Abstract
Background:
The zone of stasis that forms in acute burn is initially viable, but coagulation progresses to necrosis in the process that follows.
Objective:
This study investigates the effects of platelet-rich plasma (PRP) on apoptosis in the burn zone of stasis and on the viability of living tissue.
Methods:
Burns were established in the right ears of 20 female New Zealand rabbits using the “comb burn” model. Platelet-rich plasma was obtained from blood collected from rabbits’ ears (n = 10) and was injected subcutaneously into the zone of stasis (n = 10). The same amount of saline solution was injected into the zone of stasis of the control group rabbits (n = 10). Histological and immunohistochemical apoptosis analysis was performed to evaluate viable areas.
Results:
Apoptosis levels were higher in the control group than in the experimental group. The area of viable tissue in the zone of stasis was greater than in the control group. Infection-induced neutrophil infiltration was statistically significantly lower in the experimental group.
Conclusion:
In this animal model, apoptosis count and viable tissue area measurement and the anti-inflammatory results in the burn area confirm that PRP therapy has a statistically significant positive impact on the survival of the zone of stasis and in acute burn injury.
Keywords: platelet-rich plasma, burn, stasis zone
Abstract
Historique:
La zone de stase qui se forme après une brûlure aiguë est d’abord viable, mais la coagulation entraîne la nécrose dans le processus qui suit.
Objectif:
La présente étude porte sur les effets du plasma riche en plaquettes (PRP) sur l’apoptose dans la zone de stase d’une brûlure et sur la viabilité des tissus vivants.
Méthodologie:
Les chercheurs ont établi les brûlures dans l’oreille droite de 20 lapins de Nouvelle-Zélande au moyen du modèle de brûlure par peigne. Ils ont obtenu le PRP dans le sang prélevé dans l’oreille des lapins (n = 10) et l’ont injecté par voie sous-cutanée dans la zone de la stase (n = 10). Ils ont injecté la même quantité de soluté physiologique dans la zone de la stase du groupe témoin de lapins (n = 10). Ils ont analysé l’apoptose histologique et immunohistochimique pour évaluer les secteurs viables.
Résultats:
Les taux d’apoptose étaient plus élevés dans le groupe témoin que dans le groupe expérimental. La région de tissus viables de la zone de stase était plus étendue que dans le groupe témoin. L’infiltration de neutrophiles induite par infection était statistiquement plus basse dans le groupe expérimental.
Conclusion:
Dans ce modèle animal, la numération de l’apoptose, la mesure de la région des tissus viables et les résultats anti-inflammatoires dans la région de la brûlure confirment que le traitement par PRP a des répercussions positives statistiquement importantes pour la survie de la zone de stase en cas de brûlure aiguë.
Introduction
Thermal trauma can be conceptualized as occurring in 3 concentric zones of injury in the skin, the most severe damage occurring in the center as described by Jackson in 1953.1 Burn damage in this central area, known as the zone of coagulation, is direct and irreversible. The area immediately around the zone of coagulation is known as the zone of stasis, while the outermost area is known as the zone of hyperemia.1 The zone of hyperemia is the area characterized by vasodilation induced by mediators as a result of inflammation and that is capable of healing completely. The zone of stasis represents the at-risk area and becomes necrotic in case of progressive ischemia, edema, and infection.2 Procedures intended to save the zone of stasis may prevent this coalescing with the neighboring zone of coagulation, so secondary expansion of the burn wound can thus be limited. The zone of stasis is particularly exposed to oxidative stress resulting from ischemia–reperfusion injury, and apoptosis is observed.3 An association has been shown between tissue damage and apoptosis rates.4
Platelets are significantly involved in hemostasis and wound healing following tissue injury. Platelet activation results in the expression of more than 30 growth factors,5 such as platelet-derived growth factor, transforming growth factor (TGF-β1), platelet-derived epidermal growth factor, and platelet-derived angiogenesis factor (PDAF), insulin-like growth factor-1, platelet factor-4 (PF-4), basic fibroblast growth factor, bone morphogenetic protein, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL1α,1β,7,8), matrix metalloproteinase (MMP2,9), stem cell factor (SCF), tissue inhibitor of metalloproteinase, tissue factor, tumor necrosis factor (TNF-α), vascular endothelial growth factor (VEGF). Of these cytokines, the TGF family of proteins is able to reversibly inhibit keratinocytes and leukocytes. However, TGFs are weak in their ability to stimulate fibroblasts, and it is able to stimulate chemotaxis of inflammatory cells and to synthesize the extracellular matrix.
These stimulate migration in mesenchymal and epithelial cells and enhance collagen and matrix synthesis.6 They also trigger a cascade for the recruitment of reparative cells while at the same time suppressing apoptosis and metalloproteinase activity. Platelets are chemotactic and regulate wound healing by stimulating fibroblast and endothelial cell and progenitor cell proliferation.
The use of PRP has been proposed for the purpose of enhancing bone regeneration and soft tissue reconstruction.7 It has been identified as a viable alternative option for clinical procedures and for use in the treatment of chronic wounds8 and pressure ulcers.9 The application of PRP now extends even to the treatment of tendinopathies.10-15 Platelet-rich plasma has also been reported to exhibit antimicrobial characteristics that may assist with the prevention of infections.8 Studies have shown that PRP injection enhances the survival of cutaneous flaps and correlates well with angiogenesis.16
Close associations apply between apoptosis and inflammation. For example, inflammatory mediators increase in line with apoptosis. Studies have demonstrated that PRP administration leads to an increase in the proliferation and the secretion of anti-inflammatory cytokines and growth factors, lower levels of apoptosis, and greater autophagy.17
However, our review of the literature revealed no previous studies of the effects of PRP on reversible tissue damage in the zone of stasis zone and apoptosis. The purpose of this study was to investigate the effects of PRP on the zone of stasis following acute burn trauma.
Materials and Methods
Approval for the experimental protocol was granted by the institutional Animal Care Committee. All institutional and national guidelines for the care and use of laboratory animals were followed. Twenty adult female New-Zealand rabbits, aged 210 to 240 days, weighing mean average 2830 g, were used. All animals were housed in separate cages with ad libitum access to standard rabbit chow and water. Anesthesia was induced with ketamine (100 mg/kg) and chlorpromazine (5 mg/kg) administered intramuscularly. Rabbits were divided into experimental (n = 10) and control (n = 10) groups.
The Experimental Protocol and Burn Injury Model
The hair covering the entire anterior surface of the ear was first removed by clipping. Thermal injury was induced on the ear of all 20 rabbits using the “comb burn” technique18 involving heated brass probes containing 4 rows (2 cm × 1 cm) and 3 interspaces between them (0.5 cm × 2 cm). These interspaces represented the burn zone of stasis zone (Figure 1). Saline solution at 0.5 mL was injected into each zone of stasis in the rabbits in the control group, while 0.5 mL PRP was injected subcutaneously at angle of 40° to 45° of the zone of stasis in each rabbit in the experimental group. Sufficient blood for PRP was collected from the middle ear veins. Separate cannulae and injectors were used for each rabbit. Histological and immunohistochemical apoptosis analysis was performed to evaluate viable areas in the zone of stasis 72 hours after burn injury. No clinical signs of infection were observed in the burn areas. At the end of the experiment, rabbits were killed.
Figure 1.
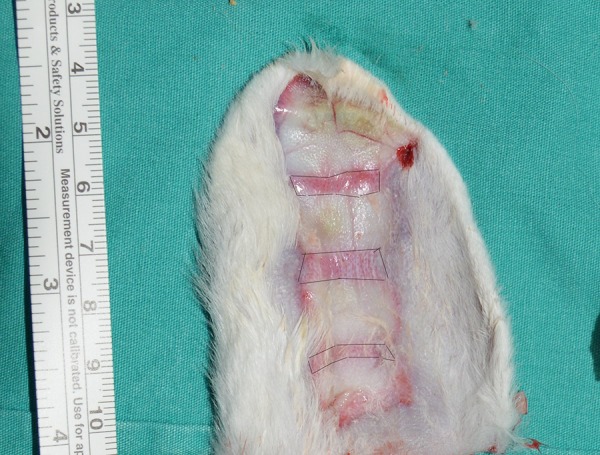
The comb-burn model established in the rabbit ear. Marked areas show stasis zone which PRP injected, biopsy specimens were collected and viable stasis zone area measured. PRP indicates platelet-rich plasma.
Preparation of PRP
A Traylife PRP kit and activator (Promoitalia, Italy) were used in the preparation of PRP; 10 mL of blood was collected from the rabbits’ left ears, of which 1 mL was used for a basal platelet count. The remaining 9 mL was (Eppendorf 5810, Hamburg, Germany) centrifuged at 2000 rpm for 10 minutes in a citrate tube. Approximately 5 to 6 mL of serum was obtained. An erythrocyte layer, a platelet, and leukocyte-rich buffy coat and plasma layers formed after centrifugation. Platelet-rich plasma was activated by the addition of 0.5 mL 5% to 10% calcium chloride solution enriched with adenosine, guanine, cytosine, and thymine nucleotides for activation of the approximately 1 mL of PRP obtained. This was injected into the experimental group zone of stasis within 8 minutes of activation.
Histopathological Analysis
Ear specimens from both the groups were divided into 3 parts containing zones of coagulation and stasis. Sections 5 μm in thickness were taken from the paraffin blocks using a fully automated microtome (Leica RM 2255, Tokyo, Japan). These were stained with hematoxylin and eosin (H&E) and with Masson trichrome to visualize the connective tissue structure. Histopathological evaluation of the preparates was performed by an experienced histologist blinded to the study groups. Macrophages and the other cells were differentiated by light microscopy with conventional dyes (H&E, Masson trichome, etc.) by morphological characteristics. Analysis 5 Research (Olympus Soft Imaging Solutions, Germany) software was used for light microscopic measurement and counts. Subepithelial neutrophil infiltration, fibroblast proliferation and macrophage density were evaluated using a scoring system: (0-5 cells (1+), 5-10 cells (2+), and more than 10 cells (3+). During evaluation of epithelialization, that part of the zone of stasis with epithelialization was calculated as a proportion of the length of the entire zone of stasis. Under the scoring system, no epithelialization was scored (0), up to 25% epithelialization was scored 1, 25% to 50% epithelialization was scored 2, 50% to 75% epithelialization was scored 3, and complete epithelialization was scored 4. Angiogenesis was scored semiquantitatively: no angiogenesis 0, mild angiogenesis 1, moderate angiogenesis 2, and strong angiogenesis 3.
Immunohistochemical Analysis
Skin specimens removed from the zones of stasis of the experimental and control groups were first fixed in 10% formaldehyde. Specimens were then subjected to immunohistochemical analysis using the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) technique. An in situ cell death detection kit (Roche, Mannheim, Germany) was used in the TUNEL technique in order to assess apoptosis in epidermis epithelium cells and dermis connective tissue cells. A kit DAB(Sigma, St. Louis, Missouri, USA) containing 3,3′-diaminobenzidine (DAB) was used to color apoptotic cells. Homogeneously stained brown cells with no areas of necrosis were regarded as apoptotic cells and defined as (+). Apoptotic and normal cells among epidermis epithelial cells and dermis connective tissue cells from both groups were counted in 100 cells in 5 different areas with 40× magnification. The percentage of apoptotic cells was then calculated.
Zone of Stasis Area Measurements
Image J (National Institutes of Health, Bethesda, Maryland) software was used to calculate zones of stasis on photographic images taken on the fourth day of trauma. Three areas of zone of stasis in each ear were calculated as square millimeter following calibration using a millimetric rule placed over the photographic image (Figure 1).
Statistical Analysis
All statistical analyses were performed on SPSS for Windows version 13.0 (SPSS, Inc, Chicago, Illinois) software. Differences between the groups in terms of categoric variables (fibroblast proliferation, neutrophil infiltration, reepithelialization, angiogenesis, macrophage density, epithelial apoptosis levels, and connective tissue apoptosis levels) were compared using the Kolmogorov-Smirnov test. Student t test was used to compare normally distributed measurement data in 2 groups, while the Mann Whitney U test was used for nonnormally distributed data. P values <.05 were regarded as statistically significant.
Results
Platelet-rich plasma prepared in our study group was higher than basal platelet levels in all experimental animals, with a mean concentration factor of 1.37 (PRP Platelet/Basal Platelet Count). There was no statistically significant difference between the experimental and control groups in terms of preexperiment blood platelet numbers (P = .068; Table 1). Platelet numbers in PRPs in all subjects in the study group were higher than basal levels.
Table 1.
Histopathological Data, Zone of Stasis Areas, and Apoptosis Levels in the 2 Groups.
| Control Group (Average ± SD) | Experimental Group (Average ± SD) | P Value (P < .05 = significant) | |
|---|---|---|---|
| Reepitelisation, 0-4 | 0.70 | 3.56 | <.001 a |
| Neutrophil infiltration, 1-3 | 2.80 | 1.78 | .007 a |
| Macrophage density, 1-3 | 1.50 | 1.11 | .076 |
| Fibroblast proliferation (1-3) | 1.20 | 2.44 | <.001 a |
| Angiogenesis, 0-3 | 0.70 | 1.00 | .203 |
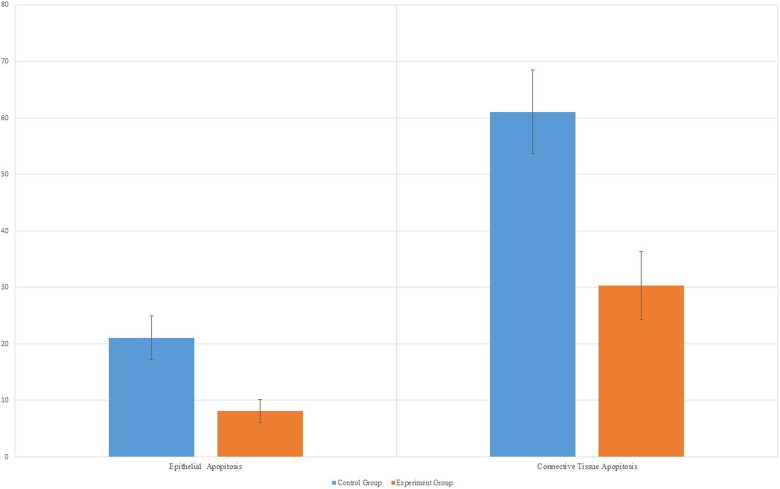
| Epithelial apoptosis, % | 21.10 ± 3.90 | 8.11 ± 2.02 | <.001 a |
| Connective tissue apoptosis, % | 61.10 ± 7.4 | 30.33 ± 6.02 | <.001 a |
| Vital stasis zone area, mm2 | 47 423 ± 20.80 | 64.29 ± 45.31 | .041 |
| Platelet count, 103/µ | 213 80 ± 88.81 | 285 89 ± 69.81 | .068 |
Abbreviation: SD, standard deviation.
a Statistically significant parameters.
Histopathological Analysis
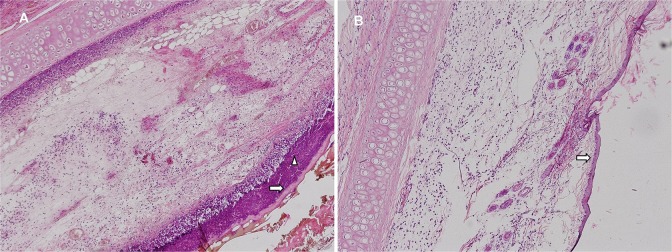
Pronounced diffuse neutrophil infiltration was observed in different zone of stasis sections in the control group preparations (Figure 2). Substantial thinning was present in the epithelial layer. Occasional areas with no epithelial layer were observed. Diffuse neutrophil infiltration and increased macrophage density were present in the dermis. Connective tissue cells in the dermis had decreased markedly, and vascular congestion was present (Figures 3).
Figure 2.
A, Control group. B, Experimental group. Epithelial (ð) and subepithelial (▹) neutrophil infiltration, (H&E ×100). H&E indicates hematoxylin and eosin.
Figure 3.
A, A microphotograph from the saline solution showing a decrease in connective tissue (ð) and neutrophil infiltration (star; Massons trichrome X200). B, A microphotograph from the PRP group showing increased connective tissue (ð), neutrophil infiltration (star), (Masson trichrome X200). PRP indicates platelet-rich plasma.
In preparations from the experimental group, although the epithelial structure was occasionally interrupted, its integrity was maintained. No subepithelial neutrophil infiltration was observed (Figure 2). Neutrophil infiltration and occasional macrophages were observed in the dermis. Diffuse fibroblast proliferation was present (Figure 3). Reepithelialization and fibroblast scores in the experimental group were statistically significantly higher than in the control group (P < .01; Mann-Whitney U test). Neutrophil infiltration, however, was significantly lower in the experimental group (P < .007; Mann-Whitney U test). Since macrophage density and angiogenesis scores were higher in the experimental group, the differences were not statistically significant (Figure 4; Table 1).
Figure 4.
Diagram showing histopathological differences between the 2 groups, *: statistically significant histopathological parameters.
Figure 5.
Chart showing apoptosis percentages in epithelium and connective tissue between the 2 groups: significantly less apoptosis was observed in both epithelial tissue and connective tissue in the experiment group compared to the control group. PRP indicates platelet-rich plasma.
Immunohistochemical Analysis
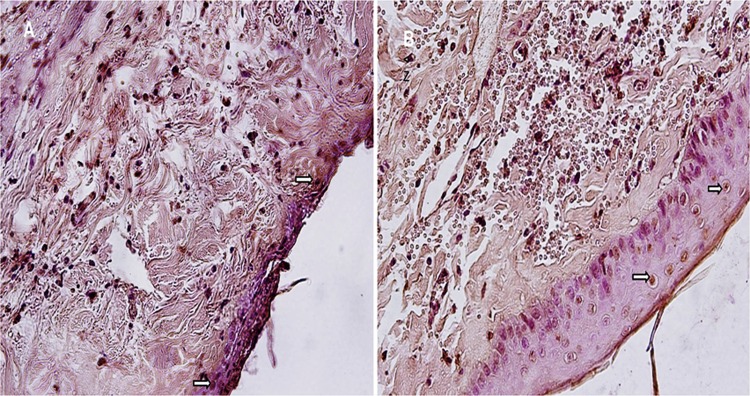
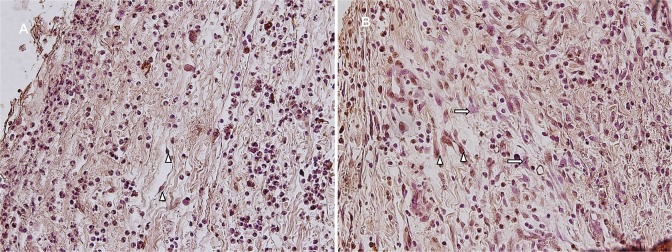
The apoptosis count in epithelial tissue was statistically significantly higher in the control group (21.1 ± 3.9) compared to that in the experimental group (8.11 ± 2.02; P < .001;Figure 6). Connective tissue apoptosis count was also statistically significantly higher in the control group (61.1 ± 7.4) compared to the experimental group (30.33 ± 6.02; P < .01; Student t test; Figure 7 and Table 1).
Figure 6.
A, Control group. B, Experimental group. Apoptotic epithelial cells (ð), (TUNEL analysis ×400). TUNEL indicates terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling.
Figure 7.
A, Control group. B, Experimental group. Apoptotic connective tissue cells (Δ), normal fibroblast cells (ð), (TUNEL analysis ×400). TUNEL indicates terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling.
Zone of Stasis Measurement Findings
Mean viable tissue in the zone of stasis areas was 64.298 ± 45.31 mm2 in the experimental group and 47.423 ± 20.80 mm2 in the control group. The difference was statistically significant (P < .041; Student t test; Figure 8; Table 1).
Figure 8.
Significantly more viable zone of stasis was observed in the PRP group. PRP indicates platelet-rich plasma.
Discussion
The damage caused in the skin by burn trauma results first from coagulation necrosis caused by heat at the time of contact and from progressive dermal ischemia-related delayed injury resulting in cell death in the following 48 to 72 hours. Apoptosis has also been implicated in increasing cell death and dysfunction in the zone of stasis in the period following ischemia and reperfusion injury.19,20 Clinical and experimental studies have reported increased apoptosis increase in the skin following burn injury.4 Increased tissue loss in the zone of stasis following burn and worsening of clinical condition have been reported to be directly associated with apoptotic cell count.21 Close correlation has been reported between apoptosis and inflammation, with inflammatory mediators rising in line with apoptosis.17 Platelet-rich plasma exhibits an anti-inflammatory effect and neovascularization, depending on the environment into which it is injected. Platelet-rich plasma therapy reduces pain and significantly improves function in patients with tendinopathies, effects that are considerably greater than those of corticosteroid injection.10,12,13 Platelet-rich plasma injection has been reported to encourage skin flap survival by stimulating capillary proliferation and lowering inflammatory cell infiltration.16 Platelets contain a range of proteins, cytokines, and other bio-active factors responsible for triggering and regulating various components of the wound healing process. These are significantly involved in cell proliferation and differentiation, chemotaxis, and angiogenesis. Transforming growth factor stimulate granulation tissue formation and activate fibroblasts, resulting in procollagen formation, which is induced by collagen deposition and wound healing.22
Although numerous agents whose effects on saving the zone of stasis are still being investigated, the side effects of the majority of drugs that regulate microcirculation and exhibit antithrombotic effects have limited their use in clinical practice.3,23-26
Moreover, no reliable, low-cost, and highly effective drug in terms of improving the zone of stasis in acute burn is still in routine clinical use today. For these reasons, this study was intended to investigate the effects on saving the zone of stasis and apoptosis of PRP, which is easy to prepare and apply, is particularly effective in wound healing and immunomodulation, and delivers various growth factors.
A hypermetabolic condition resulting in cytokine response develops in proportion to the severity of burn in the case of severe burns covering 40% or more of the body’s total surface area. Since the burn induced in each rabbit in the burn procedure established in this study represented a relatively small proportion of the total body area, we anticipated no increase in cytokine levels. Although burn shock is not anticipated in a burn with such a small surface area, it has still been reported that systematically active factors may be released, and our knowledge on the kind of effects on platelets is limited. However, IL-1 and TNF-α that increase in the systemic circulation in large and severe burns can interact with platelets in the circulation which causes increased platelet aggregation through arachidonic acid pathway.5 Tumor necrosis factor is a potent inhibitor of the clonal growth of primitive and committed human bone marrow progenitors in combination with multiple cytokines including granulocyte colony-stimulating factor (G-CSF), colony- CSF-1, erythropoietin, SCF. In contrast, TNF-α at low concentrations can synergistically and directly enhance the clonal growth of primitive and more mature human CD34+ bone marrow progenitors when combined with GM-CSF or interleukin 3.27
It has therefore been recommended that autologous PRP prepared for use in acute burn should be applied in burn involving 10% or less of the total body surface area.18,23-26
Platelet-rich plasma represents a concentration of platelets in a small amount of plasma, and 8 to 10 cm3 blood is required in order to obtain it. Since it is impossible to collect enough blood to prepare PRP in small animals such as rats, we used rabbits in this study. Quantitative measurement of growth factors in PRP has been performed in several studies, and significant variations have been reported.28-29 The amount of growth factors in PRP depends on various factors, particularly the concentration of growth factors in α-granules, and also the technique used to prepare PRP, whether or not fragmentation is present in the platelets during preparation, the presence or absence of leukocytes in the prepared PRP, and platelet activation being complete.5 Studies have generally involved plasma obtained until a concentration factor several times higher than the basal platelet factor has been obtained. A platelet count of at least 0, 8 × 106/μL is recommended by some authors in PRP prepared irrespective of existing pathology, although this is not definite.30 Concentration factors for an effective and ideal PRP vary depending on the pathological status. The idea that the higher the platelet count, the better result it will give has been changed by studies of intestinal anastomoses in rats showing that a high-concentration PRP exhibits an inhibitor effect on wound healing.8 The PRP prepared in our experiment exhibited a higher value than basal platelet values in all experimental subjects.
In the absence of a suitable alternative, quantitative measurement of growth factor represents the best method for measuring the quality of PRP in the presence of all these variables.8,30 How PRP applied to different percentage areas of burn in different concentration factors and at different times will affect various growth factors was not measured in this study, and investigation of these in further studies will shed light on the use of PRP in burn treatment.
Conclusions
In this animal model, PRP exhibited anti-inflammatory and antiapoptotic effects in the zone of stasis in acute burn. Greater reepithelialization and fibroblast proliferation were observed in the experimental group, and statistically significantly survival of a viable zone of stasis was achieved in consequence. Our hypothesis that PRP injection would reduce apoptosis in the burn zone of stasis was strongly supported by the results of this study. Our findings show that PRP application in cases of acute burn in cases involving areas smaller than 10% of the total body area is an effective treatment for potentially preventing the zone of stasis from progressing to necrosis. Further studies are now needed to determine the most effective therapeutic PRP dosage, frequency of application, length of treatment, and ideal therapeutic algorithm. After animal studies, human studies are also necessary to use clinic practice.
Footnotes
Level of Evidence: Level 2, Diagnostic
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Jackson DM. The diagnosis of the depth of burning. Br J Surg. 1953;40(164):588–596. [DOI] [PubMed] [Google Scholar]
- 2. Zawacki BE. Reversal of capillary stasis and prevention of necrosis in burns. Ann Surg. 1974;180(1):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Öksüz S, Ülkür E, Öncül O, Köse GT, Küçükodaci Z, Urhan M. The effect of subcutaneous mesenchymal stem cell injection on statis zone and apoptosis in an experimental burn model. Plast Reconstr Surg. 2013;131(3):463–471. [DOI] [PubMed] [Google Scholar]
- 4. Gravante G, Palmieri MB, Esposito G, et al. Apoptotic death in deep partial thickness burns vs. normal skin of burned patients. J Surg Res. 2007;141(2):141–154. [DOI] [PubMed] [Google Scholar]
- 5. Pallua N, Wolter T, Markowicz M. Platelet-rich plasma in burns. Burns. 2010;36(1):4–8. [DOI] [PubMed] [Google Scholar]
- 6. Anitua E, Andía I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23(2):281–286. [DOI] [PubMed] [Google Scholar]
- 7. Sun W, Sun W, Lin H, et al. Collagen membranes loaded with collagen binding human PDGF-BB accelerate wound healing in a rabbit dermal ischemic ulcer model. Growth Factors.2007;25(5):309–318. [DOI] [PubMed] [Google Scholar]
- 8. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. [DOI] [PubMed] [Google Scholar]
- 9. Sell SA, Ericksen JJ, Reis TW, et al. A case report on the use of sustained release platelet-rich plasma for the treatment of chronic pressure ulcers. J Spinal Cord Med. 2011;34(1):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774–1778. [DOI] [PubMed] [Google Scholar]
- 11. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34(6):909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. [DOI] [PubMed] [Google Scholar]
- 13. Peck E, Ely E. Successful treatment of de Quervain tenosynovitis with ultrasound-guided percutaneous needle tenotomy and platelet-rich plasma injection: a case presentation. PM R. 2013;5(5):438–441. [DOI] [PubMed] [Google Scholar]
- 14. Rha DW, Park GY, Kim YK, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(2):113–122. [DOI] [PubMed] [Google Scholar]
- 15. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255–262. [DOI] [PubMed] [Google Scholar]
- 16. Wang B, Geng Q, Hu J, Shao J, Ruan J, Zheng J. Platelet-rich plasma reduces skin flap inflammatory cells infiltration and improves survival rates through induction of angiogenesis: an experiment in rabbits. J Plast Surg Hand Surg. 2016;50(4):239–245. [DOI] [PubMed] [Google Scholar]
- 17. Moussa M, Lajeunesse D, Hilal G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146–156. [DOI] [PubMed] [Google Scholar]
- 18. Regas FC, Ehrlich HP. Elucidating the vascular response to burns with a new rat model. J Trauma. 1992;32(5):557–563. [DOI] [PubMed] [Google Scholar]
- 19. Gravante G, Filingeri V, Delogu D, et al. Apoptotic cell death in deep partial thickness burns by coexpression analysis of TUNEL and Fas. Surgery. 2006;139(6):854–855. [DOI] [PubMed] [Google Scholar]
- 20. Duan H, Chai J, Sheng Z, et al. Effect of burn injury on apoptosis and expression of apoptosis-related genes/proteins in skeletal muscles of rats. Apoptosis. 2009;14(1):52–65. [DOI] [PubMed] [Google Scholar]
- 21. Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118(6):147e–159e. [DOI] [PubMed] [Google Scholar]
- 22. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet-rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297–300. [DOI] [PubMed] [Google Scholar]
- 23. Işik S, Sahin U, Ilgan S, Güler M, Günalp B, Selmanpakoğlu N. Saving the zone of stasis in burns with recombinant tissue-type plasminogen activator (r-tPA): an experimental study in rats. Burns. 1998;24(3):217–223. [DOI] [PubMed] [Google Scholar]
- 24. Uygur F, Evinc R, Urhan M, Celikoz B, Haholu A. Salvaging the zone of stasis by simvastatin: an experimental study in rats. J Burn Care Res. 2009;30(5):872–879. [DOI] [PubMed] [Google Scholar]
- 25. Eski M, Ozer F, Firat C, et al. Cerium nitrate treatment prevents progressive tissue necrosis in the zone of stasis following burn. Burns. 2012;38(2):283–289. [DOI] [PubMed] [Google Scholar]
- 26. Deniz M, Borman H, Seyhan T, Haberal M. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns. 2013;39(2):320–325. [DOI] [PubMed] [Google Scholar]
- 27. Jacobsen SE, Jacobsen FW, Fahlman C, Rusten LS. TNF-alpha, the great imitator: role of p55 and p75 TNF receptors in hematopoiesis. Stem Cells. 1994;12(suppl 1):111–126. [PubMed] [Google Scholar]
- 28. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69. [DOI] [PubMed] [Google Scholar]
- 29. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–92. [DOI] [PubMed] [Google Scholar]
- 30. Yamaguchi R, Terashima H, Yoneyama S, Tadano S, Ohkohchi N. Effects of platelet-rich plasma on intestinal anastomotic healing in rats: PRP concentration is a key factor. J Surg Res. 2012;173(2):258–266. [DOI] [PubMed] [Google Scholar]