large adenomatous polyps, as well as in the overall polyp number.7
Inflammation may also act in FAP by influencing the microbiota. Several bacterial pathogens seem to be directly and specifically involved in promoting CRC and the gut microbiota can generate microbial metabolites that have a major influence on host physiology.8 For example, the short-chain fatty acids (SCFA) acetate, propionate, and butyrate function in the suppression of inflammation and cancer, induce apoptosis and inhibit tumor cell progression, whereas other microbial metabolites, such as secondary bile acids, promote carcinogenesis.8 The regulation of miRNA expression is also increasingly recognized as crucial to the host response to the microbial population and many miRNA families have already been linked with inflammation and diet.9
All these observations suggest that anti-inflammatory support combined with the intensive endoscopic surveillance could help reduce the risk of new adenomas in the rectal stump of FAP patients. Several trials have suggested that recurrence can be reduced by the use of nonsteroidal anti-inflammatory drugs (NSAIDs)10 and/or also essential fatty acids (omega-3).11 However, long-term treatment with NSAIDs entails a cardiological risk and the regular use of omega-3 supplements may be costly for patients. Therefore, there is a pressing need for an alternative prevention strategy in FAP that is safe and well tolerated.
Several epidemiological studies have assessed the relationship between diet and inflammation, focusing on dietary components and patterns.12 They have analyzed the low glycemic index,13 fruits and vegetables,14 red meat,15 nonhydrogenated vegetable oils,16 soy,17 micronutrients including nonheme iron and magnesium,18 and macronutrients, including omega-3 fatty acids19 and low- and high-carbohydrate diets.20 In most cases, the effects of the different dietary approaches were assessed in patients with metabolic syndrome, an insulin resistance syndrome that is thought to be a low-grade inflammatory disease.
Two recent meta-analyses of randomized controlled trials suggested that a low-fat diet (29% ± 2% energy from fats, 7% from saturated fats) or consumption of legumes contributed to lowering C-reactive protein (CRP) levels.21,22 In the colonoscopy-based Tennessee Colorectal Polyp Study, which tested the association of the main risk factors for CRC with the risk of colorectal polyps, high body mass index and high red meat consumption emerged as well-established risk factors for adenoma and CRC, while high dietary fiber and dietary calcium intake were associated with lower risk.23
An ad hoc low-inflammatory diet has given promising results in inflammatory bowel diseases24 (IBDs). IBDs and FAP have 2 distinct patterns of pathogenesis, but both conditions markedly increase the risk of CRC and the mucosal inflammation may alter the formation or expression of adenomas.25 A recent meta-analysis of randomized trials of a low-inflammatory diet in adults undergoing radical pelvic radiotherapy reported a significant reduction of common gastrointestinal symptoms, including increased frequency of defecation, urgency to open bowels, alteration in stool consistency, change in bowel habit, pain, bleeding, bloating, and nausea.26 These symptoms are very common in FAP patients too after total colectomy.
The Mediterranean diet, a food-dietary scheme closely associated with health advantages, is a lower-fat diet balanced with unrefined whole grains, legumes, nuts, vegetables, and fruits. The principal source of dietary lipids is extra virgin olive oil. The diet also includes occasional consumption of fish and fermented dairy products (yogurt and aged cheeses), moderate to small amounts of white meat and eggs, rarely other animal products (butter and red meat), and low ethanol intake, usually as wine.27 A recent meta-analysis of dietary randomized trials showed that the Mediterranean diet, in studies with interventions lasting 3 months or more, significantly reduced CRP levels compared with control diets.28 Plant-based dietary patterns (mostly fruit, vegetables, whole grains, and little red meat) were proposed as valid means for long-term inflammation control.29 The Mediterranean diet was suggested as having strong immunomodulatory effects, with even some potential for action on epigenetic mechanisms.
Recent data from the Predimed study showed that over 5 years of Mediterranean diet intervention was associated with the methylation of genes related to inflammation and exerted strong regulatory effects.30 A trial31 on people with Crohn’s disease also reported that a 6-week Mediterranean-based diet improved participants’ health, reduced markers of inflammation, normalized the microbiota and generated significant changes in gene expression.
As yet there is no information about the role of diet in FAP and preventive dietary recommendations are still lacking. Therefore, we designed a pilot study to test whether a low-inflammatory diet based on Mediterranean principles and recipes reduced intestinal markers of inflammation, improved the gut microbiota with beneficial effects on the intestine and the quality of life, and consequently reduced recurrent adenomas in the rectum.
This report describes the scientific protocol of this study and reports the feasibility data by describing the participants’ adherence to the proposed dietary recommendations.
Materials and Methods
Protocol
Study Design
This was a prospective nonrandomized pilot study in FAP patients, aimed at assessing the effects of a Mediterranean low-inflammatory diet in terms of the patterns of calprotectin marker levels in stool (dietary intervention effect). Further aims were to evaluate:
changes of other markers of inflammation (CRP, glycated hemoglobin) and dysmetabolism (insulin, glycemia, insulin-like growth factor–I [IGF-I]) linked to both diet and disease progression
changes in the bacterial composition of the gut and of gene and miRNA expression from adenomas and ileum tissue removed during rectal sigmoidoscopies
the impact of the diet on patients’ quality of life, mainly in terms of reduction of diarrheal discharges
the impact of the diet on the balance of immunosuppressive and immunostimulatory cell profiles in blood and adenomas
the impact of the diet on the expression of COX-2 (cyclo-oxygenase-2) and 15-PGDH (15-prostaglandin dehydrogenase), 2 target enzymes of the selective COX-2 inhibitor celecoxib (http://meetinglibrary.asco.org/content/175867-195).
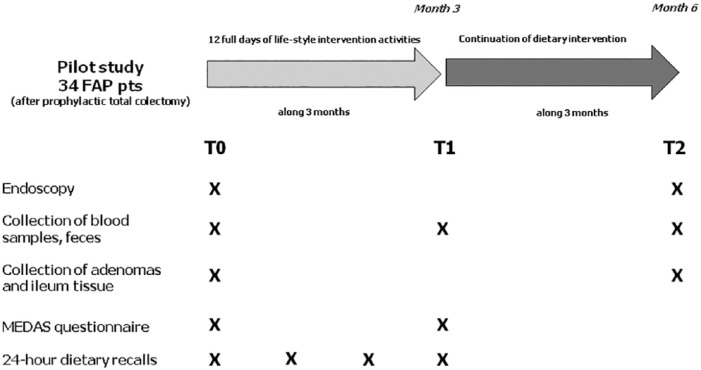
The study was approved by the Ethical Committee of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, INT78/2017, and was planned to last 6 months. A schematic representation of the study is reported in Figure 1.
Figure 1.
Graphical representation of the study.
Briefly, FAP participants were invited to take part in dietary intervention activities that included 12 days of training, cookery, supportive educational content, and meals. The activities were actively conducted by a nutritionist, a physician with a master’s degree in nutrition and a dietitian.
The dietary intervention involved groups of 20 people each. Relatives of FAP patients could participate in the dietary activities. The participants received the nutritional content of the menus provided during cooking classes, meals, and recipes.
The intervention was spread over 3 months to assist patients with lifestyle changes. At the beginning of the study (T0, baseline), at the end of the dietary intervention (T1), and 6 months after T0 (T2), participants were asked to give blood and stool samples. Each person was examined endoscopically at T0 and T2 and adenoma and ileum tissues were collected. Patients were asked to complete questionnaires and 24-hour dietary recalls throughout the 3-month dietary intervention.
Subjects
FAP patients who underwent prophylactic colectomy were identified from the Hereditary Digestive Tumor Registry at the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano (INT). All the patients in the registry had signed an informed consent form to be recalled for new studies.
Participants in the study were required
to give signed informed consent to participate in the study
to follow the prescribed dietary changes
to provide information on their health status and any intervening clinical problems
to undergo an endoscopy at baseline (T0) and 6 months later (T2)
to give blood samples and feces at baseline (T0), at the end of the active dietary intervention (T1: 3 months), and at T2 (6 months)
to complete the MEDAS questionnaire32 and 24-hour dietary recalls on the previous day’s food intake at T0 and T1. Two further 24-hour dietary recalls were requested during the 3-month active dietary intervention.
Inclusion criteria were
older than 18 years
APC gene tested
undergone prophylactic total colectomy/IRA
currently involved in the surveillance program at the INT.
Exclusion criteria were
no compliance with intensive endoscopic surveillance and patients
treatment with NSAIDs or omega 3 products.
Dietary Intervention
The diet we propose is based on our previous experience in dietary intervention trials33-35 and on the scientific evidence of the effect of diet in inflammatory bowel diseases.24,31 We aim to reduce foods that generate inflammation, to boost regular consumption of foods with anti-inflammatory properties, and to reduce any insulin resistance and metabolic disorders.
The proposed diet, mainly based on Mediterranean dietary principles and recipes, has 5 basic aims:
1. To markedly reduce the consumption of refined foods (e.g. sugar and refined grains). This helps avoiding foods that quickly raise glycaemia (sugar, sugary drinks, refined wheat flour, white bread, potatoes, and sweets). Refined grains induce a larger short-term increase of plasma glucose and insulin, thus triggering pro-inflammatory cytokines. The consumption of whole-grain foods significantly attenuates the postprandial blood glucose response and acutely improves insulin homeostasis compared to similar refined foods, in healthy subjects.36 Switching to a diet rich in whole grains may also lower circulating levels of free radicals and pro-inflammatory cytokines, such as IL-6, IL-18, tumor necrosis factor–α (TNF-α).37
Nuts and legume flours are proposed in sweet and savory cookery recipes. Desserts are prepared without adding sugars, using fruits, dates, raisins, dried apricots, and sweet potatoes.
2. To restore the balance of the intestinal flora. The consumption of fresh cultured yogurt, kefir, honey (Manuka honey in particular) as probiotics is encouraged. Onions, garlic, chicory, leeks, oats, barley—all rich in dietary prebiotics in the form of soluble fiber (containing beta-glucans and inulin)—are strongly recommended.
3. To distinguish the different types of fat in foods (saturated, trans-, mono-, and poly-unsaturated). Processed and red meats (rich in saturated fats), but also some foods of vegetable origin, such as margarine (which contains trans-fatty acids) promote inflammation and should be avoided or substantially reduced. Cold-pressed extra-virgin olive oil is the best source of fat. Cold-water fish such as salmon and mackerel, rich in omega-3 poly-unsaturated fatty acids that are strongly anti-inflammatory, are ideal.
4. To change the textures of foods (e.g. blending, grinding, or cooking) as needed depending on the patient’s symptoms, to facilitate nutrient absorption and minimize intact fiber. All whole grains have anti-inflammatory properties, especially oats, barley (hulled better than pearl) and brown rice. Since the fiber of whole grains may be problematic for these patients, they can be creamed (whole grains can be cooked for a long time and passed through a sieve).
Almond and/or hazelnut creams without sugar are recommended in the preparation of desserts.
5. To encourage higher dietary quality, as defined by macro- and micro nutrient needs.
Our goal in FAP patients is to achieve a healthier dietary pattern, mainly based on plant and unrefined foods (as in the Mediterranean diet), reducing the consumption of processed and pro-inflammatory animal foods. FAP patients have to progressively adapt and extend their diet as their tolerance and absorption improve. Given the carbohydrate malabsorption and lactose intolerance following total colectomy,38 milk- and lactose-containing dairy products (custards, ice cream, soft cheese) should be avoided. The use of plant-based unsweetened milk and creams (soy milk, almond milk, soy cream) is encouraged.
Initially, the foods recommended are soft, well-cooked, and without seeds. Many FAP patients need foods to be softened and textures mechanically altered by pureeing (to whole-grain cream) and should avoid foods with stems and seeds when starting the diet, as intact fiber can be problematic. As their symptoms improve, patients can gradually advance to eating more whole foods.
All varieties of cabbages, borage, nettles, purslane oleracea (also known as portulaca) and other weeds with anti-inflammatory properties are good. Patients are encouraged to eat small fruits (like berries, or pomegranate, initially as juices without added sugars), apples, pears, plums, peaches—best cooked with a pinch of salt to enhance the sweet taste, and other fruit in season. The consumption of all seasonal fruit and vegetables is encouraged because fruits and vegetables grown in season are richer in nutrients (especially vitamins and minerals) and usually undergo less processing, packaging, transportation and storage.39,40 Raw tomatoes, eggplants, and peppers all contain substances (glycoalkaloids and polyamines) that can increase inflammation and intestinal cancerogenesis, so are best avoided.41-43
Japanese fermented foods (miso, soy sauce, tempeh) are included too, to facilitate digestion and serve as a reliable reservoir for microbiota.44
Questionnaires
FAP participants were asked to complete the MEDAS questionnaire, a short questionnaire on adherence to the Mediterranean diet,32 at T0 and at the end of the 3-month dietary intervention (T1). MEDAS consists of 12 questions on food consumption frequency and 2 on eating habits. Each question is scored 0 or 1 depending on whether participants adhere to each Mediterranean component or not. The total is given by the sum of the single scores and ranges from 0 to 14. In addition, as FAP patients who receive a prophylactic TC/IRA usually suffer from diarrhea, dysbiosis, malabsorption, and/or food intolerances, which limit the variety in their diet and their quality of life, we added to the questionnaire questions about (a) the number of diarrheal discharges in a typical day, (b) whether they had taken any NSAID the previous day, and (c) antibiotics in the past week.
Participants were also asked to complete four 24-hour dietary recalls for the previous day: one at baseline, before receiving any dietary recommendation, one at the end of the 3-month active dietary intervention, and 2 others during the intervention. The 24-hour dietary recall does not include information on portion size or weight, or recipes. Participants have to indicate only whether, the previous day, they had or had not eaten the specified food at breakfast, lunch, dinner, or during breaks. We decided not to ask for portion sizes, because these are too problematic to collect and classify, and our aim was to measure the compliance of FAP participants with the diet. As we previously reported, the 24-hour dietary recall enables us to create qualitative scores, so we can measure compliance.
The 24-hour dietary recall contains a list of 65 food items organized in the following 6 groups:
Drinks (with and without alcohol, with and without added sugar), milk, dairy products. This group includes a separate item for plant-based unsweetened milk.
Sweets and confectionery. This group includes separate items for sugar, whole sugar, honey and malt.
Bread and grains. This group includes separate items for refined and whole grains and grain products.
Meat, fish, eggs, and meat substitutes. This group includes a separate item for meat substitutes made from wheat gluten protein.
Legumes, vegetables, fresh and dried fruit, nuts and seeds. This group includes separate items for soy products.
Sauces, animal and vegetable fats. This group includes a separate item for miso and soy sauce.
One question about the number of diarrheal discharges the previous day was added in the 24-hour dietary recall for the present study. Diarrheal discharges were classified according to the Bristol Stool Chart as type 6 and 7 feces.45
Endoscopy
Rectal sigmoidoscopies were performed with an Olympus (Evis Exera II 160 and 180 series) high-definition video endoscope. Adenomas and normal rectal tissues were sampled at the T0 and at T2 endoscopies (6 months after the baseline endoscopy). Tissue specimens collected at endoscopy were immediately frozen and stored at −80°C. The number and size of the largest small bowel polyps from the proximal, medial, and distal rectum were recorded. The size of the largest polyp in each segment was estimated with open-biopsy forceps.
Inflammation Markers
Markers of inflammation in blood and stool were analyzed at each time point for all subjects (T0, T1, and T2). Stool calprotectin was measured using the Alegria technique. As with blood samples, we measured markers of inflammation and dysmetabolism linked both to diet and to the progression of the disease, such as IGF, CRP, insulin, glycated hemoglobin, glycemia and plasma lipids. Serum IGF-I will be measured with radioimmunoassay kits from Biosource (Nivelles, Belgium); microparticle enzyme immunoassay kits from Abbot (Abbot Park, IL, USA) will be used for insulin.
Microbiota
Dietary habits and composition have an important effect on the gut microbiota. Changes in the fecal microbiota are detectable as early as a few days after switching between carefully controlled diets. The gut microbiota were analyzed in stool at T0, T1, and T2. Stool samples collected 15 days after the beginning of the diet will be analyzed to study the rate of change of the microbiota. Subjects were asked to collect naturally evacuated stool samples in empty tubes designed to be kept several hours at room temperature without altering the nucleic acids of the samples (Norgen Biotek). DNA will be isolated and metagenomic libraries will be prepared using the Illumina Nextera XT kit; shotgun sequencing will be done at a target depth of 5GB/sample.
Gene and miRNA Profile
Gene and miRNA expression analyses will be done on adenomas and ileum tissue collected at colonoscopies (T0 and T2). Gene expression will be analyzed on Affymetrix Human Clariom S arrays (20K distinct RefSeq and Ensembl transcripts). Normalization and data analysis will be done using Partek Genomics Suite. MiRNA profiling will be done through miRNA-seq on Ion Proton (Thermo Fisher Scientific). Multiplexed sequencing of 8 samples/run will be conducted on Ion PITM Chip v3, to generate >107 miRNA raw sequences/sample. Reads will be aligned using STAR to miRBase and quantified. Results will be analyzed in relation to the diet and also to changes in the microbiota composition.
Immunological Markers
Peripheral blood was collected for future assessment of any changes due to the diet in the frequency of circulating inflammatory and immunosuppressive cells, including low-density granulocytes, CD11b+CD15+HLA-DRnegCD33+ gMDSC, CD15+PDL-1+ cells, CD14+ PDL-1+ monocytes, CD14+HLA-DRneg mMDSC, and lineage-negative cells. Concomitantly, the frequency of activated or exhausted T and NK cells (CD3+, CD4+, CD8+, CD3negCD16+CD56+, expressing PD-1 and/or CD69), and regulatory T cells (CD4+CD25high Foxp3+CD127+) will be recorded. Multiparametric cytofluorimetry will be used for the analysis. Representative myeloid and T cell markers will also be used to characterize immune and inflammatory infiltrate in polyp tissue biopsies.
Immunohistochemistry
The expression of COX-2 and 15-PGDH, 2 target enzymes of the selective COX-2 inhibitor celecoxib (http://meetinglibrary.asco.org/content/175867-195) will be investigated if the study proves successful. Adenomas removed during the pilot study (T0 and T2) will be analyzed to assess the expression of COX-2 (high or low) and 15-PGDH (present or absent) by immunohistochemistry (IHC).
Sample Size and Statistical Analysis
A total of 30 patients were considered for this pilot study. This number can be considered appropriate according to both logistical and timeline issues. This size is in accordance with both the general rule of thumb provided by Browne46 and with the systematic review by Billingham et al47 in which 30 is the median number of patients enrolled in such pilot and/or feasibility studies.
Considering a 15% drop-out rate, we enrolled 34 subjects.
To evaluate changes in calprotectin concentrations, a mixed model48 will be employed, modeling the calprotectin level (dependent variable) as a function of “time” (fixed factor, T0, T1, T2) and “patients” (random factor). For the secondary endpoints (gut microbiota and miRNA quantities) a similar strategy will be adopted.
To examine the relationship between miRNA expression and the type of tissue (polyps and normal counterpart), we shall use the bootstrap simultaneous confidence interval (SCI).49,50 Finally, the number of adenomas at the baseline colonoscopy (T0) will be compared with that at the end of the diet (T2).
Results
The 34 FAP patients selected for their convenient distance from the INT, who met the inclusion criteria and gave signed informed consent, were enrolled. Baseline clinical data and endoscopic findings are available for them all. Six patients decided to drop out before starting the dietary intervention, so 28 participated in the study. Two other FAP patients, despite participating the entire dietary intervention period, did not complete the MEDAS questionnaire (one did not complete the first, the other the final one). Questionnaire responses are therefore available for 26 subjects. The cohort consisted of 13 men and 13 women with a median age at baseline of 43 years (range 18-77 years); all patients were tested for APC and the median interval between TC/IRA and starting the study was 11 years (range 5 months to 47 years).
To check adherence to the Mediterranean diet, we examined the questions on food consumption frequency and eating habits to compute a score according to the MEDAS criteria. We used the nonparametric Wilcoxon signed-rank test to compare baseline and 3-month MEDAS score distributions in FAP patients. A similar approach was adopted to compare the number of diarrheal discharges between T0 and T1. All statistical analyses were carried out with SAS software (Version 9.4; SAS Institute, Inc, Cary, NC), adopting a significance level of α = .05.
As regards adherence to the Mediterranean diet, Table 1 describes participants’ baseline food consumption according to the MEDAS criteria to assess the use of each dietary item. FAP patients used olive oil as their main cooking fat (84.62%), but in moderate quantities (only 30.77% used more than 4 tablespoons per day) and had low (19.23%) or no consumption of vegetables or fruit. Most participants (92.31%) preferred white meat to red or processed meat, with an intake of red meat less than one portion per day in 61.54% of cases. However, more than 80% reported a high consumption of commercial sweets (4 portions per week) and very low consumption of legumes (only 7.69% ate 3 or more portions per week). More than 70% of the participants ate at least 2 portions per week of durum wheat pasta.
Table 1.
Frequency Distribution of Adherence to the Mediterranean Diet (MedDiet) in the 26 Patients With Familial Adenomatous Polyposis at T0 (Baseline Examination) and at T1 (End of Dietary Intervention).
| Item No. | Questions | Criterion | T0 (26 Patients) | T1 (26 Patients) |
|---|---|---|---|---|
| % Adherence to MedDiet | % Adherence to MedDiet | |||
| 1 | Do you use olive oil as main cooking fat? | Yes | 84.62 | 96.15 |
| 2 | How much olive oil do you consume in a day (including oil for frying, or on salads)? | ≥4 tbsp | 30.77 | 46.15 |
| 3 | How many servings of vegetable do you eat per day? (1 serving about 200 g) | ≥ 2 | 19.23 | 30.77 |
| 4 | How many fruit portions do you eat per day ? (1 serving about 100-150g) | ≥3 | 0.00 | 3.85 |
| 5 | How many servings of red meat, hamburger, or meat products do you eat per day? | <1 | 61.54 | 80.77 |
| 6 | How many servings of butter, margarine, or cream do you eat per day? (1 serving = 12 g) | <1 | 92.31 | 92.31 |
| 7 | How many sweet beverages do you drink per day? | <1 | 73.08 | 84.62 |
| 8 | How much wine do you drink per week? (glasses) | ≥7 | 0.00 | 0.00 |
| 9 | How many servings of legumes do you eat per week? (1 portion = 150 g) | ≥3 | 7.69 | 19.15 |
| 10 | How many servings of fish or shellfish do you eat per week? (1 portion = 150/200 g) | ≥3 | 26.92 | 57.69 |
| 11 | How many times per week do you eat commercial sweets or pastries (not homemade)? | <2 | 19.23 | 57.69 |
| 12 | How many servings of nuts do you eat per week? (1 portion = 30 g) | ≥3 | 26.92 | 26.92 |
| 13 | Do you preferably eat chicken, turkey, or rabbit meat instead of veal, pork, hamburger, or sausage? | Yes | 92.31 | 96.15 |
| 14 | How many servings of pasta do you eat per week? (1 portion about 80g) | >2 | 73.08 | 76.92 |
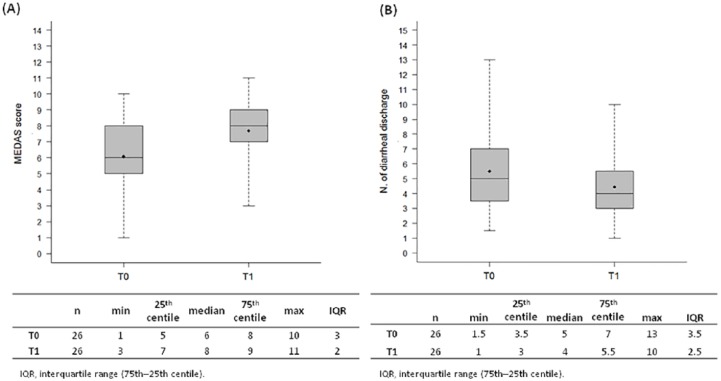
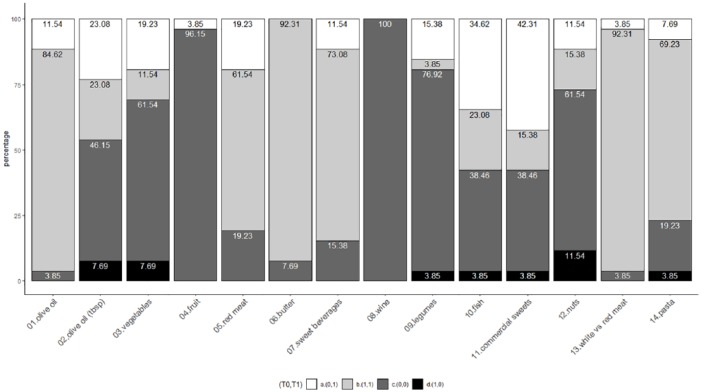
At baseline, adherence to the Mediterranean diet was poor (Figure 2a), with a median score of 6 (range 1-10). Patients also reported 5 diarrheal discharges per day on average (Figure 2b). At T1, there was a general improvement in adherence to the Mediterranean diet for each item considered (Table 1). There was a significant increase (Wilcoxon signed rank test P < .001) for the MEDAS score distributions at T0 and T1, with 2 MEDAS points of increment (looking at the median values) (Figure 2a). Figure 3 shows the impact of the dietary intervention for each item, jointly considering T0 and T1 data. About 20% of participants reduced their consumption of red/processed meat and 42% that of commercial sweets; more than a third (34.62%) increased their consumption of fish, followed by that of vegetables (19.23%), legumes (15.38%), and fruit (3.85%). Only a few patients worsened their habits by reducing their consumption of legumes, pasta, fish (3.85%), olive oil, vegetables (7.69%), and nuts (11.54%) and increasing their intake of commercial sweets (3.85%). As regards diarrhea, a significant decrease (Wilcoxon signed rank test P < .001) was observed with a reduction of an average of one discharge per day. Figure 2b presents the number of episodes of diarrheal discharges at T0 and T1.
Figure 2.
Distribution of the MEDAS score and number of diarrheal discharges per day at baseline and at the end of the dietary intervention. Box plots reflecting the distribution of the MEDAS score (A) and the number of diarrheal discharges per day (B) at baseline (T0) and at the end of the dietary intervention (T1) together with the corresponding descriptive statistics. Each box indicates the 25th and 75th centiles. The horizontal line inside the box indicates the median, the black dot represents the mean values and the whiskers indicate the extreme values measured.
Figure 3.
Impact of the dietary intervention. Bar chart representing the impact of the proposed dietary intervention comparing the T0 and T1 MEDAS data. On the x-axis are reported the 14 dietary items considered (as reported in Table 1) and on the y-axis the percentage of subjects according to a 4-class categorization: (0,0) subjects who did not satisfy the MEDAS criteria both at T0 and T1 (dark gray rectangle), (1,1) subjects who fulfilled the adherence to the MEDAS criteria at both time points (light gray rectangle), (0,1) subjects who showed a positive change (ie, compliant with the MEDAS criteria at T1, white rectangle) and (1,0) subjects with a negative change (ie, compliant with the MEDAS criteria at T0, only—black rectangle).
Discussion
Living with a lifelong disease such as FAP can be stressful. FAP patients are obliged to call on healthcare services throughout their lives; they are dependent on a lifetime program of endoscopic surveillance, with prophylactic surgery to prevent gastrointestinal cancer. The impact of diet and nutrition are major concerns for these patients who, although normally free of symptoms before surgery, report problems related to bowel function postoperatively. These include leakage, difficulty in distinguishing gas from feces, perianal skin problems, symptoms of small bowel obstruction, an increase in defecation frequency, and dietary restrictions.
During outpatient check-ups, patients systematically ask for dietary or nutritional support for the management of symptoms, sometimes for too many discharges, sometimes for a bloated stomach, and sometimes for fear that some foods can hurt them or worsen their condition and promote the formation of new adenomas. Then, too, most physicians are currently not provided with specific dietary treatments to offer their FAP patients and recommendations are often based on a “if it hurts, don’t do it” philosophy. Therefore, FAP patients usually try various ingredients or dishes to see what they can eat without problems and, as a result, they start planning social activities differently.
Observational and experimental data have shown that the Mediterranean diet has anti-inflammatory effects in several chronic diseases.51-55 Our hypothesis is that a low-inflammatory diet mainly based on Mediterranean diet principles and recipes may reduce the intestinal inflammation in FAP participants that is related to both their bowel function and progression of the disease. To our knowledge, this is the first dietary intervention in FAP patients. The intervention also aims to improve the participants’ quality of eating. The reduction of certain items, including lactose and processed complex carbohydrates, the promotion of pre- and probiotics, and the distinction between saturated, trans-, mono-, and polyunsaturated fats may simultaneously change their nutrients intake and diet quality, ultimately lowering circulating levels of free radicals, pro-inflammatory cytokines, and CRP.31,32,56
Our preliminary findings are encouraging as regards the feasibility, adherence (>80% overall compliance) dietary outcome measures, safety, and satisfaction with the study. FAP patients reported a perception of improvement in their general physical state. During the 3-month dietary intervention, they consumed more of the Mediterranean diet foods (vegetables, fruits, fish, and legumes), and less pro-inflammatory foods (red/processed meat and sweets); this led to significantly higher MEDAS scores. This improvement, despite the increased consumption of foods with higher fiber content (vegetables, fruits, and legumes), was accompanied by a decrease in the number of diarrheal discharges. One explanation lies in the reduced consumption of certain carbohydrates that cause dysbiosis (lactose and in general refined sugars), the use of prebiotics and probiotics and the initial changes in food texture to enhance absorption and reduce intact fiber. The overall results will help answer the research questions posed. Based on the MEDAS results, we expect a significant reduction of the inflammatory markers. Furthermore, understanding if and how the microbiota can be modified by diet will enable us to hypothesize treatments with pre/probiotics that directly act on the microbiota to specifically increase the number of bacteria that can interfere with polyp development and, ultimately, with CRC progression.
Finally, some notes on the possible limitations of the current study should be mentioned. This study is a nonrandomized trial with the main purpose of assessing the feasibility and impact of a low-inflammatory dietary intervention on FAP patients. Accordingly, we implemented a single arm trial, with all patients treated with the dietary recommendations. Therefore, as reported,46,47 a sample size of ~30 subjects was considered suitable for such pilot/feasibility studies, also to generate information on the effect of the intervention. The data generated by this study can be seen as the first step toward a more in-depth assessment of the impact of the low-inflammatory diet on inflammatory markers, and consequently on adenoma recurrence/CRC in these patients. Taking advantage of the information acquired in this first study, we are planning to design new randomized studies involving larger cohorts of patients to eventually improve the transferability and generalizability of the results observed. The main difficulty we encountered with these patients was the initial mistrust of the proposed diet due to their problems related to bowel function. The concern about the possibility of worsening bowel symptoms by changing their dietary and eating habits was the reason why six participants dropped out before starting the intervention. To monitor any adverse effects or difficulties in following the diet, we created a group chat among participants, nutritionists and clinicians and no relevant difficulties emerged. The chat was also used to share and comment on food recipes, and the participants actively participated by sending comments and images of the food they prepared.
The strength of this project is the multidisciplinary nature of the team, which involves different departments and research groups from the INT, all with consolidated experience in FAP patients’ management, high through-put studies on CRC, and dietary intervention trials.
It is our belief that the success of this project will significantly improve the FAP patient’s quality of life and will help achieve a breakthrough in knowledge and management of this disease.
Conclusion
The impact of diet and nutrition are major concerns for FAP patients after colectomy. There is scientific evidence from animal models and epidemiologic studies that dietary factors may influence both intestinal mucosal inflammation and the risk of developing adenoma/CRC. Here we describe the first dietary intervention in FAP patients. It is worth stressing the importance of dietary manipulation as an adjunct to the existing management options for FAP.
Acknowledgments
The authors wish to acknowledge and express sincere gratitude to Martina Stroscia, research nurse, for her valuable contribution in recruiting patients, to Roberto Fiocco, endoscopy nurse, for his valuable contribution to the endoscopic studies during pilot study. We also thank Mariangela Di Ceglie and Ornella Galuppo from the Hereditary Digestive Tract Tumors Unit, for prospectively maintaining patient’s database, Angela Angarano and Antonella Maule for the kitchen activities and Maria Grazia Guerrini for the editorial support. Finally, the authors would like to acknowledge with much appreciation all the volunteers who participated in the study.
Footnotes
Ethical Approval: All participants signed an informed consent. We obtained the ethical approval to conduct this study from the institutional review board and ethical committee of the Fondazione IRCCS Istituto Nazionale dei Tumori di Milano. All data are available through Fondazione IRCCS Istituto Nazionale dei Tumori di Milano.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds obtained through an Italian law that allows taxpayers to allocate 0.5% share of their income tax contribution to a research institution of their choice.
ORCID iD: Patrizia Pasanisi  https://orcid.org/0000-0001-6278-3491
https://orcid.org/0000-0001-6278-3491
References
- 1. Signoroni S, Vitellaro M, Sala P, Bertario L. Biomarkers in familial adenomatous polyposis: role and significance. Front Biosci (Schol Ed). 2010;2:413-421. [DOI] [PubMed] [Google Scholar]
- 2. Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [DOI] [PubMed] [Google Scholar]
- 3. Vitellaro M, Ferrari A, Trencheva K, et al. Is laparoscopic surgery an option to support prophylactic colectomy in adolescent patients with familial adenomatous polyposis (FAP)? Pediatr Blood Cancer. 2012;59:1223-1228. [DOI] [PubMed] [Google Scholar]
- 4. Provenzale D, Gupta S, Ahnen DJ, et al. Genetic/Familial High-Risk Assessment: Colorectal Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1010-1030. [DOI] [PubMed] [Google Scholar]
- 5. Vitellaro M, Bonfanti G, Sala P, et al. Laparoscopic colectomy and restorative proctocolectomy for familial adenomatous polyposis. Surg Endosc. 2011;25:1866-1875. [DOI] [PubMed] [Google Scholar]
- 6. Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [DOI] [PubMed] [Google Scholar]
- 7. McClellan JL, Davis JM, Steiner JL, et al. Intestinal inflammatory cytokine response in relation to tumorigenesis in the ApcMin/+ mouse. Cytokine. 2012;57:113-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gill PA, van Zelm MC, Muir JG, Gibson PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48:15-34. [DOI] [PubMed] [Google Scholar]
- 9. Quintanilha BJ, Reis BZ, Duarte GBS, Cozzolino SMF, Rogero MM. Nutrimiromics: role of microRNAs and nutrition in modulating inflammation and chronic diseases. Nutrients. 2017;9:E1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313-1316. [DOI] [PubMed] [Google Scholar]
- 11. Hull MA. Nutritional agents with anti-inflammatory properties in chemoprevention of colorectal neoplasia. Recent Results Cancer Res. 2013;191:143-156. [DOI] [PubMed] [Google Scholar]
- 12. Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res. 2017;61(1). doi: 10.1002/mnfr.201600129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuhouser ML, Schwarz Y, Wang C, et al. A low-glycemic load diet reduces serum C-reactive protein and modestly increases adiponectin in overweight and obese adults. J Nutr. 2012;142:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489-1497. [DOI] [PubMed] [Google Scholar]
- 15. Azadbakht L, Esmaillzadeh A. Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr. 2009;139:335-339. [DOI] [PubMed] [Google Scholar]
- 16. Esmaillzadeh A, Azadbakht L. Home use of vegetable oils, markers of systemic inflammation, and endothelial dysfunction among women. Am J Clin Nutr. 2008;88:913-921. [DOI] [PubMed] [Google Scholar]
- 17. Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30:967-973. [DOI] [PubMed] [Google Scholar]
- 18. de Oliveira Otto MC, Alonso A, Lee DH, et al. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141:1508-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flock MR, Rogers CJ, Prabhu KS, Kris-Etherton PM. Immunometabolic role of long-chain omega-3 fatty acids in obesity-induced inflammation. Diabetes Metab Res Rev. 2013;29:431-445. [DOI] [PubMed] [Google Scholar]
- 20. Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007;26:163-169. [DOI] [PubMed] [Google Scholar]
- 21. Salehi-Abargouei A, Saraf-Bank S, Bellissimo N, Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition. 2015;31:631-639. [DOI] [PubMed] [Google Scholar]
- 22. Steckhan N, Hohmann CD, Kessler C, Dobos G, Michalsen A, Cramer H. Effects of different dietary approaches on inflammatory markers in patients with metabolic syndrome: a systematic review and meta-analysis. Nutrition. 2016;32:338-348. [DOI] [PubMed] [Google Scholar]
- 23. Fu Z, Shrubsole MJ, Smalley WE, et al. Lifestyle factors and their combined impact on the risk of colorectal polyps. Am J Epidemiol. 2012;176:766-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J. 2014;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samadder NJ, Gornick M, Everett J, Greenson JK, Gruber SB. Inflammatory bowel disease and familial adenomatous polyposis. J Crohns Colitis. 2013;7:e103-e107. [DOI] [PubMed] [Google Scholar]
- 26. Henson CC, Burden S, Davidson SE, Lal S. Nutritional interventions for reducing gastrointestinal toxicity in adults undergoing radical pelvic radiotherapy. Cochrane Database Syst Rev. 2013;(11):CD009896. [DOI] [PubMed] [Google Scholar]
- 27. Del CF, Vernocchi P, Dallapiccola B, Putignani L. Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci. 2014;15:11678-11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neale EP, Batterham MJ, Tapsell LC. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res. 2016;36:391-401. [DOI] [PubMed] [Google Scholar]
- 29. Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant-based diets on obesity-related inflammatory profiles: a systematic review and meta-analysis of intervention trials. Obes Rev. 2016;17:1067-1079. [DOI] [PubMed] [Google Scholar]
- 30. Arpon A, Riezu-Boj JI, Milagro FI, et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J Physiol Biochem. 2016;73:445-455. [DOI] [PubMed] [Google Scholar]
- 31. Marlow G, Ellett S, Ferguson IR, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics. 2013;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schroder H, Fitó M, Estruch R, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140-1145. [DOI] [PubMed] [Google Scholar]
- 33. Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the Diet and Androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25-33. [PubMed] [Google Scholar]
- 34. Bruno E, Manoukian S, Venturelli E, et al. Adherence to Mediterranean diet and metabolic syndrome in BRCA mutation carriers. Integr Cancer Ther. 2018;17:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasanisi P, Gargano G, Gaetana Di, Mauro M, et al. A randomized controlled trial of Mediterranean diet and metformin to prevent age-related diseases in people with metabolic syndrome. Tumori. 2018;104:137-142. [DOI] [PubMed] [Google Scholar]
- 36. Musa-Veloso K, Poon T, Harkness LS, O’Shea M, Chu Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2018;108:759-774. [DOI] [PubMed] [Google Scholar]
- 37. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685. [DOI] [PubMed] [Google Scholar]
- 38. Croagh C, Shepherd SJ, Berryman M, Muir JG, Gibson PR. Pilot study on the effect of reducing dietary FODMAP intake on bowel function in patients without a colon. Inflamm Bowel Dis. 2007;13:1522-1528. [DOI] [PubMed] [Google Scholar]
- 39. Phillips KM, Tarrago-Trani MT, McGinty RC, Rasor AS, Haytowitz DB, Pehrsson PR. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J Sci Food Agric. 2018;98:4191-4204. [DOI] [PubMed] [Google Scholar]
- 40. Stevenson TJ, Visser ME, Arnold W, et al. Disrupted seasonal biology impacts health, food security and ecosystems. Proc Biol Sci. 2015;282:20151453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rial NS, Meyskens FL, Gerner EW. Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention. Essays Biochem. 2009;46:111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iablokov V, Sydora BC, Foshaug R, et al. Naturally occurring glycoalkaloids in potatoes aggravate intestinal inflammation in two mouse models of inflammatory bowel disease. Dig Dis Sci. 2010;55:3078-3085. [DOI] [PubMed] [Google Scholar]
- 43. Sucha L, Tomsik P. The steroidal glycoalkaloids from Solanaceae: toxic effect, antitumour activity and mechanism of action. Planta Med. 2016;82:379-387. [DOI] [PubMed] [Google Scholar]
- 44. Rezac S, Kok CR, Heermann M, Hutkins R. fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920-924. [DOI] [PubMed] [Google Scholar]
- 46. Browne RH. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933-1940. [DOI] [PubMed] [Google Scholar]
- 47. Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCulloch CE, Neuhaus JM. Generalized Linear Mixed Models. New York, NY: John Wiley & Sons, Inc; 2005. [Google Scholar]
- 49. Pizzamiglio S, Cossa G, Gatti L, et al. Simultaneous confidence intervals to compare gene expression profiles using ABC transporter TaqMan microfluidic cards. Oncol Rep. 2010;23:853-860. [PubMed] [Google Scholar]
- 50. Reid JF, Sokolova V, Zoni E, et al. miRNA profiling in colorectal cancer highlights miR-1 involvement in MET-dependent proliferation. Mol Cancer Res. 2012;10:504-515. [DOI] [PubMed] [Google Scholar]
- 51. Casas R, Estruch R, Sacanella E. The protective effects of extra virgin olive oil on immune-mediated inflammatory responses. Endocr Metab Immune Disord Drug Targets. 2018;18:23-35. [DOI] [PubMed] [Google Scholar]
- 52. Mena MP, Sacanella E, Vazquez-Agell M, et al. Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr. 2009;89:248-256. [DOI] [PubMed] [Google Scholar]
- 53. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440-1446. [DOI] [PubMed] [Google Scholar]
- 54. Fragopoulou E, Panagiotakos DB, Pitsavos C, et al. The association between adherence to the Mediterranean diet and adiponectin levels among healthy adults: the ATTICA study. J Nutr Biochem. 2010;21:285-289. [DOI] [PubMed] [Google Scholar]
- 55. Mayr HL, Thomas CJ, Tierney AC, et al. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: the AUSMED Heart Trial. Nutr Res. 2018;55:94-107. [DOI] [PubMed] [Google Scholar]
- 56. McCarty MF. Low-insulin-response diets may decrease plasma C-reactive protein by influencing adipocyte function. Med Hypotheses. 2005;64:385-387. [DOI] [PubMed] [Google Scholar]