Abstract
Background
This study sought to re-estimate the cumulative incidence of perianal or rectovaginal fistulas and the associated proctectomy rate in the prebiologic era vs the biologic era using a population-based cohort of Crohn’s disease (CD) patients.
Methods
The medical records of 414 residents of Olmsted County, Minnesota, who were diagnosed with CD between 1970 and 2010 were reviewed. The cumulative incidence of perianal or rectovaginal fistulas from time of CD diagnosis and the cumulative rate of proctectomy from date of first perianal or rectovaginal fistula diagnosis were estimated using the Kaplan-Meier method.
Results
Eighty-five patients (20.5%) diagnosed with CD between 1970 and 2010 had at least 1 perianal or rectovaginal fistula episode between January 1, 1970, and June 30, 2016. The cumulative incidence of perianal or rectovaginal fistulas was 18% after 10 years, 23% after 20 years, and 24% after 30–40 years from CD diagnosis. The cumulative incidence of perianal or rectovaginal fistulas was significantly lower in patients diagnosed in 1998 or after than in patients diagnosed before 1998 (P = 0.03, log-rank). Among 85 patients developing perianal or rectovaginal fistulas, 16 patients (18.8%) underwent proctectomy for the treatment of perianal or rectovaginal fistulas during follow-up.
Conclusions
In a population-based inception cohort of CD, one-fifth of patients were diagnosed with at least 1 perianal or rectovaginal fistula. The cumulative probability of perianal or rectovaginal fistulizing disease has decreased over time.
Keywords: Crohn’s disease, perianal fistula, rectovaginal fistula, natural history
INTRODUCTION
Crohn’s disease (CD), 1 of the subtypes of inflammatory bowel disease (IBD), is a chronic relapsing inflammatory gastrointestinal disorder that may lead to increased morbidity and mortality over time. The natural history of CD is characterized by periods of remission and relapse, with an increased risk of gastrointestinal complications when intestinal inflammation is not well controlled.1 In a population-based cohort of Olmsted County residents who were diagnosed between 1970 and 2004, 50% of patients with CD developed an intestinal complication within 20 years of diagnosis,2 and 60% of patients with CD experienced at least 1 major abdominal surgery after 20 years of disease.3
Perianal fistulas are 1 of the most common complications of CD and usually require a multidisciplinary approach with both medical and surgical therapy because they are notoriously difficult to treat and recurrence is common. Since Penner and Crohn described the concomitant presence of perianal fistulas in “regional enteritis” in 1938,4 population-based studies have confirmed that perianal fistulas are the most common manifestation of fistulizing CD, developing in 20% of patients with CD and recurring in approximately 30% of cases.5, 6 In a population-based cohort of 169 Olmsted County patients diagnosed with CD from 1970 to 1993, the cumulative incidence of perianal fistulizing CD was 12% after 1 year, and it doubled after 20 years from the diagnosis.6
However, previous incidence studies of perianal fistulizing CD have been largely performed in the prebiologic era, and there remains a paucity of data regarding the natural course and long-term outcome of perianal fistulizing CD in the modern era after the introduction of tumor necrosis factor–α antagonists (anti-TNF agents) in 1998.7 This study, therefore, sought to re-estimate the cumulative incidence of perianal or rectovaginal fistulas and the associated proctectomy rate in the prebiologic era (before 1998) vs the biologic era (1998 or later) using a population-based cohort of CD patients.
METHODS
Rochester Epidemiology Project
The Rochester Epidemiology Project (REP) is a unique medical records linkage system developed in the 1960s and supported by the National Institutes of Health.8 It exploits the fact that virtually all of the health care for the residents of Olmsted County is provided by 2 organizations: Mayo Medical Center, consisting of the Mayo Clinic and its 2 affiliated hospitals (Rochester Methodist Hospital and Saint Marys Hospital), and Olmsted Medical Center, consisting of a smaller multispecialty group and its affiliated hospital (Olmsted Community Hospital). In any 4-year period, >95% of county residents are examined at either of the 2 health care systems.8–11 Diagnoses generated from all outpatient visits, emergency room visits, hospitalizations, nursing home visits, surgical procedures, autopsy examinations, and death certificates are recorded in a central diagnostic index. Thus, it is possible to identify virtually all diagnosed cases of a given disease for which patients seek medical attention.
Case Ascertainment
The institutional review boards of both Mayo Medical Center and Olmsted Medical center approved this study. The medical records of only patients who did not withdraw research authorization were reviewed. The resources of the REP were used to identify all residents of Olmsted County who were diagnosed with CD from 1970 to 2010. Previously used definitions for CD diagnosis were employed in this study.12–14 The complete (including inpatient and outpatient) medical records of 414 pediatric and adult residents from Olmsted County diagnosed with CD between 1970 through 2010 were reviewed until June 30, 2016, for development of active perianal or rectovaginal fistulas and medical or surgical therapy for fistulas. The diagnosis of a fistulizing episode was made if there was clinical, radiographic, endoscopic, or surgical evidence of a fistula.6 The diagnosis of perianal fistulas included both trans-sphincteric and extrasphincteric perianal fistulas, and the diagnosis of rectovaginal fistulas included both rectovaginal and anovaginal fistulas. For purposes of the data analysis, patients who were diagnosed with a fistula before CD diagnosis were assumed to have developed their first fistula on the day after diagnosis.
Statistical Analysis
The data were summarized with percentages, medians, and ranges. Patients were followed up from the time of diagnosis of CD until development of the first fistula, date of last follow-up, or June 30, 2016. The cumulative incidence (1 minus survival free) of perianal or rectovaginal fistulas from time of CD diagnosis and the cumulative rate of proctectomy from the date of first perianal or rectovaginal fistula were estimated using the Kaplan-Meier method. Results were stratified by diagnosis of CD in 2 time periods, before 1998 vs 1998 and later, for cumulative incidence of perianal or rectovaginal fistulas, and they were stratified by date of first perianal or rectovaginal fistula before 1998 or 1998 or later for cumulative rate of proctectomy and compared using the log-rank and Breslow tests. A P value <0.05 was considered statistically significant. All statistical analyses were carried out using SPSS, version 21 (IBM Corp., Armonk, NY, USA).
RESULTS
Demographic Characteristics
Among 414 pediatric (n = 59) and adult (n = 355) CD patients, 210 patients were female (50.7%), and the median age at diagnosis of CD (range) was 30 (4–93) years. The median duration of follow-up (range) was 16.2 (0.1–46.1) years. Eighty-five patients (20.5%) diagnosed with CD between 1970 and 2010 had at least 1 perianal or rectovaginal fistula episode between January 1, 1970, and June 30, 2016. Of them, 42 patients with perianal or rectovaginal fistulas were women (49.4%), resulting in a female-to-male ratio of 1:1. Among the patients with perianal or rectovaginal fistulas, the median age at diagnosis of CD (range) was 26 (9–61) years, and the median interval from diagnosis of CD to the first perianal or rectovaginal fistula (range) was 5 months (0 days–22.6 years). A total of 34 patients (40%) developed their first perianal or rectovaginal fistula before or at the time of CD diagnosis. Excluding them, the median interval from CD diagnosis to the first perianal or rectovaginal fistula (range) was 4.4 years (range, 6 days–22.6 years). Thirteen patients (15.3%) among the 85 patients with perianal or rectovaginal fistulas developed rectovaginal fistulas during follow-up.
Cumulative Incidence of Perianal or Rectovaginal Fistulas
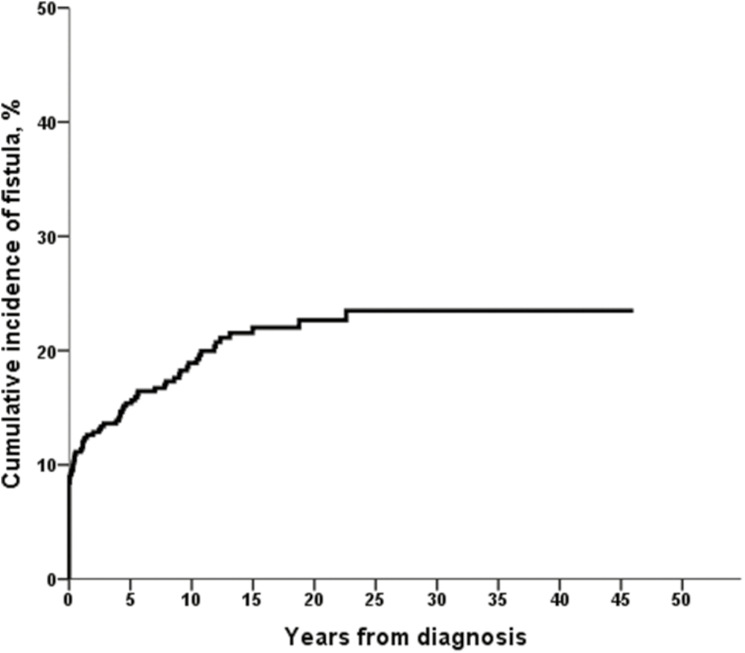
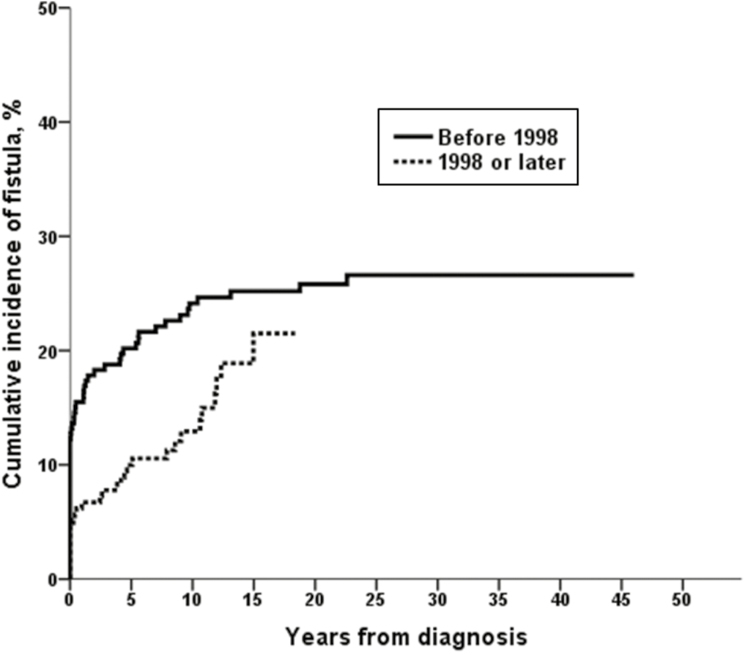
The cumulative incidence of perianal or rectovaginal fistulas was 11% (95% confidence interval [CI], 8%–14%) after 1 year, 15% (95% CI, 11%–18%) after 5 years, 18% (95% CI, 15%–22%) after 10 years, 23% (95% CI, 18%–27%) after 20 years, and 24% (95% CI, 19%–29%) after 30–40 years from CD diagnosis (Fig. 1). Fifty-seven patients (25.8%) among the 221 patients diagnosed before 1998 and 28 patients (14.5%) among the 193 patients diagnosed in 1998 or after had at least 1 perianal or rectovaginal fistula episode. The cumulative incidence of perianal or rectovaginal fistulas was significantly lower in patients diagnosed in 1998 or after (n = 193) than in patients diagnosed before 1998 (n = 221; P = 0.03, log-rank; P = 0.005, Breslow) (Fig. 2). For example, the 10-year risk of perianal or rectovaginal fistula was 24% among patients diagnosed with CD before 1998 and 12% in those diagnosed with CD in 1998 or later.
FIGURE 1.
Cumulative incidence of perianal or rectovaginal fistulas among 414 Olmsted County residents diagnosed with CD between 1970 and 2010.
FIGURE 2.
Cumulative incidence of perianal or rectovaginal fistulas stratified by date of CD diagnosis (221 CD patients were diagnosed before 1998, and 193 were diagnosed with CD in 1998 or later; P = 0.03, log-rank; P = 0.005, Breslow).
Outcome of Perianal or Rectovaginal Fistulas
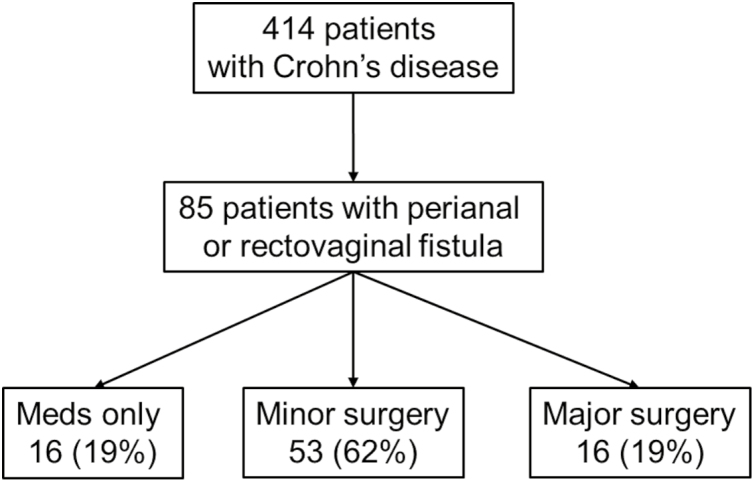
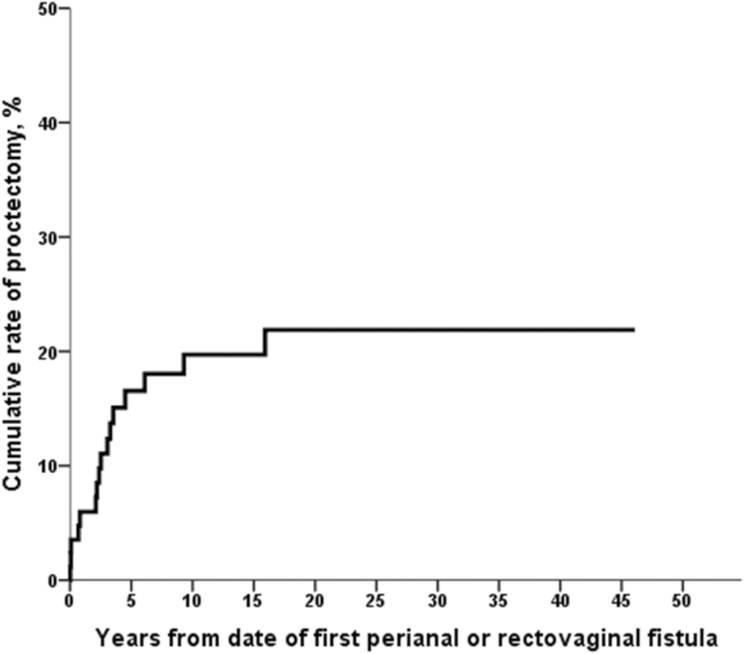
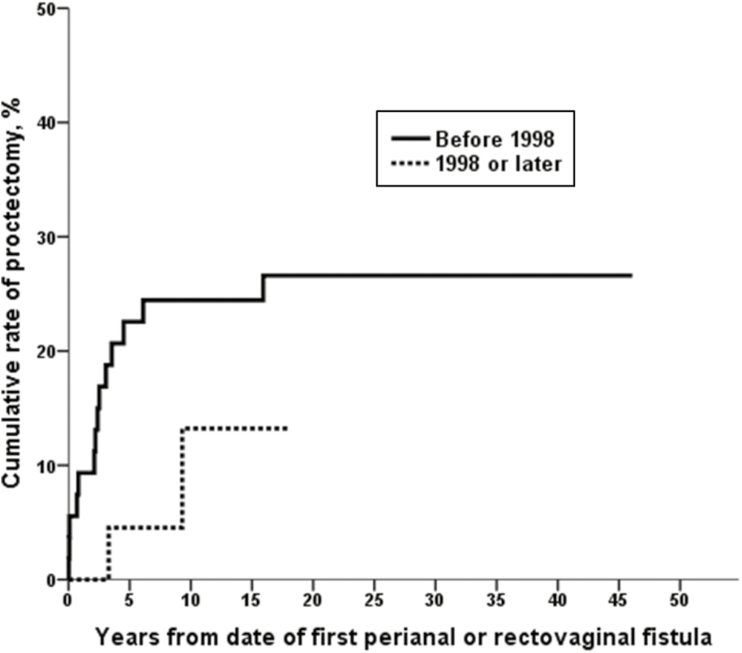
Among 85 patients developing perianal or rectovaginal fistulas, 16 patients (18.8%) received medical treatment such as antibiotics without surgery, 53 patients (62.4%) underwent minor surgery including incision and drainage, fistulotomy, and fistulectomy, and the remaining 16 patients (18.8%) underwent proctectomy for the treatment of perianal or rectovaginal fistulas during follow-up (Fig. 3). Among 85 patients who developed perianal or rectovaginal fistulas, 5 patients (5.9%) underwent diverting ileostomy for the treatment of perianal or rectovaginal fistulas during follow-up. Two of these diverted patients later underwent proctectomy, and 3 patients did not undergo proctectomy during follow-up. The cumulative rate of proctectomy was 20% at 10 years and 22% at 20–40 years after the date of first perianal or rectovaginal fistula (Fig. 4). Fourteen patients (25.9%) among the 54 patients who had a first perianal or rectovaginal fistula episode before 1998 and 2 patients (6.5%) among the 31 patients who had a first perianal or rectovaginal fistula episode in 1998 or later underwent proctectomy. The cumulative rate of proctectomy was lower among patients who had a first perianal or rectovaginal fistula episode in 1998 or later (n = 31) than among patients who had a first perianal or rectovaginal fistula episode before 1998 (n = 54; eg, 13% at 10 years vs 24% at 10 years); however, this did not meet statistical significance (P = 0.09, log-rank; P = 0.056, Breslow) (Fig. 5). Among the 13 patients who had rectovaginal fistula episodes, 4 patients (30.8%) underwent proctectomy.
FIGURE 3.
Flowchart showing the natural history of 85 patients with CD developing perianal or rectovaginal fistulas.
FIGURE 4.
Cumulative rate of proctectomy among 85 patients developing perianal or rectovaginal fistulas.
FIGURE 5.
Cumulative rate of proctectomy stratified by date of first perianal or rectovaginal fistula (54 CD patients with fistula before 1998 and 31 CD patients with fistula in 1998 or later; P = 0.09, log-rank; P = 0.056, Breslow).
DISCUSSION
In the present study, the disease burden of perianal or rectovaginal fistulas in a population-based inception cohort of CD was stable in the long term (up to 40 years after CD diagnosis). The cumulative incidence of perianal or rectovaginal fistulas was 24% after 30–40 years from CD diagnosis. Estimates on the incidence and prevalence of perianal disease vary widely according to the study design and study populations. In referral center studies and in the surgical literature, incidence rates exceeding 80% have been reported,15 and a higher rate of perianal fistulas has been reported in East Asian studies.16 However, we confirmed the stable incidence of perianal or rectovaginal fistulas in CD patients in the long term over the 40 years from CD diagnosis, in accordance with our previous report and other studies from population-based cohorts.5, 6 Also, we observed a decreased cumulative incidence of perianal or rectovaginal fistulas in the biologic era compared with the prebiologic era. The reasons for this decreasing incidence of perianal or rectovaginal fistulas in the biologic era are unknown. However, earlier detection and diagnosis of CD and a changing treatment paradigm to a top-down approach, with increased early use of biologic therapy, may account for this decreasing incidence. Further investigation will be needed to fully elucidate this phenomenon.
Another important finding of this study was that the outcome of perianal or rectovaginal fistulas, as measured by the cumulative rate of proctectomy for the treatment of fistulas, has improved in the biologic era (1998 or after) compared with the prebiologic era (before 1998), although this did not quite reach statistical significance. There has been a lack of data analyzing the change of disease outcome of IBD in the prebiologic and biologic eras. A recent review summarized the data regarding surgical trends in IBD in the prebiologic and biologic eras (before and after 1998), and it suggested a protective effect of biologics (anti-TNF agents) from surgery from population-based studies; however, the authors did not focus on surgery for perianal or rectovaginal fistulas specifically.17 A recent study from a population-based cohort of IBD patients in the Netherlands evaluated the incidence and outcome of perianal and rectovaginal fistulas in CD patients over the past 2 decades, and the risk of developing perianal fistulas was stable, along with their outcome, which was measured by fistula recurrence rate.18 The cumulative 5-year perianal fistula recurrence rate was not different between eras distinguished by the year of CD diagnosis: 1991–1998, 1999–2005, and 2006–2011 (19.5% vs 25.5% vs 33.1%; P = 0.28). On the other hand, we used the rate of proctectomy as an outcome of perianal or rectovaginal fistulas because proctectomy represents a significant milestone in the natural history of perianal or rectovaginal fistulas in CD. We observed a trend of decreased risk of proctectomy in the biologic era compared with the prebiologic era, and this trend reflects the possibility of a disease-modifying effect of biologics on the natural history of perianal and rectovaginal fistulas in CD.
Perianal fistulas are not necessarily associated with intestinal fistulizing complications, which is 1 of the complicated phenotypes of CD; thus, it has been considered that perianal disease alone requires a separate subclassification.19 This is reflected in the Montreal classification, which provides a separate modifier for perianal disease.20 However, perianal disease may be associated with significant impairment in quality of life and is an independent predictor of IBD-related work disability.21, 22 Also, the presence of perianal disease generally heralds a more aggressive disease course in patients with CD. For example, in a French referral center–based study, the presence of perianal disease was 1 of the risk factors predictive of subsequent 5-year “disabling” disease course, defined by the need for repeated doses of corticosteroids, corticosteroid dependence, hospitalizations for disease flares, immunosuppressive medications, surgical resection, and/or the presence of chronic disabling symptoms.23 Studies from East Asia show a high rate of anorectal malignancies in patients with long-standing CD and suggest that the high risk of anorectal cancer may be attributable to the high prevalence of perianal fistulas in this population and that adequate treatment of perianal fistulas may diminish this risk.24
Over the past decades, immunosuppressive agents and anti-TNF agents were introduced and have been widely used in the management of CD, including the treatment of perianal fistulas. This change in treatment paradigm for CD may be altering the natural history of perianal fistulas in CD. Although immunomodulators, including thiopurines,25 tacrolimus,26 and cyclosporine,27 have been shown to have a moderate effect in the treatment of perianal fistulizing CD and can be considered adjunctive treatment, anti-TNF agents (especially infliximab) have been studied independently in randomized controlled trials for the treatment of perianal disease. In the first trial, an induction regimen of infliximab resulted in closure of all fistulas in 55% of patients receiving 5 mg/kg of infliximab and 38% of patients receiving 10 mg/kg of infliximab, as compared with 13% of patients assigned to placebo (P = 0.001; P = 0.04).28 In the ACCENT II trial, which evaluated infliximab maintenance therapy for perianal CD, the time to loss of response (defined as any 1 of a number of events including the recrudescence of draining fistulas, the need for a change in medication for CD or the need for additional therapy for persistent or worsening luminal disease activity, the need for a surgical procedure for CD, or the discontinuation of the study medication owing to a perceived lack of efficacy) was significantly longer in patients on infliximab maintenance therapy than among those who received placebo (>40 weeks vs 14 weeks; P < 0.001).29
In our study, 19% of patients (16/85) underwent proctectomy, which has been considered a last resort for the treatment of perianal or rectovaginal fistulas, during follow-up. This result was comparable with a previous study, in which proctectomy was required in 21% of patients with fistulizing CD (18/87).30 On the other hand, a study using an older cohort from Stockholm, Sweden, between 1995 and 1974 reported a higher risk of proctectomy in this subset of patients, in which 23% of 826 patients with CD developed perianal fistulas, 40% of whom underwent proctocolectomy.5 This discrepancy may reflect the development of more effective medical treatments for perianal fistulizing CD over the decades. In 2014, a global consensus on the classification, diagnosis, and multidisciplinary treatment of perianal fistulas in CD was published.31 The authors emphasized the multidisciplinary approach in the diagnosis and treatment of perianal fistula in CD and recommended combined medical and surgical treatment for most cases.
Recently, new treatment modalities, such as mesenchymal stem cell (MSC) therapy for the treatment of perianal fistulas in CD, have been developed. Several phase I,32–34 phase II,34–36 and now phase III trials37 have been published demonstrating improved healing rates compared with conventional therapy with the direct injection of MSCs into the fistula tract. Other phase I trials have demonstrated even higher rates of healing when MSCs are impregnated on a bioabsorbable matrix plug.38 Thus, cell-based treatment therapies may offer promising new treatment approaches.
The strengths of this study include the fact that it utilizes the REP, which allows access to the medical records of a population-based cohort of patients diagnosed with IBD. Thus, all case ascertainment was based on clinical diagnoses and medical record review rather than administrative data. This provided us with accurate population-based estimates of the incidence of perianal or rectovaginal fistulas in patients with CD. However, there are a few limitations of this study. As this was a retrospective chart review, data could have been missed if they were not included in the medical record. Also, we could not retrieve the data regarding medical therapies for CD. A detailed classification of simple and complex fistulas could not be ascertained because the study period had started in 1970 and the study was retrospective. The sample size was smaller than what could have been obtained from a national database. However, our total population size is >400 patients with CD, and we feel that this is a sufficient size for significant findings, as we were able to develop significant findings in a previous population-based study6 with a much smaller cohort of patients.
In summary, in a population-based inception cohort of CD, one-fifth of patients were diagnosed with at least 1 perianal or rectovaginal fistula in the long term. The cumulative incidence of perianal or rectovaginal fistulas has significantly decreased in patients diagnosed in 1998 or after. The cumulative risk of proctectomy following onset of perianal or rectovaginal fistulas in CD seems to have decreased in patients who developed perianal or rectovaginal fistulas in 1998 or after. This might indicate a potential beneficial disease-modifying effect of biologics (anti-TNF agents) on the natural course of perianal or rectovaginal fistulas in CD. However, well-designed disease-modifying trials are needed to confirm this.
ACKNOWLEDGMENTS
The authors are grateful to Lawrence Timmons and Debra Jewell for data abstraction.
Conflicts of interest: Dr. Loftus has consulted for AbbVie, Janssen, Takeda, UCB, Amgen, Pfizer, Eli Lilly, Celgene, Celltrion Healthcare, and Napo Pharmaceuticals and has received research support from AbbVie, Janssen, Takeda, UCB, Genentech, Amgen, Pfizer, Receptos, Celgene, Gilead, Seres Therapeutics, MedImmune, and Robarts Clinical Trials. The others have no conflicts of interest to disclose.
Author contributions: Sang Hyoung Park, MD, original concept, study design, data abstraction, analysis, manuscript draft, and final revision; Satimai Aniwan, MD, manuscript review and editing; W. Scott Harmsen, MS, data abstraction, manuscript review and editing; William J. Tremaine, MD, manuscript review and editing; Amy L. Lightner, MD, manuscript review and editing; William A. Faubion, MD, manuscript review and editing; Edward V. Loftus Jr., MD, original concept, study design, manuscript review, and final editing.
Supported by: This research was supported in part by the Mayo Foundation for Medical Education & Research and the Rochester Epidemiology Project (grant number R01 AG034676 from the National Institute on Aging, National Institutes of Health).
Disclaimer: The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
REFERENCES
- 1. Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. [DOI] [PubMed] [Google Scholar]
- 2. Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, et al. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. 2012;107:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penner A, Crohn BB. Perianal fistulae as a complication of regional ileitis. Ann Surg. 1938;108:867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellers G, Bergstrand O, Ewerth S, et al. Occurrence and outcome after primary treatment of anal fistulae in Crohn’s disease. Gut. 1980;21:525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122:875–880. [DOI] [PubMed] [Google Scholar]
- 7. Gecse KB, Sebastian S, Hertogh Gd, et al. Results of the fifth scientific workshop of the ECCO [II]: clinical aspects of perianal fistulising Crohn’s disease-the unmet needs. J Crohns Colitis. 2016;10:758–765. [DOI] [PubMed] [Google Scholar]
- 8. Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loftus EV Jr, Silverstein MD, Sandborn WJ, et al. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. [DOI] [PubMed] [Google Scholar]
- 13. Loftus CG, Loftus EV Jr, Harmsen WS, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13:254–261. [DOI] [PubMed] [Google Scholar]
- 14. Shivashankar R, Tremaine WJ, Harmsen WS, et al. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ingle SB, Loftus EV Jr. The natural history of perianal Crohn’s disease. Dig Liver Dis. 2007;39:963–969. [DOI] [PubMed] [Google Scholar]
- 16. Ye BD, Yang SK, Cho YK, et al. Clinical features and long-term prognosis of Crohn’s disease in Korea. Scand J Gastroenterol. 2010;45:1178–1185. [DOI] [PubMed] [Google Scholar]
- 17. Olivera P, Spinelli A, Gower-Rousseau C, et al. Surgical rates in the era of biological therapy: up, down or unchanged?Curr Opin Gastroenterol. 2017;33:246–253. [DOI] [PubMed] [Google Scholar]
- 18. Göttgens KW, Jeuring SF, Sturkenboom R, et al. Time trends in the epidemiology and outcome of perianal fistulizing Crohn’s disease in a population-based cohort. Eur J Gastroenterol Hepatol. 2017;29:595–601. [DOI] [PubMed] [Google Scholar]
- 19. Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mahadev S, Young JM, Selby W, et al. Quality of life in perianal Crohn’s disease: what do patients consider important?Dis Colon Rectum. 2011;54:579–585. [DOI] [PubMed] [Google Scholar]
- 22. Ramos A, Calvet X, Sicilia B, et al. IBD-related work disability in the community: prevalence, severity and predictive factors. A cross-sectional study. United European Gastroenterol J. 2015;3:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656. [DOI] [PubMed] [Google Scholar]
- 24. Lee HS, Park SH, Yang SK, et al. The risk of colorectal cancer in inflammatory bowel disease: a hospital-based cohort study from Korea. Scand J Gastroenterol. 2015;50:188–196. [DOI] [PubMed] [Google Scholar]
- 25. Present DH, Korelitz BI, Wisch N, et al. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987. [DOI] [PubMed] [Google Scholar]
- 26. Sandborn WJ, Present DH, Isaacs KL, et al. Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trial. Gastroenterology. 2003;125:380–388. [DOI] [PubMed] [Google Scholar]
- 27. Egan LJ, Sandborn WJ, Tremaine WJ. Clinical outcome following treatment of refractory inflammatory and fistulizing Crohn’s disease with intravenous cyclosporine. Am J Gastroenterol. 1998;93:442–448. [DOI] [PubMed] [Google Scholar]
- 28. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. [DOI] [PubMed] [Google Scholar]
- 29. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 30. Bell SJ, Williams AB, Wiesel P, et al. The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145–1151. [DOI] [PubMed] [Google Scholar]
- 31. Gecse KB, Bemelman W, Kamm MA, et al. ; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials, World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. [DOI] [PubMed] [Google Scholar]
- 32. García-Olmo D, García-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. [DOI] [PubMed] [Google Scholar]
- 33. Cho YB, Lee WY, Park KJ, et al. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279–285. [DOI] [PubMed] [Google Scholar]
- 34. de la Portilla F, Alba F, Garcia-Olmo D, et al. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. [DOI] [PubMed] [Google Scholar]
- 35. Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. [DOI] [PubMed] [Google Scholar]
- 36. Lee WY, Park KJ, Cho YB, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–2581. [DOI] [PubMed] [Google Scholar]
- 37. Panés J, García-Olmo D, Van Assche G, et al. ; ADMIRE CD Study Group Collaborators Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]
- 38. Dietz AB, Dozois EJ, Fletcher JG, et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2017;153:59–62.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]