Abstract
Objective
To evaluate the impact of health professionals’ intervention on adherence to statins, the influence on total cholesterol levels, and lifestyle patterns in patients with hypercholesterolemia and analyze the differences according to the center of recruitment.
Study Setting
Forty‐six community pharmacies and 50 primary care centers of Spain.
Study Design
Randomized controlled trial design (n = 746). Patients were assigned into adherent (ADH) or nonadherent group depending on their initial adherence to statins. Nonadherent patients were randomly assigned to intervention (INT) or nonintervention (NOINT) group. Patients enrolled in the INT group received an intervention depending on the cause of nonadherence. Patients in the ADH and NOINT groups received usual care. Intention‐to‐treat (ITT) analysis was performed with multiple imputation to replace the missing data.
Data Collection
Adherence, total cholesterol levels, and lifestyle behaviors.
Findings
The odds of becoming adherent during the 6 months was higher in the INT group compared to the NOINT group (OR = 1,49; 95% CI: 1.30‐1.76; P < 0.001), especially in the community pharmacy group (OR = 2.34; 95% CI: 1.81‐3.03; P < 0.001). Adherent patients showed lower values of total cholesterol compared with nonadherent patients at baseline (ADH: 200.3 mg/dL vs NOADH: 216.7 mg/dL; P < 0.001) and at the endpoint (ADH: 197.3 mg/dL vs NOADH: 212.2 mg/dL; P < 0.001). More patients enrolled in the INT group practices exercise at the end of the study (INT: +26.6 percent; P = 0.002), and a greater number of patients followed a diet to treat hypercholesterolemia (+30.2 percent; P < 0.001).
Conclusions
The intervention performed by health professionals, especially by community pharmacists, improved adherence to statins by hypercholesterolemic patients, and this improvement in adherence was accompanied by a reduction in total cholesterol levels and a healthier lifestyle.
Keywords: clinical outcomes, community pharmacy, compliance, hypercholesterolemia, lipid‐lowering drugs
1. INTRODUCTION
Hypercholesterolemia is one of the most important risk factors in the development of cardiovascular diseases (CVD), which are responsible for more than one‐third of all deaths worldwide.1 Key factors in the management of dyslipidemia include physical activity, diet, and compliance with therapy.2, 3 However, lipid‐lowering therapies remain underused4 and unhealthy lifestyle is common in hypercholesterolemic patients.5
Currently, nonadherence is a problem of outstanding magnitude that particularly affects those with chronic diseases.6 Hypercholesterolemia is a symptomless condition, and as a consequence, nonadherence rates are high.7 While it is difficult to determine the exact magnitude of statin nonadherence, it is estimated to be around 50 percent during the initial stages of prescription and has been observed to increase with time.8 Moreover, nonadherence has been found to be directly related to higher rates of hospitalizations,9 increased morbidity and mortality,10, 11 and overall increases in health care costs.12, 13 However, the relationship between nonadherence to lipid‐lowering drugs and risk of cardiovascular events remains unclear.
Causes of nonadherence, either intentional or unintentional, may be related to a patient's health care system, community, financial resources, therapy regimen, and other patient‐related factors.6 A wide range of interventions have been studied to improve adherence to lipid‐lowering drugs, including simplification of treatment regimens,14, 15, 16, 17 use of reminder systems,18, 19, 20 and delivery of educational and informational content to patients.21, 22 However, no single intervention has been shown to improve adherence in patients affected by chronic diseases. Rather, a combination of strategies is necessary.23
Community pharmacists (CPs) and general practitioners (GPs) are ideally positioned to detect nonadherence and to provide patient‐centered interventions to those with chronic diseases.24, 25 In the last few years, interventions by several types of health professionals have been reported, and these have focused on improving adherence to lipid‐lowering medicines.26, 27 However, only a few of these studies assessed the impact of adherence on clinical outcomes.28, 29 In a recently published systematic review, patient‐centered interventions were found to improve adherence to lipid‐lowering drugs and cholesterol levels.7 However, these interventions were complex, they were composed of multiple components that involved a combination of different types of strategies, and the measures and outcomes that were assessed were not consistent. Thus, direct comparisons among these data are challenging.30 Services that focus on detecting causes of nonadherence and then delivering the best intervention to address these causes may help clarify the relationship between improved adherence and clinical outcome.
In many cases, lifestyle patterns are related to statin nonadherence.31 Although a new prescription of statin usually involves assessment of healthy lifestyle, unhealthy habits are common in patients treated with statins.32 Apart from adherence to lipid‐lowering drugs, physical activity and healthy diet are key factors in the management of hypercholesterolemia.2, 3 CPs and GPs are the most accessible health professionals who could play a major role in health‐promoting activities and providing health education to patients.33, 34
In this context, the aim of this study was to evaluate the impact of interventions that were administered by CPs and GPs in Spain to promote adherence to statins. The relationship of these interventions to total cholesterol (TC) levels in patients with hypercholesterolemia and their lifestyle patterns were also examined.
2. METHODS
2.1. Study design and ethical approval
This study was a six‐month randomized controlled trial. It was conducted with the participation of 46 community pharmacies and 50 primary care centers in 10 provinces in Spain (Andalusia, Aragon, Asturias, Basque Country, Castile‐La Mancha, Catalonia, Extremadura, Galicia, Madrid, and Valencia) between February 2014 and June 2016. This study was not registered in advance, but it was classified as a postauthorization observational prospective study (EPA‐SP) by the Spanish Medicines and Sanitary Product Agency (AEMPS) (OAT‐HIP‐2013‐01, 07/05/2013). The study started once the AEMPS issued the authorization and the research ethic committees gave the approval (these data can be verified through the promoters of the study: contacto@oatobservatorio.com; cofgipuzkoa@redfarma.org).
The protocol for this study was in agreement with the Helsinki Declaration. All of the participating patients provided informed consent at the time of their enrollment.
2.2. Participants
Patients were recruited according to the following criteria: aged 18 years or older; a prescription of at least one statin was received within the previous 3 months; and an informed consent form was completed. Patients who had participated in other adherence‐promotion or cardiac‐rehabilitation programs, those who were not able to communicate with the health professionals, those who could not self‐administer statins, those who were dependent or living in long‐term care facilities, or those who had suffered a stroke in the previous 6 months were excluded from this study.
Each health professional involved in this study was responsible for recruiting a minimum of six patients, including two patients who were adherent to treatment (ADH) and four patients who were nonadherent to treatment (Figure 1). If six patients (two adherent and four nonadherent) were recruited and a chance to include more patients remained, they were recruited following the sequence of “adherent—nonadherent—nonadherent” or “nonadherent—adherent—nonadherent,” in order to preserve proportionality. Initial adherence was assessed at recruitment using the Morisky‐Green‐Levine test. The nonadherent patients were randomly allocated to the intervention group (INT) or the nonintervention group (NOINT; Figure 1). Randomization was performed by an external researcher according to the SAS software program (SAS (r) 9.2; Copyright 2002‐2003 by SAS Institute Inc., Cary, NC, USA).
Figure 1.
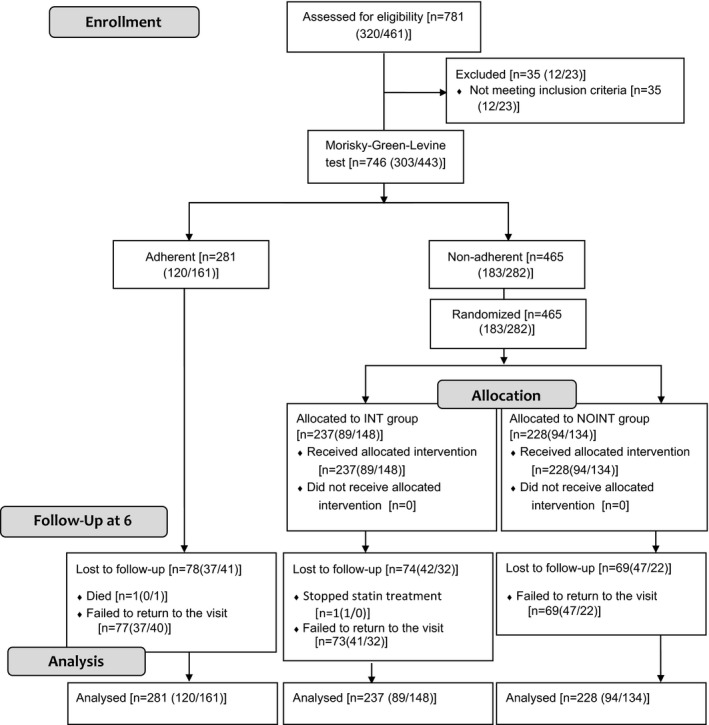
CONSORT flow diagram of the progress through the phases of the study of three groups (ADH: adherent group; INT: intervention group; NOINT: nonintervention group). Data are shown as [total (community pharmacy data/general practitioner data)]
2.3. Study procedure
Participants in the INT group received an intervention that was codesigned by pharmacists and primary care doctor experts on adherence for this study. Based on patient feedback and the cause of nonadherence, a multicomponent strategy was proposed (Table 1). Firstly, the CP or GP identified the cause of nonadherence. The cause of nonadherence could be intentional or unintentional. The possible causes within the group of unintentional nonadherence were disability and forgetfulness. The possible causes within the group pf intentional nonadherence were lack of knowledge about the disease or treatment, related to medication, psychological, related to health system, and economic. After identifying the cause, the CP or GP chose the most appropriate intervention for the patient. At the subsequent visit, the adherence and therefore effectiveness of each strategy were evaluated using the Morisky‐Green‐Levine test. Participants in the NOINT and ADH groups received usual care. All data were entered into online electronic case report forms (e‐CRF) that provided encryption of data, real‐time assessment completeness, and patient follow‐up.
Table 1.
Description of interventions provided to the INT group patients
| Causes of nonadherence | Proposed interventions | |
|---|---|---|
| Nonintentional nonadherence | ||
|
Disability Forgetfulness |
|
|
| Intentional nonadherence | ||
| Knowledge about the disease or treatment |
|
|
| Related to the medication |
|
|
|
|
|
| Psychological |
|
|
| Related to health system |
|
|
|
|
|
| Economic |
|
|
Abbreviation: GP, general practitioner.
aRefers only to community pharmacist intervention. bRefers only to GP intervention, since in Spain, pharmacists are not allowed to change dose regimens.
2.4. Training
Community pharmacists and GPs attended a 2‐hour workshop that presented and described the study protocol. They also received information regarding hypercholesterolemia, statin treatment, and strategies to detect the cause of nonadherence and possible interventions to increase adherence. For the duration of the study, the CPs and GPs were supported by phone by a lead researcher for this study.
2.5. Outcome measures
Adherence to statin therapy was the primary outcome, and it was assessed with the Morisky‐Green‐Levine test.35 For statistical purposes, patients were classified as adherent (0 questions answered differently) or nonadherent (≥1 question answered differently). Causes of nonadherence and intervention provided were registered at each visit. Causes of nonadherence were classified as intentional or unintentional. Since one patient could receive more than one intervention, for statistical purpose, they were categorized in two groups: (a) interventions to improve unintentional nonadherence (when the cause of nonadherence is forgetfulness); and (b) interventions to improve intentional nonadherence (when the cause of nonadherence is related to the knowledge about the disease or treatment, or factors related to medication, the patient's psychological state, the health system, or economic circumstances).
Total cholesterol levels were measured at community pharmacies with Reflotron® Plus (Roche) and according to the usual analytical process in the reference hospital laboratory of each primary care center. The therapeutic objective was dichotomized into achievement of the TC goal (<200 mg/dL) and nonachievement of the TC goal (≥200 mg/dL).
Physical activity and dietary intake were both evaluated. Based on previous recommendations for populations in Spain,36 patients were dichotomized into those who exercised and those who did not. Dietary intake was also dichotomized into those who followed a diet low in sugar and fats or had healthy eating habits and those who did not, based on previously published criteria.36
2.6. Sample size
Adherence to a statin regimen was previously estimated to be <50 percent.37 To detect an improvement in adherence from 50 to 65 percent with 80 percent power and a two‐sided P‐value of 0.05, 160 patients were needed for each group. The sample size was estimated to obtain differences in adherence, and randomization was done in order to classify nonadherent patients in the INT and NOINT groups in a 1:1 distribution. The OpenEpi 20 software (http://www.openepi.com/Menu/OpenEpiMenu.htm) was used.
2.7. Statistical analyses
The Kolmogorov‐Smirnov and Shapiro‐Wilk tests were used to evaluate data distribution. Changes in clinical characteristics were evaluated and compared between groups with paired t tests or Student's t test for parametric variables. Nonparametric variables were analyzed by ANOVA (Friedman) for repeated‐measurement analysis. Chi‐square (χ2) and Fisher's exact tests were used to analyze the frequency distribution of the studied variables and the relationship between groups according to outcome. Changes in cholesterol levels during the study period were analyzed and adjusted according to baseline values by factorial analysis of covariance (ANCOVA).
Multiple regression analysis was used to evaluate the impact of professional intervention on cholesterol levels and was adjusted for variables related to outcome. Binary logistic regression was also performed to evaluate the impact of the studied variables and professional intervention on adherence during this study. The results are expressed as n and percentage (percent) for the categorical variables and as the mean ± standard deviation (SD) for the continuous variables.
Analyses were performed in the intention‐to‐treat population.38 Data were analyzed on an intention‐to‐treat (ITT) and a per‐protocol basis. For the ITT analyses, values were calculated based on the multiple imputation system. Information of patients lost to follow‐up was imputed for all the variables. The fully conditioned method using a logistic model was used to generate 50 multiple imputation data for each condition. For the per‐protocol analyses, patients were considered to have complied with the study if they completed the first‐month and the sixth‐month visit after the baseline visit.
Statistical analyses were performed with the SPSS 18.0 program for Windows XP (Microsoft). A two‐tailed P‐value <0.05 was designated as the level of statistical significance.
3. RESULTS
3.1. Participant recruitment
A total of 746 patients were recruited for the study, with 303 patients recruited by CPs and 443 patients recruited by GPs. Figure 1 lists the number of patients in each group. There were 281 patients (37.6 percent) enrolled in the ADH group (CP: 120; GP: 161) and 465 nonadherent patients who were randomly assigned to the INT group or the NOINT group: 237 patients (31.8 percent; CP: 148; GP: 237) and 228 patients (30.6 percent; CP: 94; GP: 134), respectively. There were 221 patients who did not complete a follow‐up visit. When demographic data of the patients who dropped out of the study were compared with the patients who remained in the study, no significant differences were found (P > 0.05). Moreover, the proportions of patients enrolled from community pharmacies and primary health centers were similar (Figure 1).
Baseline analyses show that adherent patients had lower values of total cholesterol (ADH: 200.3 mg/dL vs NOADH: 216.72 mg/dL; P < 0.001) than nonadherent patients. Patients’ age and time since diagnosis also differed significantly between groups (Table 2).
Table 2.
Baseline characteristics of the patients studieda
| ADH(n = 281) | NOADH | P b | P c | ||
|---|---|---|---|---|---|
| NOINT(n = 228) | INT(n = 237) | ||||
| Total (n = 746) | |||||
| Age, y | 65.8 (10.6) | 61.9 (11.8) | 63.7 (11.3) | <0.001 | 0.833 |
| Females | 148 (52.7) | 128 (56.1) | 122 (51.5) | 0.636 | 0.426 |
| Total cholesterol (mg/dL) [ (SD)] | 200.3 (42.8) | 219.3 (46.3) | 211.7 (52.7) | <0.001 | 0.862 |
| Time since diagnosis, y [ (SD)] | 7.6 (7.1) | 6.3 (6.1) | 6.1 (5.6) | 0.023 | 0.986 |
| Phytosterol intake, yes | 15 (5.3) | 12 (5.2) | 16 (6.8) | 0.720 | 0.423 |
| Dieting, yes | 132 (47.0) | 103 (45.2) | 99 (41.8) | 0.482 | 0.216 |
| Exercising, yes | 196 (70.0) | 147 (64.5) | 149 (62.9) | 0.488 | 0.188 |
| Recruited by CPs (n = 303) | (n = 120) | (n = 94) | (n = 89) | ||
|---|---|---|---|---|---|
| Age, y | 65.2 (11.9) | 61.5 (12.7) | 63.9 (12.9) | 0.098 | 0.898 |
| Females | 66 (55.0) | 62 (66.0) | 54 (60.7) | 0.299 | 0.327 |
| Total cholesterol (mg/dL) [ (SD)] | 207.2 (41.7) | 222.1 (44.8) | 217.0 (48.9) | 0.052 | 0.415 |
| Time since diagnosis, y [ (SD)] | 8.2 (8.4) | 6.1 (5.5) | 5.2 (4.8) | 0.018 | 0.605 |
| Phytosterol intake, yes | 7.0 (5.8) | 6 (6.3) | 5 (5.6) | 0.984 | 0.554 |
| Dieting, yes | 43 (35.8) | 29 (30.9) | 33 (37.1) | 0.431 | 0.268 |
| Exercising, yes | 82 (68.3) | 54 (57.4) | 53 (59.6) | 0.408 | 0.844 |
| Recruited by GPs (n = 443) | (n = 161) | (n = 134) | (n = 148) | ||
|---|---|---|---|---|---|
| Age, y | 66.3 (9.6) | 62.2 (11.2) | 61.8 (10.5) | <0.001 | 0.478 |
| Females | 82 (50.9) | 66 (49.3) | 68 (45.9) | 0.685 | 0.351 |
| Total cholesterol (mg/dL) [ (SD)] | 196.0 (40.7) | 218.9 (44.0) | 214.4 (46.9) | <0.001 | 0.480 |
| Time since diagnosis, y [ (SD)] | 7.3 (6.2) | 6.3 (6.4) | 6.6 (6.0) | 0.397 | 0.678 |
| Phytosterol intake, yes | 8 (5.0) | 6 (4.5) | 11 (7.4) | 0.494 | 0.212 |
| Dieting, yes | 89 (55.3) | 74 (55.2) | 66 (44.6) | 0.314 | 0.884 |
| Exercising, yes | 114 (70.8) | 93 (69.4) | 96 (64.9) | 0.709 | 0.576 |
Abbreviations: ADH, adherent group; INT, intervention group; NOINT, nonintervention group; , mean; SD: standard deviation; CPs: community pharmacists; GPs: General practitioners.
aData are reported as n (%) except where indicated as mean [ (SD)]. bAnalysis of the ADH, INT, and NOINT groups was performed by using ANOVA or the chi‐square test. cAnalysis of the INT and NOINT groups was performed by using Student's t test or Fisher's exact test.
3.2. Health professional intervention
Adherence throughout the study was analyzed at 0, 3, and 6 months after the start of the study. The Friedman test for repeated measures showed a significant increase in the percentage of patients who became adherent during the period analyzed, and this percentage was significantly higher in the INT group (Figure 2). The proportion of adherent patients was 9.5 percent higher after six months of intervention (χ2 = 22.87, P < 0.001) in the INT group compared with the NOINT group (Figure 2A). Logistic regression analysis was performed to evaluate the impact of baseline characteristics on adherence (ADH group vs INT and NOINT groups) firstly and to analyze the impact of different variables and professional intervention on adherence secondly. Interventions provided by the health professionals improved adherence to statins throughout the 6 months of study (OR = 1.49 [95% CI: 1.30‐1.76; P < 0.001]; Table S1). Age and gender were slightly significantly associated with adherence at follow‐up. Per‐protocol analysis results did not differ qualitatively from those in the ITT analysis (see Supporting Information).
Figure 2.
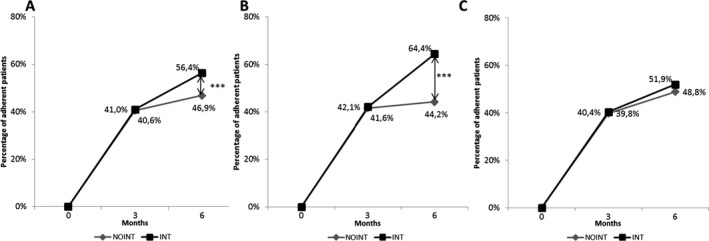
Variation in adherence to statins in patients who were nonadherent at baseline. A, Total (CP and GP); B, CP; and C, GP groups were analyzed. The Friedman test for repeated measures was used to evaluate the evolution of adherence with time and intervention‐related effects according to group. NOINT: nonadherent patients with usual care; INT: nonadherent patients with intervention. *P < 0.05; **P < 0.01. Analysis of adherent patients of the INT and NOINT groups was performed by using chi‐square test
Causes of nonadherence were analyzed at baseline. Unintentional nonadherence (55.7 percent, n = 132) was more prevalent than intentional nonadherence (44.3 percent, n = 105) among the studied patients. The most provided intervention for unintentional nonadherence (80.7 percent of the unintentional nonadherence causes) was directed toward forgetfulness like using drug packaging (74.6 percent), including the posology in the box (59.8 percent), and using reminders (31.2 percent). The most frequently provided intervention for intentional nonadherence was directed toward improving knowledge about the disease or treatment (60.9 percent of the intentional nonadherence causes) and providing written and oral standardized information (92.6 percent).
Cholesterol levels decreased in both groups over the course of the study (INT: −11.06 mg/dL, P < 0.001; NOINT: −10.4 mg/dL, P < 0.001). Per‐protocol analysis also showed a decrease in both groups, yet a statistically significant decrease was only observed in the INT group (INT: 210.18 mg/dL vs 197.59 mg/dL, P = 0.028; NOINT: 223.32 mg/dL vs 214.42 mg/dL, P = 0.127). In order to evaluate the relationship between adherence and clinical outcome, data were stratified in patients that achieved adherence at the end of the study and patients who remain nonadherent. Adherent patients at endpoint showed lower values of total cholesterol compared with nonadherent patients (adherent: 197.33 ± 35.32 mg/dL vs nonadherent: 212.23 ± 40.68 mg/dL; P < 0.001; Figure 3). When a factorial ANCOVA was adjusted for baseline cholesterol levels, the effect of professional intervention on the decrease in cholesterol levels during the six‐month period exhibits statistical significance as well (P < 0.001).
Figure 3.
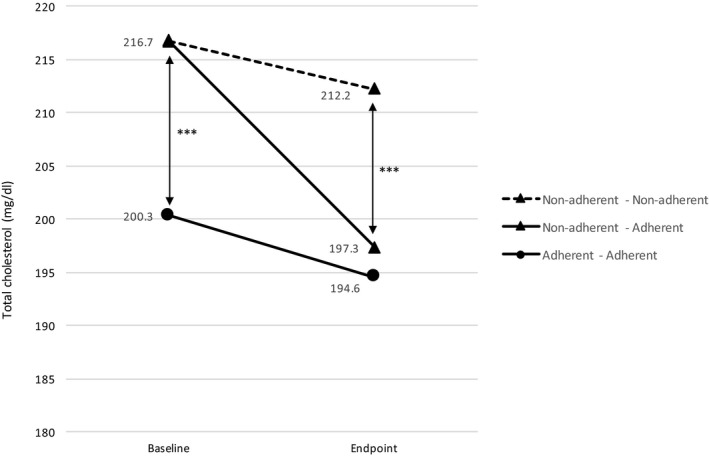
Total cholesterol variation between baseline (0 months) and endpoint (6 months) based on patients’ adherence variation. Analysis was performed using Student's t test. Statistical differences between baseline and endpoint were only observed in the nonadherent‐adherent group (P < 0.001). ***P < 0.001
Between the INT and NOINT groups, there were no differences in the proportion of patients showing normal cholesterol levels at baseline (Table 2). However, this proportion was significantly higher (χ2 = 21.78, P < 0.001) in the INT group (52.1 percent) compared to the NOINT group (45.0 percent) at the endpoint of the study. Per‐protocol analysis results did not differ qualitatively from those in the ITT analysis (see Supporting Information).
No differences were observed in the physical activity or dietary intake of the three studied groups at baseline (Table 2). However, patients in the INT group had a significant increase in their overall amount of exercise over the six‐month study period (baseline: 62.9 percent vs endpoint: 93.1 percent, P < 0.001) compared with the patients in the NOINT group (baseline: 64.5 percent vs endpoint: 65.7 percent min, P = 0.998) and ADH group (baseline: 70.0 percent vs endpoint: 69.7 percent, P = 0.985) who did not have an increase in their overall amount of exercise. Regarding dietary intake, a greater proportion of the patients in the INT group stated that they were following a diet to reduce cholesterol levels (baseline: 41.8 percent vs endpoint: 68.4 percent; χ2 = 5.45, P = 0.002), while the proportion of patients who stated that they were following a diet to reduce cholesterol levels in the NOINT group (baseline: 45.2 percent vs endpoint: 51.3 percent, χ2 = 0.47, P = 0.627) and ADH group (baseline: 47.0 percent vs endpoint 48.2 percent, χ2 = 0.73, P = 0.712) remained unchanged. Per‐protocol analysis results did not differ qualitatively from those in the ITT analysis (see Supporting Information).
3.3. Community pharmacists’ and general practitioners’ intervention
The CP INT group exhibited a 20.1 percent increase in the proportion of adherent patients at the endpoint of the study (χ2 = 40.27, P < 0.001; Figure 2B), compared with the NOINT group showing that CPs’ intervention improved adherence to statins throughout the six months of study (OR = 2.34 [95% CI: 1.87‐3.03]; P < 0.001; Table S1). Although the intervention provided by CPs did not reach statistical significance in cholesterol level decrease between groups (INT: −5.1 mg/dL vs NOINT: −4.7 mg/dL; P = 0.571), at endpoint, adherent patients (209.7 ± 29.50 mg/dL) showed lower values of total cholesterol compared with nonadherent (221.7 ± 45.14 mg/dL) patients (P < 0.001).
In the GP group, the proportion of patients adherent after GPs’ intervention did not reach significance (P < 0.05; Figure 2C), and total cholesterol decrease did not show differences between the INT and NOINT groups (INT: −12.7 mg/dL; P < 0.001; NOINT: −15.2 mg/dL; P = 0.303). However, adherent patients (191.05 ± 36.38 mg/dL) at endpoint showed lower values of total cholesterol compared with nonadherent (207.9 ± 37.71 mg/dL) patients (P = 0.047).
Unintentional nonadherence was more prevalent than intentional nonadherence in the CP and GP groups. Drug packaging was the most frequent intervention used in CP to improve unintentional nonadherence, whereas adapting the dose regimen to the patient's situation was the most provided intervention in GP. Providing written and oral information was the most used intervention to improve intentional nonadherence in both centers.
Percentage of patients that followed a diet to reduce cholesterol and that increased their overall amount of exercise at endpoint compared to baseline improved in both groups showing the same trend as in the global analyses (CP + GP).
4. DISCUSSION
This six‐month interventional program with CPs and GPs studied variation in adherence to statins in hypercholesterolemic patients and its relationship with total cholesterol levels and lifestyle patterns. The present research shows that CPs’ intervention improved adherence to statin in patients who were nonadherent at baseline. Moreover, it suggests that adherence could be related to total cholesterol reduction and to an improvement on the studied lifestyle patterns.
After health professional intervention, the percentage of patients that finished the study being adherent to statins was higher compared with patients that did not receive the intervention, concluding that intervention provided by CPs and GPs throughout the 6‐months period was effective. When adherent patients were studied independently to the intervention group, a total cholesterol reduction was determined. These findings are in accordance with previously published reports, which analyzed the impact of adherence on lipid profiles.7, 39 Some authors state that total cholesterol level decrease could be greater in longer studies,7, 40 so a longer intervention period could also provide greater reduction than that observed in the study. Moreover, patients who were adherent at baseline showed lower values of total cholesterol compared with nonadherent patients, reinforcing that adherence to statins could be related to improvement in clinical values, in total cholesterol in this case. Considering that high total cholesterol levels have been related to an increased rate of major cardiovascular events and mortality, a total cholesterol level reduction would probably lead to a reduction in cardiovascular risk for these patients.41 Our study also suggests that CP and GP intervention increases the number of patients that reach total cholesterol level objective. Reaching total cholesterol level under 200 mg/mL is considered to have normal level of total cholesterol decreasing, as well, cardiovascular risk in those patients.41
Among previously published works studying interventions delivered by health professionals to improve adherence and clinical outcomes, only a few focused on hypercholesterolemic patients. For example, Aslani et al42 analyzed adherence to lipid‐lowering drugs and total cholesterol levels using two validated questionnaires, and no changes due to intervention were observed. In a study performed by Faulkner et al,43 the intervention was focused on adherence in patients who underwent cardiac surgery, and improvements in adherence to treatment and lipid profiles were observed after two years. In another study, improvements in adherence and lipid profiles were observed when a calendar reminder‐based intervention was conducted.44 The results of the present study are consistent with those of a recently published Cochrane review that analyzed adherence to a lipid‐lowering medication in the context of various types of interventions.7 The interventions delivered in our study were based on an identification of the causes of nonadherence and selection of the best intervention in each situation. Therefore, customizing interventions depending on the cause of the nonadherence and situation of the patient could be an effective way to reduce nonadherence in chronic diseases.
To the best of our knowledge, this study represents the first major hypercholesterolemia adherence trial to evaluate interventions delivered by CPs and GPs. In fact, the intervention was codesigned by community pharmacists and primary care doctors with the goal of establishing a standard intervention that would be able to be implemented in both of these health professional fields. In the case of interventions where reminders were used, there are studies where adherence improves after intervention in the community pharmacy19, 42 and in the hospital setting.45, 46 On the other hand, when the intervention is about providing education on the importance of adherence to treatment and other issues related to the disease, there are studies that do not find improvement at the end of the study in community pharmacy21 nor in the hospital environment.22, 26 Data suggest that in order to obtain the improvement in adherence, identifying the cause of nonadherence and choosing the most appropriate intervention for the patient's situation should be part of the intervention.
In the present study, intervention provided by CPs showed a greater improvement on adherence compared to the GP group. It is worth highlighting that in our study, the patients enrolled in the nonintervention group in both settings, especially those recruited by the GPs, showed an unexpected enhancement in adherence. This result may be attributed to different factors, including a Hawthorne effect by which a simple observation modifies patients’ behavior.47 However, when the type of interventions provided was studied, our data show that drug packaging and including the posology in the box were the most used intervention to improve unintentional nonadherence, while providing written and oral standardized information was the one to improve intentional nonadherence. Those interventions have been classically offered in the CP and has already showed their effectiveness, reinforcing the idea that CP could be one of the most appropriate health professionals in improving adherence.48, 49
Among baseline data of recruited patients, the ADH group patients were older at baseline. Although several studies have reported that nonadherence rates increase with time,50 a systematic review of 102 studies found that elderly people might have higher compliance.51 This could show that although nonadherence has been usually related to the elderly, in middle‐age patients other factors like priorities on life or lack of time can influence on nonadherence and become less likely to be compliant to therapy. For these patients, the CP could be an accessible health center and the actions toward implementing this type of services in the CP could be in this way also justified.
Dietary and exercise habits were also modified at the end of the study. The number of patients following a diet and doing exercise was higher in the INT group compared with the NOINT group. Our results are in accordance with other previous studies since the relationship between a healthy diet and adherence has previously been described.52, 53 It has been established that changes in lifestyle, in addition to pharmacological treatment, are related to a decrease in the prevalence and progression of chronic diseases.51 Thus, the intervention proposed in the present study could potentially improve both clinical and lifestyle patterns.
There were some limitations associated with the present study. For example, there were a substantial number of patients who did not complete the study and follow‐up. This rate is comparable to other intervention trials which analyzed adherence outcome for various chronic diseases,54, 55 and to the rates reported for lipid‐lowering interventional trials,42 and a multiple imputation analysis was used to take into account the uncertainty of the imputed values. It may be worth considering that if participating health professionals had received reimbursement, they may have provided better patient recruitment and the number of dropouts could be reduced.44 Being a nonclustered randomized controlled trial, the risk of concealment of an allocation is major. Knowledge of treatment group assignment may influence the professionals’ way of acting or may behave in a compensatory way to the nonintervention group patients that may diminish differences between the intervention and the control groups. Finally, adherence and lifestyle outcomes were measured using patient‐reported information. However, all available adherence measures have their limitations12 and the Morisky‐Green test is one of the most accepted self‐report measures for identifying nonadherence.56
Considering that adherence to statins may change over time, the impact of an intervention should be reevaluated at different time points. Studies evaluating other variables such as morbidity, mortality, quality of life, and/or cost‐effectiveness could also be useful in providing guidance for health care systems and establishing cost‐effectiveness of the intervention.
In summary, the findings of this study show that intervention delivered by health professionals increased adherence to statins after 6 months, especially among patients who were enrolled in the community pharmacy group, and this improvement on adherence was related to a decrease in total cholesterol levels and a healthier lifestyle.
Supporting information
ACKNOWLEDGMENTS
Joint Acknowledgment/Disclosure Statement: We gratefully acknowledge all community pharmacists, general practitioners, other health professionals, and patients that participated in the study. The coauthors would like to acknowledge the support of TEVA S.L.
Oñatibia‐Astibia A, Malet‐Larrea A, Larrañaga B, et al. Tailored interventions by community pharmacists and general practitioners improve adherence to statins in a Spanish randomized controlled trial. Health Serv Res. 2019;54:658–668. 10.1111/1475-6773.13152
REFERENCES
- 1. World Health Organization . The World Health Report 2002 ‐ Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; 2002. http://www.who.int/whr/2002/en/whr02en.pdf?ua=1. Accessed December 18, 2016. [Google Scholar]
- 2. Colantonio LD, Monda KL, Huang L, et al. Patterns of statin use and outcomes following myocardial infarction among Medicare beneficiaries. Presented at ESC, London, UK. 2015.
- 3. Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. Attaining United Kingdom‐European Atherosclerosis Society low‐density lipoprotein cholesterol guideline target values in the GREek Atorvastatin and Coronary‐heart. [DOI] [PubMed]
- 4. Rosenson RS, Kent ST, Brown TM, et al. Underutilization of high‐intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol. 2015;65(3):270‐277. [DOI] [PubMed] [Google Scholar]
- 5. Mannu GS, Zaman MJS, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev. 2013;9(1):2‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization (WHO) . Adherence to long‐term therapies. Evidence for action. 2003. http://apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf.
- 7. van Driel ML, Morledge MD, Ulep R, Shaffer JP, Davies P, Deichmann R. Interventions to improve adherence to lipid‐lowering medication. Cochrane Database Syst Rev 2016;230‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huser MA, Evans TS, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 9. Peterson AM, Takiya LFR. Meta‐analysis of trials of interventions to improve medication adherence. Am J Heal Syst Pharm. 2003;60(7):657‐665. [DOI] [PubMed] [Google Scholar]
- 10. DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes: a meta‐analysis. Med Care. 2002;40(9):794‐811. [DOI] [PubMed] [Google Scholar]
- 11. Schiff GD, Fung S, Speroff T, et al. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114(8):625‐630. [DOI] [PubMed] [Google Scholar]
- 12. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487‐497. [DOI] [PubMed] [Google Scholar]
- 13. Mahoney JJ, Ansell BJ, Fleming WK, Butterworth SW. The unhidden cost of noncompliance. J Manag Care Pharm. 2008;14(6b):S1‐S29. [Google Scholar]
- 14. Castellano JM, Gines S, Penalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS Project. J Am Coll Cardiol. 2014;64(20):2071‐2082. [DOI] [PubMed] [Google Scholar]
- 15. Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill‐based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22:920‐930. [DOI] [PubMed] [Google Scholar]
- 16. Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ. 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
- 17. Thom S, Poulter N, Field J, et al. Effects of a fixed‐dose combination strategy on adherence and risk factors in patients with or at high risk of CVD (UMPIRE). JAMA. 2013;310(9):918‐929. [DOI] [PubMed] [Google Scholar]
- 18. Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol‐lowering medications. JAMA Intern Med. 2013;173(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 19. Eussen SRBM, van der Elst ME, Klungel OH, et al. A pharmaceutical care program to improve adherence to statin therapy: a randomized controlled trial. Ann Pharmacother. 2010;44(12):1905‐1913. [DOI] [PubMed] [Google Scholar]
- 20. Nieuwkerk PT, Nierman MC, Vissers MN, et al. Intervention to improve adherence to lipid‐lowering medication and lipid‐levels in patients with an increased cardiovascular risk. Am J Cardiol. 2012;110:666‐672. [DOI] [PubMed] [Google Scholar]
- 21. Gujral G, Winckel K, Nissen LM, Cottrell WN. Impact of community pharmacist intervention discussing patients’ beliefs to improve medication adherence. Int J Clin Pharm. 2014;36(5):1048‐1058. [DOI] [PubMed] [Google Scholar]
- 22. Willich SN, Englert H, Sonntag F, et al. Impact of a compliance program on cholesterol control: results of the randomized ORBITAL study in 8108 patients treated with rosuvastatin. Eur J Cardiovasc Prev. 2009;16:180‐187. [DOI] [PubMed] [Google Scholar]
- 23. de Almeida Neto AC, Aslani P, Chen TF. Improving adherence to prescribed drugs. BMJ. 2009;339:b3282. [DOI] [PubMed] [Google Scholar]
- 24. National Community Pharmacists Association . Medication Adherence in America: A National Report. Alexandria, VA: National Community Pharmacists Association; 2013. [Google Scholar]
- 25. Bronner C, Bruce C. Medication compliance problems in general practice: detection and intervention by pharmacists and doctors. Aust J Rural Health. 2002;10:32‐38. [DOI] [PubMed] [Google Scholar]
- 26. Park LG, Howie‐Esquivel J, Chung ML, Dracup K. A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Educ Couns. 2014;94(2):261‐268. [DOI] [PubMed] [Google Scholar]
- 27. Vrijens B, Belmans A, Matthys K, de Klerk E, Lesaffre E. Effect of intervention through a pharmaceutical care program on patient adherence with prescribed once‐daily atorvastatin. Pharmacoepidemiol Drug Saf. 2006;15(2):115‐121. [DOI] [PubMed] [Google Scholar]
- 28. Ho PM, Lambert‐Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2013;80220(2):658‐8. [DOI] [PubMed] [Google Scholar]
- 29. Conthe P, Márquez‐Contreras E. Documento de Consenso. Una Proximación Multidisciplinar Al Problema de La Adherencia Terapéutica En Las Enfermedades Crónicas: Estado de La Situación Y Perspectivas de Futuro; 2012.
- 30. Viswanathan M, Golin CE, Jones CD. Closing the quality gap: revisiting the state of the science (vol. 4: medication adherence interventions: comparative effectiveness). Evid Rep Technol Assess. 2012;2084:658‐685. [PMC free article] [PubMed] [Google Scholar]
- 31. Halava H, Korhonen M, Huupponen R, et al. Lifestyle factors as predictors of nonadherence to statin therapy among patients with and without cardiovascular comorbidities. CMAJ. 2014;186(12):E449‐E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Warren J, Falster M, Fox D, Jorm L. Factors influencing adherence in long‐term use of statins. Pharmacoepidemiol Drug Saf. 2013;22(12):1298‐1307. [DOI] [PubMed] [Google Scholar]
- 33. Ogbonna B, Ndukwe H. Community pharmacists and health promotion activities in the 21st century; maximizing the expanded roles for universal health coverage and population health optimization. MOJ Public Heal. 2017;6(3):174‐179. [Google Scholar]
- 34. Beshir SA, Bt Hamzah NH. Health promotion and health education: perception, barriers and standard of practices of community pharmacists. Int J Heal Promot Educ. 2014;52(4):174‐180. [Google Scholar]
- 35. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care. 1986;24(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 36. Ministerio de Sanidad Servicios Sociales e Igualdad . Actividad física para la salud y reducción del sedentarismo. 2016:658‐28. https://www.msssi.gob.es/profesionales/saludPublica/prevPromocion/Estrategia/docs/Recomendaciones_ActivFisica_para_la_Salud.pdf.
- 37. Rosenson RS. Statin non‐adherence: clinical consequences and proposed solutions. F1000 Res. 2016;5:658‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta S. Intention‐to‐treat concept: a review. Perspect Clin Res. 2011;2(3):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Vera MA, Bhole V, Burns LC, Lacaille D. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol. 2014;78(4):684‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simpson RJ, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J Clin Lipidol. 2010;4(6):462‐471. [DOI] [PubMed] [Google Scholar]
- 41. Cholesterol Treatment Trialists’ (CTT) Collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aslani P, Rose G, Chen TF, Whitehead PA, Krass I. A community pharmacist delivered adherence support service for dyslipidaemia. Eur J Public Health. 2011;21(5):567‐572. [DOI] [PubMed] [Google Scholar]
- 43. Faulkner MA, Wadibia EC, Lucas BD, Hilleman DE. Impact of pharmacy counseling on compliance and effectiveness of combination lipid‐lowering therapy in patients undergoing coronary artery revascularization: a randomized, controlled trial. Pharmacotherapy. 2000;20(4):410‐416. [DOI] [PubMed] [Google Scholar]
- 44. IOM (Institute of Medicine) . Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 45. Kardas P. An education‐behavioural intervention improves adherence to statins. Open Med. 2013;8(5):580‐585. [Google Scholar]
- 46. Nieuwkerk PT, Nierman MC, Vissers MN, et al. Intervention to improve adherence to lipid‐lowering medication and lipid‐levels in patients with an increased cardiovascular risk. Am J Cardiol. 2012;110(5):666‐672. [DOI] [PubMed] [Google Scholar]
- 47. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67(3):267‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clifford S, Garfield S, Eliasson L, Barber N. Medication adherence and community pharmacy: a review of education, policy and research in England. Pharm Pract (Granada). 2010;8(2):77‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Conn VS, Ruppar TM, Enriquez M, Cooper PS. Packaging interventions to increase medication adherence: systematic review and meta‐analysis. Curr Med Res Opin. 2015;31(1):145‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Turin A, Pandit J, Stone NJ. Statins and nonadherence: should we RELATE better? J Cardiovasc Pharmacol Ther. 2015;20(5):447‐456. [DOI] [PubMed] [Google Scholar]
- 51. Jin J, Sklar GE, Sen Oh VM, Chuen LS. Factors affecting therapeutic compliance: a review from the patient's perspective. Ther Clin Risk Manag. 2008;4(1):269‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Johal S, Jamsen KM, Bell JS, et al. Do statin users adhere to a healthy diet and lifestyle? The Australian Diabetes, Obesity and Lifestyle Study. Eur J Prev Cardiol. 2017;24(6):621‐627. [DOI] [PubMed] [Google Scholar]
- 53. Lytsy P, Burell G, Westerling R. Cardiovascular risk factor assessments and health behaviours in patients using statins compared to a non‐treated population. Int J Behav Med. 2012;19(2):134‐142. [DOI] [PubMed] [Google Scholar]
- 54. Stewart K, George J, Mc Namara KP, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster‐randomized, controlled trial (HAPPy trial). J Clin Pharm Ther. 2014;39(5):527‐534. [DOI] [PubMed] [Google Scholar]
- 55. Armour CL, Reddel HK, LeMay KS, et al. Feasibility and effectiveness of an evidence‐based asthma service in Australian community pharmacies: a pragmatic cluster randomized trial. J Asthma. 2013;50(3):302‐309. [DOI] [PubMed] [Google Scholar]
- 56. Stirratt MJ, Dunbar‐Jacob J, Crane HM, et al. Self‐report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


