Abstract
BACKGROUND
Epidemiology can assess the effect of Chiari I malformation (CM1) on the neurological health of a population and evaluate factors influencing CM1 development.
OBJECTIVE
To analyze the regional and ethnic differences in the prevalence of CM1.
METHODS
The population of the Republic of Tatarstan (RT) in the Russian Federation was evaluated for patients with CM1 symptoms over an 11-yr period. Typical symptoms of CM1 were found in 868 patients. Data from neurological examination and magnetic resonance imaging (MRI) measurement of posterior cranial fossa structures were analyzed.
RESULTS
MRI evidence of CM1, defined as cerebellar tonsils lying at least 5 mm inferior to the foramen magnum, was found in 67% of symptomatic patients. Another 33% of symptomatic patients had 2 to 4 mm of tonsillar ectopia, which we defined as “borderline Chiari malformation type 1 (bCM1).” The period prevalence in the entire RT for symptomatic CM1 was 20:100 000; for bCM1 was 10:100 000; and for CM1 and bCM1 together was 30:100 000. Prevalence of patients with CM1 symptoms was greater in the northern than southern districts of Tatarstan, due to a high prevalence (413:100 000) of CM1 in the Baltasy region in one of the northern districts.
CONCLUSION
One-third of patients with typical symptoms of CM1 had less than 5 mm of tonsillar ectopia (bCM1). Assessments of the health impact of CM1-type symptoms on a patient population should include the bCM1 patient group. A regional disease cluster of patients with Chiari malformation was found in Baltasy district of RT and needs further study.
Keywords: Chiari malformation type 1, Epidemiology, Population groups, Syringomyelia
ABBREVIATIONS
- bCM1
borderline Chiari malformation type 1
- CL
clivus
- CM1
Chiari I malformation
- CSF
cererbrospinal fluid
- FM
foramen magnum
- MRI
magnetic resonance imaging
- NDRT
northern districts of Republic of Tatarstan
- PF
posterior cranial fossa
- RT
Republic of Tatarstan
- SDRT
southern districts of Republic of Tatarstan
- SO
supraoccipital bone
At the end of the 19th century, Professor Hans Chiari described the postmortem features of 4 types of craniocervical junction anomalies.1,2 The Chiari I malformation (CM1) was initially described as deformed cerebellar tonsils herniating through the foramen magnum (FM) accompanied by hydrocephalus. Later, magnetic resonance imaging (MRI) showed noninvasively that CM1-related tonsillar herniation often occurs without hydrocephalus; therefore, hydrocephalus became a nonessential element of CM1.3,4 Chiari's description of CM1 was further modified by an MRI diagnostic threshold requiring cerebellar herniation to extend at least 5 mm below the FM. Later, 2 subsets of patients with CM1-type semiology, CM0 and CM 1.5, were described based on their unique MRI findings.5–10 Patients with CM0 had less than 2 mm of tonsillar ectopia and syringomyelia.5–9 Patients with CM1.5 had brainstem descent in addition to the 5 mm of tonsillar descent seen in CM1 patients.10
Morphological measurements in many patients with CM1 revealed a small posterior fossa, a normal-sized hindbrain, and obliteration of the cererbrospinal fluid (CSF) of the cisterna magna by the cerebellar tonsils extending below the FM. A primary genetic abnormality or secondary injury presumably impeded development of the cephalic paraxial mesoderm, causing premature fusion of the spheno-occipital synchondrosis. The latter stunted growth of the basiocciput (the inferior segment of the clivus) and supraocciput,11–13 reduced posterior cranial fossa (PF) volume,11,13–18 and led to herniation of the cerebellar tonsils and CM1.19–21 Genetic factors were involved because 12% of symptomatic CM1 patients had a positive history of CM1; 21% of asymptomatic first-degree relatives of symptomatic CM1 patients had CM1 and/or syringomyelia.15,22,23 Studies of families in the United States with more than 1 member affected by CM1 identified several plausible candidate genes for CM1.17,24 The small posterior fossa trait, sometimes referred to as occipital hypoplasia, was more consistently inherited than tonsillar descent and CM1 expression.7,24–26 In nonfamilial cases of CM1, posterior fossa dimensions may be influenced by the expression of many genes, as occurs with morphometric features such as height and head circumference.27 Epigenetic modifications of somatic DNA and environmental factors could also restrict development of the posterior fossa and lead to CM1.28–30
Factors related to developing symptoms in CM1 patients include: (1) greater amounts of tonsillar herniation; (2) hypoplastic PF dimensions; (3) PF overcrowding characterized by constriction of CSF spaces including the cisterna magna; and (4) physiological measures of reduced and distorted CSF flow.11,31–33 Symptoms of CM1 rarely improve spontaneously, but can if tonsillar ectopia decreases 34–36 from PF growth in children or other factors.37–40 Tonsillar herniation greater than 12 mm almost invariably causes symptoms,4 but lesser amounts of tonsillar herniation may or may not evoke symptoms. Therefore, factors other than tonsillar herniation must be involved in symptom production in this group. Some asymptomatic CM1 patients have “spacious” subarachnoid spaces and no crowding of neural structures.41 Patients with typical CM1-symptoms but tonsillar herniation even less than the 5-mm threshold for CM1 often have MRI evidence of a small posterior fossa and hindbrain overcrowding and cine-MRI findings of abnormal CSF velocity/flow.4,8,42–44 Some asymptomatic CM1 patients (incidental CM1) later became symptomatic34–36 after minor trauma, Valsalva maneuver, and heavy manual labor.15,45,46 Quantitative, imaging-based physiological factors may more accurately predict positive outcomes after surgery for CM1 than morphological measures.11,13,17,24,31,47 Up to 40% of adults with CM1 and 12% to 23% of children with CM1 have syringomyelia.15,34,36,48,49
The CM0 malformation is characterized by clinical and MRI findings of cervical syringomyelia, a crowded posterior fossa, CSF obstruction around the FM, and less than 2 mm of tonsillar ectopia.5–9,42,50–53 In one study, normal subjects had no more than 2 mm of tonsillar ectopia below the FM and more than 2 mm of ectopia was 100% sensitive in capturing patients with CM1-type symptoms.3 Symptoms in patients with 2 to 4 mm of tonsillar descent below the FM may be identical to those with CM1.34,43,44,51,54 Such symptomatic patients may be diagnosed with “variant CM1, borderline CM1, or Chiari I-like syndrome.”15,44,55
Our current study investigated the prevalence and regional, ethnic, and gender distribution of symptomatic CM1 (Figure 1), Chiari I-like syndrome associated with minimal borderline tonsillar ectopia borderline Chiari malformation type 1 (bCM1), and CM-associated syringomyelia in the Republic of Tatarstan (RT). RT is a federal subject of the Russian Federation, found in the center of the East European Plain about 800 km east of Moscow. It lies between the Volga and Kama Rivers, and extends east to the Ural Mountains.
FIGURE 1.
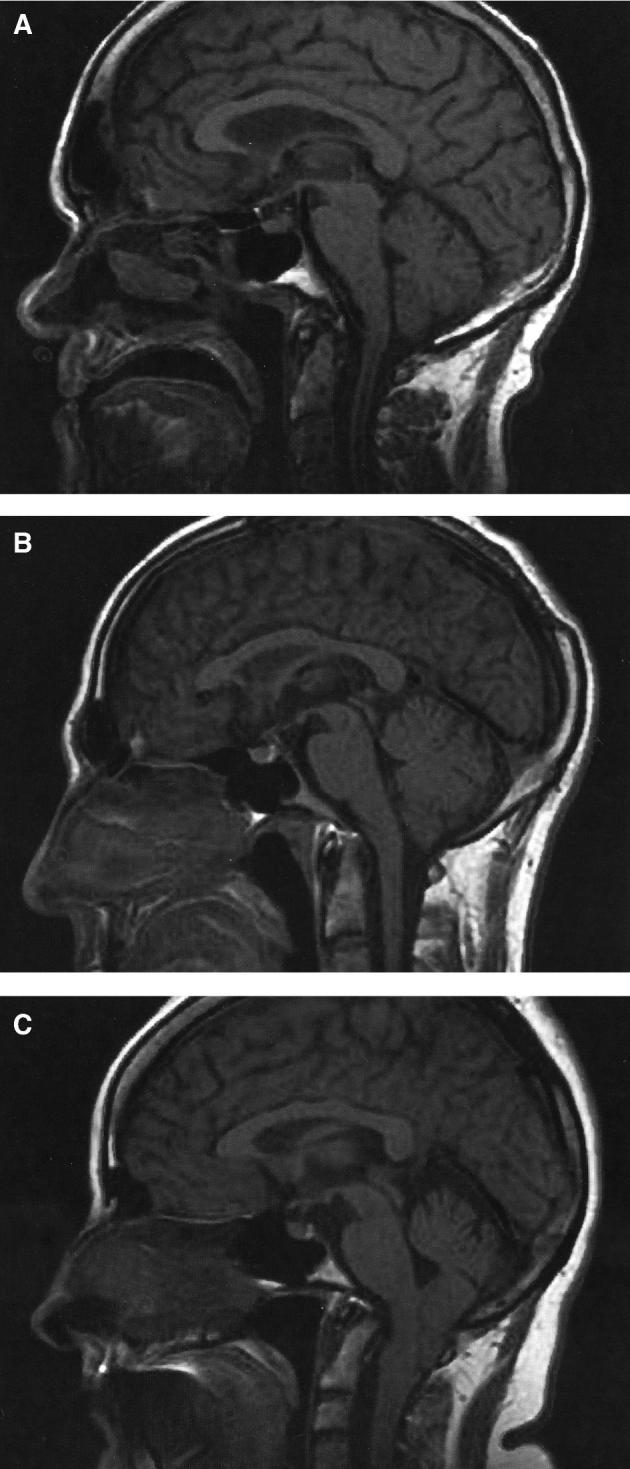
Typical Chiari I (CM1), bCM1, and Chiari 0 malformation (CM0) on the sagittal cut MRI scans (from RT population). A, M70, CM0 with syringomyelia (the tonsils are at the level of the FM). B, F40, bCM1 with syringomyelia (tonsillar herniation = 3 mm). C, F41, CM1 without syringomyelia (tonsillar herniation = 17 mm).
Currently, there are few epidemiologic studies of CM1 and Chiari1-like syndrome.28,56,57 The existing research has detection bias because: (1) the prevalence of CM1 diagnosed by MRI criteria alone is considerably higher than CM1 diagnosed by coexistent CM1 semiology and MRI criteria; and (2) not all patients with CM1 semiology undergo MRI scanning.28,57
Previous reports suggest that CM1 has a female preponderance in the United States but not in Europe or Asia.28 A regional preponderance of syringomyelia in some areas of the RT was previously reported.56,58–60 The present study seeks to identify population groups in RT that are prone to developing symptomatic CM1, CM1-like syndrome (bCM1), and CM-associated syringomyelia.
METHODS
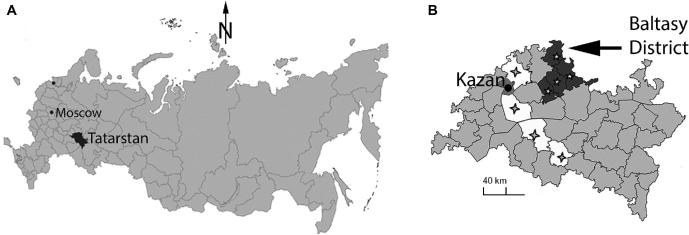
The study was conducted at the Hospital of RT (Figure 2). Patient consent was not required because this retrospective study presents only aggregated archival data. An institutional review board approved this study. In our epidemiological research, we followed STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) rules. This hospital cares for the entire RT population. It is a large, integrated tertiary medical center with 1300 inpatient beds, including 50 neurological and 80 neurosurgical beds, and weekday visits for 100 to 120 referred neurological and neurosurgical outpatients. The population of adults in RT during the study was 2876 000, with an ethnic mix of 54% Tatars, 39% ethnic Russians, and 7% others.61–63 The ethnic mix seen by the neurological and neurosurgical departments was 58% Tatars, 40% ethnic Russians, and 2% others. Patients lived in one of the 44 separate districts of RT, or Kazan city, the capital of RT (Figure 2).
FIGURE 2.
Maps of the Russian Federation, RT, and Baltasy district. A, Map of the Russian Federation. Tatarstan is shown in black. Kazan, the capital of The RT, is found 720 km East-Southeast of Moscow. B, Map of Tatarstan. The southern districts of Tatarstan are shown in white with crosses and the northern districts in dark gray with stars. The Baltasy district (arrow) is Northeast of Kazan and on the northern border of Tatarstan.
In the first stage of the study, we analyzed archival data of patients hospitalized in our clinic between January 1998 and December 2008. During the study period, data were processed from 29 008 hospitalizations to select cases with ectopia of the cerebellar tonsils and CM1-like symptoms, including: (1) CM1-type headaches, such as suboccipital headache, pseudotumor headache with visual phenomena, and cough headache; (2) dizziness; and (3) symptoms of cervical syringomyelia.15,64 Patients who had secondary causes of cerebellar herniation such as craniosynostosis and tumors were excluded. A total of 868 patients with tonsillar ectopia and CM1-like symptoms were found. We documented age, gender, nationality, residency in RT, district (Northern, Southern, or other), radiological, physical, and neurological findings (Tables 1–4).
TABLE 1.
Population of Adult Patients With CM1 and bCM1 in Hospital of RT Between January 1998 and December 2008
| Male | Female | All | |
|---|---|---|---|
| (n = 438) | (n = 430) | (n = 868) | |
| Age, years | 41 ± 15 | 44 ± 13 | 43 ± 13 |
| Tatars, n (%) | 319 (73%) | 292 (68%) | 611 (70%) |
| Russians, n (%) | 101 (23%) | 122 (28%) | 223 (26%) |
| Others, n (%) | 18 (4%) | 16 (4%) | 34 (4%) |
| Syringomyelia, n (%) | 212 (48%) | 150 (35%) | 362 (42%) |
TABLE 4.
Characteristics of Patients With CM1 and bCM1
| Male | Female | All | |
|---|---|---|---|
| (n = 108) | (n = 92) | (n = 200) | |
| Age, years | 41 ± 15 | 47 ± 11 | 43 ± 13 |
| Tatars, n (%) | 85 (79%) | 73 (79%) | 158 (79%) |
| Non-Tatars, n (%) | 23 (21%) | 19 (21%) | 42 (21%) |
| CM 1, n (%) | 61 (56%) | 73 (79%) | 134 (67%) |
| CM 1 with syrinx, n (%) | 35 (32%) | 33 (36%) | 68 (34%) |
| bCM1, n (%) | 47 (44%) | 19 (21%) | 66 (33%) |
| bCM1 with syrinx, n (%) | 17 (16%) | 8 (9%) | 25 (13%) |
MRI was performed using 1.0 and 1.5 T scanners (EXCITE, GE Healthcare, Waukesha, Wisconsin). One trained researcher (A.F.) took linear measurements of the PF structures on a midsagittal T1-weighted image. Measurements were defined as follows: (1) clivus (CL) length was the length of a line drawn from the dorsum sella to the basion; (2) supraoccipital bone (SO) was the distance between the opisthion and the internal occipital protuberance; (3) the McRae line was the line connecting the opisthion and basion; and (4) tonsillar herniation was the distance from the McRae line to the most inferior extent of the cerebellar tonsils. The diagnosis of CM1 was given to all symptomatic patients with one or both cerebellar tonsils extending 5 mm or more below the FM, with and without syringomyelia.3,4 Tonsillar herniation was commonly asymmetrical.65–67 All symptomatic patients with one or both cerebellar tonsils extending 2 to 4 mm below the FM (Figure 1), with and without syringomyelia, were diagnosed with bCM1.3,4 CM0 patients were not included in this study.42
In the second phase of the study (after detecting regional differences in the CM prevalence), we performed a more extensive MRI morphometric study of 228 of the 868 patients. This included 200 patients from different districts of RT (male/female 108/92, mean age 43 ± 13 yr, Tatars 79%) and 28 patients from the Baltasy district (male/female 12/16, mean age 41 ± 15 yr, Tatars 100%) to measure the length of their posterior fossa bones (CL, SO). We also measured the length of the posterior fossa bones in a control group of 100 individuals (male/female 39/61, mean age 47 ± 7 yr, Tatars 58%) who had previously undergone brain MRI after presenting with (1) nonspecific symptoms and no neurological impairment or (2) symptoms suggestive of multiple sclerosis or other conditions not affecting intracranial volume or PF anatomy.
Measurements from CM patients and control subjects were compared using the U-test (IBM SPSS Statistics 23, Armonk, New York). P values < .05 were considered statistically significant.
RESULTS
The Period Prevalence of CM1 and bCM1 for the Entire RT
The period prevalence of CM1 and bCM1 for the entire RT population was 30:100 000. Further analysis of the 868 RT patients showed that 118 (13.6%) were from 4 northern districts of Republic of Tatarstan (NDRT) with a population of 95 000 (Figure 2; Table 2) and 53 (6.1%) were from 4 southern districts of Republic of Tatarstan (SDRT) with a population of 104 000.61–63 The period prevalence of symptomatic CM1 and bCM1 patients in NDRT was therefore significantly more (122:100 000) than in SDRT (49:100 000; Figure 2, Table 2). Syringomyelia, a disorder often associated with CM1 and bCM1, was previously reported to be more prevalent in NDRT than SDRT.56 The proportion of ethnic Tatars among CM1 and bCM1 patients in NDRT was 87% and in SDRT was 70%, whereas the proportion of ethnic Tatars in the entire population of NDRT compared to SDRT was 87% and SDRT was 41%, respectively (Table 2).
TABLE 2.
Characteristics of Northern and Southern (Control) Districts of RT
| Northern districts | Southern districts | |
|---|---|---|
| Adults (during 2002) | ||
| Tatars (n, %) | 83 000 (87%) | 44 000 (41%) |
| Non-Tatars (n, %) | 12 000 (13%) | 61 000 (59%) |
| Total adult population | 95 000 | 104 000 |
| Frequency of referral to our center, per 1000 adult inhabitants | 29 | 28 |
| Patients with CM (n, %): | ||
| Tatars | 103 (87%) | 37 (70%) |
| Non-Tatars | 15 (13%) | 16 (30%) |
| Total | 118 | 53 |
| Patients with CM and Syringomyelia (n, % of CM): | ||
| Tatars | 54 (52%) | 17 (46%) |
| Non-Tatars | 4 (27%) | 5 (31%) |
| Total | 58 (49%) | 22 (42%) |
| Period prevalence of CM, per 100 000 adult inhabitants: | ||
| Tatars | 124 | 76 |
| Non-Tatars | 112 | 26 |
| Total RT population | 122 | 49 |
The Period Prevalence of CM1 and bCM1 for the Baltasy District
Of the 118 CM1 and bCM1 patients from the NDRT, 95 inhabited its Baltasy district (Figure 2), of whom 33 (35%) were first-degree relatives from 13 families. The 33 familial cases included 23 (64%) with CM1 and 10 (36%) with bCM1. The population of adults in the entire Baltasy district during this period was 23 000, with an ethnic composition of 84% Tatars, 10% Russians, and 6% others. Period prevalence of symptomatic CM1 and bCM1 patients in the Baltasy district was 413:100 000 (CM1 275:100 000; bCM1 138:100 000). The higher period prevalence in the Baltasy district more than accounted for the higher period prevalence of symptomatic CM1 and bCM1 in NDRT compared to SDRT, with the period prevalence in NDRT outside the Baltasy district being 32 per 100 000 compared to 49 per 100 000 in SDRT. The mean lengths of CL and SO in the CM1 patients in Baltasy were similar to CM1 patients in other RT regions and countries (Tables 5 and 6), but the mean length of CL in the bCM1 patients in Baltasy was significantly shorter than in other RT regions (respectively, 35 mm and 40 mm; P = .002).
TABLE 5A.
Results of Radiological Study SO in Patients With CM1 and bCM1
| Groups of patients, n | SO, mm (M ± m) | P a |
|---|---|---|
| CM1 (RT), 134 | 38 ± 5 | .0001 |
| bCM1 (RT), 66 | 40 ± 4 | .059 |
| CM1 (Baltasy district), 18 | 37 ± 6 | .001 |
| bCM1 (Baltasy district), 10 | 38 ± 3 | .013 |
| Control group, 100 | 42 ± 5 |
aStatistical significance of differences with control group (Mann–Whitney test).
TABLE 6.
Linear Measurements of CL and SO in CM1 Patients and Control Group Compared to Findings in Other Studies
| CM1 | Control | |||||
|---|---|---|---|---|---|---|
| Region or Country | n | CL, mm | SO, mm | n | CL, mm | SO, mm |
| Baltasy district | 28 | 37 ± 6 | 37 ± 6 | 100 | 41 ± 4 | 42 ± 5 |
| RT | 200 | 38 ± 4 | 38 ± 5 | 100 | 41 ± 4 | 42 ± 5 |
| Milhorat (USA)15 | 50 | 37 ± 4 | 38 ± 6 | 50 | 40 ± 5 | 42 ± 5 |
| Karagoz (Turkey)18 | 22 | 36 ± 7 | 38 ± 5 | 21 | 40 ± 4 | 41 ± 7 |
| Heiss (USA)20 | 48 | 39 ± 3 | 40 ± 4 | 18 | 43 ± 4 | 42 ± 4 |
Clinical and Radiological Findings
Tonsillar descent in the evaluated 200 patients with CM symptoms met the CM1 threshold (5 mm or more tonsillar ectopia) in 134 (67%) and the bCM1 threshold (2-4 mm of tonsillar ectopia) in 66 (33%; Table 5). There were 108 males, 61 (56%) with CM1, of whom 35 (57%) had syringomyelia; and 47 (44%) with bCM1, of whom 17 (36%) had syringomyelia (Table 4). There were 92 females, 73 (79%) with CM1, of whom 33 (45%) had syringomyelia; and 19 (21%) with bCM1, of whom 8 (42%) had syringomyelia (Table 4). The frequency and types of symptoms in the CM1 and bCM1 subgroups were similar (Table 3).44 Valsalva-related and other typical headaches were identified in 86% of CM1 and 88% of bCM1 patients. The MRI measurements for these 200 patients showed that the clivus and supraocciput lengths were significantly shorter in the CM1 group compared to the control group (Table 5). The cisterna magna was small or obliterated in all patients in the CM1 and bCM1 subgroups, but was preserved in controls.15,44
TABLE 3.
Results of Clinical Study of Patients With CM1 and bCM1
| bCM1 | CM1 | All | |
|---|---|---|---|
| (n = 66) | (n = 134) | (n = 200) | |
| Age, years | 42 ± 16 | 44 ± 12 | 43 ± 13 |
| M/F | 47/19 | 61/73 | 108/92 |
| All CM-related headaches, n (%) | 58 (88%) | 115 (86%) | 173 (87%) |
| Suboccipital headache | 32 (48%) | 69 (51%) | 101 (50%) |
| Pseudotumor headache with visual phenomena | 27 (41%) | 51 (38%) | 78 (39%) |
| Cough headache | 7 (11%) | 19 (14%) | 26 (13%) |
| Dizziness, n (%) | 26 (39%) | 77 (57%) | 103 (52%) |
| Syringomyelia, n (%) | 25 (38%) | 68 (51%) | 93 (46%) |
| Numbness, n (%) | 22 (33%) | 50 (37%) | 72 (36%) |
| Paresthesia, n (%) | 18 (27%) | 42 (31%) | 60 (30%) |
DISCUSSION
Ethnic Differences
This study of the RT population enrolled 868 patients with symptomatic CM1, CM1-like syndrome (bCM1), and CM-associated syringomyelia over an 11-yr period. The predominant ethnic group affected was Tatars, consisting of 70% of RT patients, including 70% of SDRT, 87% of NDRT, and 91% of Baltasy district patients. Tatars was disproportionally affected by CM compared to their percentage in the population in all but NDRT because they make up 54%, 41%, 87%, and 84% of the population of RT, SDRT, NDRT, and Baltasy district, respectively. Referral patterns cannot account for this disparity because the proportion of Tatars referred to the Hospital of RT reflected the proportion of Tatars in the population. Syringomyelia is more prevalent in Ethnic Tatars than in other ethnic groups in RT and neighboring regions of the Russian Federation, which may result because Tatars are more likely to have CM1 and bCM1, which predispose to syringomyelia.56,68,69
Clinical–Radiological Associations
In the second phase group of 200 patients, 66 (33%) had bCM1 (tonsillar descent of 2-4 mm) and 134 (67%) had CM1 (tonsillar descent of 5 mm or more). The proportion (33%) of patients with symptoms typical of CM1 but tonsillar ectopia less than 5 mm (bCM1) was much greater in our study compared to other studies (7%-9%).15,70 Milhorat reported that in 364 patients with typical CM1 symptoms, 32 (9%) had tonsillar ectopia less than 5 mm.15 In another study of 200 CM1 patients, 14 (7%) had tonsillar descent of 3 mm or less below the FM.70 The percentage of symptomatic bCM1 (33%) in our study was considerably higher than in these other studies, possibly reflecting a higher proportion of patients with a small PF in our population. The case-control evaluation of posterior fossa measurements revealed similar changes in bone phenotype in CM1 and bCM1, manifested as reduced posterior fossa bone lengths (Tables 5 and 6). Symptoms and MRI evidence of PF hypoplasia were identical in CM1 and bCM1 patients, differing only in the extent of tonsillar descent (Table 4).
TABLE 5B.
Results of Radiological Study CL in Patients With CM1 and bCM1
| Groups of patients, n | CL, mm (M ± m) | P a |
|---|---|---|
| CM1 (RT), 134 | 38 ± 4 | .0001 |
| bCM1 (RT), 66 | 40 ± 4 | .068 |
| CM1 (Baltasy district), 18 | 37 ± 6 | .0001 |
| bCM1 (Baltasy district), 10 | 35 ± 4 | .0001 |
| Control group, 100 | 41 ± 4 |
aStatistical significance of differences with control group (Mann–Whitney test).
Geographical Differences
Ethnic, racial, and geographical differences in the prevalence of tonsillar ectopia were studied in other countries. In Japan, tonsillar ectopia 1 to 4 mm below FM occurred in only 12 of 5000 subjects (0.24%).43 In a study of 200 American patients with clinical signs or symptoms unrelated to CM1, 14% had tonsils extending 1 to 3 mm below the FM.3 In a multicenter European-American study of 600 normal individuals, more than 5% had tonsils 1 to 5 mm below FM, while only 0.5% had greater than 5 mm of tonsillar ectopia.47 Based on these studies, it appears that the prevalence of tonsillar ectopia in the 1 to 5 mm range is much greater (5%-14%) in the Euro-American population than in Japan (0.24%). This conclusion agrees with observations that tonsillar position is generally higher in Japanese than Americans.43 The higher position of the cerebellar tonsils in the Japanese population could explain why Japanese are affected less often than Europeans by CM1 and bCM1-related obstruction of CSF pathways at the FM, leading to a lower prevalence of syringomyelia in Japan (1.9 per 100 000) than in England or New Zealand (8.4 and 8.2 per 100 000, respectively).71–73 In the US and European populations, 0.77% to 0.90% of normal adults and 0.97% to 3.6% of children undergoing MRI scanning have cerebellar tonsillar ectopia of 5 mm or more, which is radiologically diagnostic of CM1.22,34,36,74,75 The estimated prevalence of symptomatic CM1 in the general American population is 9 to 36 cases per 100 000.28 Closer examination of our CM1 patients showed a prevalence of CM1 in the entire RT of 20 per 100 000, which is similar to that of the American population. Our study included separate CM1 and bCM1 subgroups while the CM1 group in the studies mentioned above, in fact had 7% to 9% of bCM1 cases. Patients in the bCM1 group had symptoms compatible with CM1, tonsillar herniation less than 5 mm, and PF hypoplasia, consistent with a variant of CM1.7,26,44 Determining population prevalence accurately requires evaluation of every individual within a population. The prevalence of symptomatic CM1 would have been higher if all symptomatic or minimally symptomatic patients sought medical care at our center during the study.
Regional “Clusters” of CM1 and Syringomyelia
The prevalence of symptomatic CM1 in the Baltasy district was much higher than in other regions of the RT. The prevalence of adults with symptomatic CM1 in the Baltasy district of NDRT was over 10 times that of the rest of the RT. Clusters of CM1 and syringomyelia in other regions and ethnic groups have been reported. A higher prevalence of syringomyelia was noted in one area of Germany and regions of Russia with a large proportion of Tatars.56,60,68,69,76 Increased prevalence of CM1, basilar impression and syringomyelia was found in Northeastern Brazil and in some states in India.77,78 In New Zealand, the prevalence of CM1-associated syringomyelia was greater among Maori and Pacific people than in those of European descent.72 Familial clustering of CM1 patients in the state of Utah supported a genetic predisposition to CM1 in those American families.79
CONCLUSION
Epidemiological studies can measure the prevalence of CM1 symptoms in different countries and regions. In this study, two-thirds of patients with CM1 symptomatology had 5 mm or more tonsillar ectopia and met the MRI diagnostic standard for CM1. Another one-third of patients who manifested CM1-type symptoms had only 2 to 4 mm of tonsillar ectopia. A regional disease cluster with a high prevalence of symptomatic CM1 and bCM1 patients and a Tartar ethnic preponderance was found in the Baltasy district of RT. In that district, about one-third of affected patients had an affected first-degree relative. Further study of this disease cluster for genetic and environmental factors predisposing to CM1 and bCM1 is necessary.
Disclosures
Dr Heiss’ work on the manuscript was supported by the Intramural Research Program at the National Institute of Neurological Disorders and Stroke at the National Institutes of Health, USA. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENTS
If we are to eventually understand the etiologies of congenital malformations with coexisting familial and environmental predispositions, a thorough analysis of epidemiological factors along with cultural, ethnic, dietary, and other influences is required. Understanding geographic influences, especially in endemic regions in industrialized and developing countries alike, carries special significance and may point to non-Mendelian heritable factors yet to be identified. This article is a step forward in that direction.
Bermans J. Iskandar
Madison, Wisconsin
The authors detail a group of individuals in central Asia that appear to have a high incidence of Chiari I Malformations, syrinx associated with the CIM, and a diminished posterior fossa volume. In addition to the patients with more than 5 mm of caudal descent of their cerebellar tonsils and a syrinx, the authors have included a group with less than 5 mm of descent, no syrinx, and predominantly subjective symptoms. In the setting of a high prevalence Tartar population, inclusion of the less objective group of patients seems reasonable. Using these same, less objective, criteria of tonsillar descent less than 5 mm, no syrinx and totally subjective clinical symptoms to recommend surgery to other populations is problematic.
W. Jerry Oakes
Birmingham, Alabama
REFERENCES
- 1. Chiari H. Ueber veränderungen des kleinhirns infolge von hydrocephalie des grosshirns (concerning changes in the cerebellum due to hydrocephalus of the cerebrum). Dtsch Med Wochenschr. 1891;17(42):1172–1175. [Google Scholar]
- 2. Chiari H. Über veränderungen des kleinhirns, des pons und der medulla oblongata in folge von congenitaler hydrocephalie des grosshirns (concerning changes in the cerebellum, pons, and medulla oblongata due to hydrocephalus of the cerebrum). Denkschr Akad Wissensch. 1896;63:71–116. [Google Scholar]
- 3. Barkovich AJ, Wippold FJ, Sherman JL, Citrin CM. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol. 1986;7(5):795–799. [PMC free article] [PubMed] [Google Scholar]
- 4. Elster AD, Chen MY. Chiari I malformations: clinical and radiologic reappraisa. Radiology. 1992;183(2):347–353. [DOI] [PubMed] [Google Scholar]
- 5. Chern JJ, Gordon AJ, Mortazavi MM, Tubbs RS, Oakes WJ. Pediatric chiari malformation type 0: a 12-year institutional experience. J Neurosurg Pediatr. 2011;8(1):1–5. [DOI] [PubMed] [Google Scholar]
- 6. Iskandar BJ, Hedlund GL, Grabb PA, Oakes WJ. The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg. 1998;89(2):212–216. [DOI] [PubMed] [Google Scholar]
- 7. Markunas CA, Tubbs RS, Moftakhar R et al.. Clinical, radiological, and genetic similarities between patients with Chiari type I and type 0 malformations. J Neurosurg Pediatr. 2012;9(4):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tubbs RS, Elton S, Grabb P, Dockery SE, Bartolucci AA, Oakes WJ. Analysis of the posterior fossa in children with the Chiari 0 malformation. Neurosurgery. 2001;48(5):1050–1054; discussion 1054-1055. [DOI] [PubMed] [Google Scholar]
- 9. Tubbs RS, Smyth MD, Wellons JC 3rd, Oakes WJ. Arachnoid veils and the Chiari I malformation. J Neurosurg. 2004;100(5 suppl pediatrics):465–467. [DOI] [PubMed] [Google Scholar]
- 10. Tubbs RS, Iskandar BJ, Bartolucci AA, Oakes WJ. A critical analysis of the Chiari 1.5 malformation. J Neurosurg. 2004;101(2 suppl):179–183. [DOI] [PubMed] [Google Scholar]
- 11. Alperin N, Loftus JR, Oliu CJ et al.. Magnetic resonance imaging measures of posterior cranial fossa morphology and cerebrospinal fluid physiology in Chiari malformation type I. Neurosurgery. 2014;75(5):515–522; discussion 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noudel R, Jovenin N, Eap C, Scherpereel B, Pierot L, Rousseaux P. Incidence of basioccipital hypoplasia in Chiari malformation type I: comparative morphometric study of the posterior cranial fossa. J Neurosurg. 2009;111(5):1046–1052. [DOI] [PubMed] [Google Scholar]
- 13. Urbizu A, Poca MA, Vidal X, Rovira A, Sahuquillo J, Macaya A. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of Chiari malformation type I. J Neuroimaging. 2014;24(3):250–256. [DOI] [PubMed] [Google Scholar]
- 14. Marin-Padilla M, Marin-Padilla TM. Morphogenesis of experimentally induced arnold-chiari malformation. J Neurol Sci. 1981;50(1):29–55. [DOI] [PubMed] [Google Scholar]
- 15. Milhorat TH, Chou MW, Trinidad EM et al.. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44(5):1005–1017. [DOI] [PubMed] [Google Scholar]
- 16. Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86(1):40–47. [DOI] [PubMed] [Google Scholar]
- 17. Urbizu A, Toma C, Poca MA et al.. Chiari malformation type I: a case-control association study of 58 developmental genes. PLoS One. 2013;8(2):e57241, doi: 10.1371/journal.pone.0057241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karagoz F, Izgi N, Kapijcijoglu Sencer S. Morphometric measurements of the cranium in patients with Chiari type I malformation and comparison with the normal population. Acta Neurochir (Wien). 2002;144(2):165–171; discussion 171. [DOI] [PubMed] [Google Scholar]
- 19. Buell TJ, Heiss JD, Oldfield EH. Pathogenesis and cerebrospinal fluid hydrodynamics of the Chiari I malformation. Neurosurg Clin N Am. 2015;26(4):495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heiss JD, Suffredini G, Bakhtian KD, Sarntinoranont M, Oldfield EH. Normalization of hindbrain morphology after decompression of Chiari malformation type I. J Neurosurg. 2012;117(5):942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD. Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir. 2010;152(7):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Speer MC, George TM, Enterline DS, Franklin A, Wolpert CM, Milhorat TH. A genetic hypothesis for Chiari I malformation with or without syringomyelia. Neurosurg Focus. 2000;8(3):1–4. [DOI] [PubMed] [Google Scholar]
- 23. Speer MC, Enterline DS, Mehltretter L et al.. Review article: Chiari type I malformation with or without syringomyelia: prevalence and genetics. J Genet Couns. 2003;12(4):297–311. [DOI] [PubMed] [Google Scholar]
- 24. Markunas CA, Enterline DS, Dunlap K et al.. Genetic evaluation and application of posterior cranial fossa traits as endophenotypes for Chiari type I malformation. Ann Hum Genet. 2014;78(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyles AL, Enterline DS, Hammock PH et al.. Phenotypic definition of Chiari type I malformation coupled with high-density SNP genome screen shows significant evidence for linkage to regions on chromosomes 9 and 15. Am J Med Genet. 2006;140(24):2776–2785. [DOI] [PubMed] [Google Scholar]
- 26. Markunas CA, Lock E, Soldano K et al.. Identification of Chiari type I malformation subtypes using whole genome expression profiles and cranial base morphometrics. BMC Med Genomics. 2014;7(1):39, doi: 10.1186/1755-8794-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith BW, Strahle J, Bapuraj JR, Muraszko KM, Garton HJ, Maher CO. Distribution of cerebellar tonsil position: implications for understanding Chiari malformation. J Neurosurg. 2013;119(3):812–819. [DOI] [PubMed] [Google Scholar]
- 28. Heiss JD. Epidemiology of the Chiari I malformation. In: Tubbs RS, Oakes WJ, eds. The Chiari Malformation. New York: Springer Science+Business Media; 2013:82–91. [Google Scholar]
- 29. Markunas CA, Soldano K, Dunlap K et al.. Stratified whole genome linkage analysis of Chiari type I malformation implicates known Klippel-Feil syndrome genes as putative disease candidates. PLoS One. 2013;8(4):e61521, doi: 10.1371/journal.pone.0061521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Markunas CA, Ashley-Koch AE, Gregory SG. Genetics of Chiari I and II malformations. In: Tubbs RS, Oakes WJ, eds. The Chiari Malformation. New York: Springer Science+Business Media; 2013:93–101. [Google Scholar]
- 31. Alperin N, Loftus JR, Bagci AM et al.. Magnetic resonance imaging-based measures predictive of short-term surgical outcome in patients with Chiari malformation type I: a pilot study. J Neurosurg Spine. 2017;26(1):28–38. [DOI] [PubMed] [Google Scholar]
- 32. Alperin N, Loftus JR, Oliu CJ et al.. Imaging-based features of headaches in Chiari malformation type I. Neurosurgery. 2015;77(1):96–103; discussion 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hofkes SK, Iskandar BJ, Turski PA, Gentry LR, McCue JB, Haughton VM. Differentiation between symptomatic Chiari I malformation and asymptomatic tonsilar ectopia by using cerebrospinal fluid flow imaging: initial estimate of imaging accuracy. Radiology. 2007;245(2):532–540. [DOI] [PubMed] [Google Scholar]
- 34. Aitken LA, Lindan CE, Sidney S et al.. Chiari type I malformation in a pediatric population. Pediatr Neurol. 2009;40(6):449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Novegno F, Caldarelli M, Massa A et al.. The natural history of the Chiari type I anomaly. J Neurosurg Pediatr. 2008;2(3):179–187. [DOI] [PubMed] [Google Scholar]
- 36. Strahle J, Muraszko KM, Kapurch J, Bapuraj JR, Garton HJ, Maher CO. Chiari malformation type I and syrinx in children undergoing magnetic resonance imaging. J Neurosurg Pediatr. 2011;8(2):205–213. [DOI] [PubMed] [Google Scholar]
- 37. Bogdanov EI, Heiss JD, Mendelevich EG. The post–syrinx syndrome: stable central myelopathy and collapsed or absent syrinx. J Neurol. 2006;253(6):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kyoshima K, Bogdanov EI. Spontaneous resolution of syringomyelia: report of two cases and review of the literature. Neurosurgery. 2003;53(3):762–769; discussion 768-769. [DOI] [PubMed] [Google Scholar]
- 39. Perrini P. Spontaneous resolution of syringomyelia in an adult patient with tight cisterna magna. Neurol Sci. 2012;33(6):1463–1467. [DOI] [PubMed] [Google Scholar]
- 40. Waldau B, Domeshek LF, Leigh FA et al.. Spontaneous resolution of a 13-mm Chiari malformation type I in relation to differential growth of the posterior fossa volume. J Neurosurg Pediatr. 2009;3(2):110–114. [DOI] [PubMed] [Google Scholar]
- 41. Taylor DG, Mastorakos P, Jane JA Jr, Oldfield EH. Two distinct populations of Chiari I malformation based on presence or absence of posterior fossa crowdedness on magnetic resonance imaging. J Neurosurg. 2017;126(6):1934–1940. [DOI] [PubMed] [Google Scholar]
- 42. Bogdanov EI, Heiss JD, Mendelevich EG, Mikhaylov IM, Haass A. Clinical and neuroimaging features of “idiopathic” syringomyelia. Neurology. 2004;62(5):791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Furuya K, Sano K, Segawa H, Ide K, Yoneyama H. Symptomatic tonsillar ectopia. J Neurol Neurosurg Psychiatry. 1998;64(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sekula RF Jr, Jannetta PJ, Casey KF, Marchan EM, Sekula LK, McCrady CS. Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res. 2005;2(1):11, doi: 10.1186/1743-8454-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meeker J, Amerine J, Kropp D, Chyatte M, Fischbein R. The impact of Chiari malformation on daily activities: a report from the National Conquer Chiari Patient Registry database. Disabil Health J. 2015;8(4):521–526. [DOI] [PubMed] [Google Scholar]
- 46. Wan MJ, Nomura H, Tator CH. Conversion to symptomatic Chiari I malformation after minor head or neck trauma. Neurosurgery. 2008;63(4):748–753; discussion 753. [DOI] [PubMed] [Google Scholar]
- 47. Freeman MD, Rosa S, Harshfield D et al.. A case-control study of cerebellar tonsillar ectopia (Chiari) and head/neck trauma (whiplash). Brain Inj. 2010;24(7-8):988–994. [DOI] [PubMed] [Google Scholar]
- 48. Klekamp J. Surgical treatment of Chiari I malformation-analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery. 2012;71(2):365–380; discussion 380. [DOI] [PubMed] [Google Scholar]
- 49. Sekula RF Jr, Arnone GD, Crocker C, Aziz KM, Alperin N. The pathogenesis of Chiari I malformation and syringomyelia. Neurol Res. 2011;33(3):232–239. [DOI] [PubMed] [Google Scholar]
- 50. Heiss JD, Patronas N, DeVroom HL et al.. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91(4):553–562. [DOI] [PubMed] [Google Scholar]
- 51. Mariwalla NR, Boydston WR, Chern JJ. Newer Subsets: Chiari 0 and Chiari 1.5 malformations. In: Tubbs RS, Oakes WJ, eds. The Chiari Malformations. New York: Springer Science+Business Media; 2013:241–246. [Google Scholar]
- 52. Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. J Neurosurg. 1994;80(1):3–15. [DOI] [PubMed] [Google Scholar]
- 53. Struck AF, Haughton VM. Idiopathic syringomyelia: phase-contrast MR of cerebrospinal fluid flow dynamics at level of foramen magnum. Radiology. 2009;253(1):184–190. [DOI] [PubMed] [Google Scholar]
- 54. Ventureyra EC, Aziz HA, Vassilyadi M. The role of cine flow MRI in children with Chiari I malformation. Childs Nerv Syst. 2003;19(2):109–113. [DOI] [PubMed] [Google Scholar]
- 55. McVige JW, Leonardo J. Imaging of Chiari type I malformation and syringohydromyelia. Neurol Clin. 2014;32(1):95–126. [DOI] [PubMed] [Google Scholar]
- 56. Bogdanov EI, Faizutdinova AT. Epidemiology. In: Flint G, Rusbridge C, eds. Syringomyelia. Berlin: Springer-Verlag; 2014:11–24. [Google Scholar]
- 57. Kahn EN, Muraszko KM, Maher CO. Prevalence of Chiari I malformation and syringomyelia. Neurosurg Clin N Am. 2015;26(4):501–507. [DOI] [PubMed] [Google Scholar]
- 58. Bogdanov EI, Mendelevich EG. Syrinx size and duration of symptoms predict the pace of progressive myelopathy: retrospective analysis of 103 unoperated cases with craniocervical junction malformations and syringomyelia. Clin Neurol Neurosurg. 2002;104(2):90–97. [DOI] [PubMed] [Google Scholar]
- 59. Sirotkin VM, Pazoni I, Gimadeeva PM. The probable character of heredity in familial syringomyelia. Zh Nevropatol Psikhiatr Im S S Korsakova. 1973;73(6):831–836. [PubMed] [Google Scholar]
- 60. Sirotkin VM. Regional peculiarities of syringomyelia. Genetica. 1970;6(6):166–172. [Google Scholar]
- 61. Statistics on the Health of Population and Healthcare of the Republic of Tatarstan for 1997-2001. Kazan, Russia: Republic of Tatarstan; 2002:1- 234. [Google Scholar]
- 62. Statistics on the Health of Population and Healthcare of the Republic of Tatarstan for 2001-2005. Kazan, Russia: Republic of Tatarstan; 2006:1- 276. [Google Scholar]
- 63. Statistics on the Health of Population and Healthcare of the Republic of Tatarstan for 2005-2009. Kazan, Russia: Republic of Tatarstan; 2010:1- 267. [Google Scholar]
- 64. Mueller DM, Oro JJ. Prospective analysis of presenting symptoms among 265 patients with radiographie evidence of Chiari malformation type I with or without syringomyelia. J Amer Acad Nurse Practitioners. 2004;16(3):134–138. [DOI] [PubMed] [Google Scholar]
- 65. Deng X, Wang K, Wu L et al.. Asymmetry of tonsillar ectopia, syringomyelia and clinical manifestations in adult Chiari I malformation. Acta Neurochir. 2014;156(4):715–722. [DOI] [PubMed] [Google Scholar]
- 66. Tubbs RS, Wellons JC 3rd, Oakes WJ. Asymmetry of tonsillar ectopia in Chiari I malformation. Pediatr Neurosurg. 2002;37(4):199–202. [DOI] [PubMed] [Google Scholar]
- 67. Tubbs RS. Definitions and anatomic considerations in Chiari I malformation and associated syringomyelia. Neurosurg Clin N Am. 2015;26(4):487–493. [DOI] [PubMed] [Google Scholar]
- 68. Borisova NA, Mirsaev TR. Syringomyelia in Bashkortostan: epidemiologic and pathogenesis data. Zh Nevrol Psikhiatr Im S S Korsakova. 2007;107(3):56–60. [PubMed] [Google Scholar]
- 69. Borisova NA, Vilikova IV, Kutchaeva GA. Syringomyelia. Medicina (B Aires). 1989. [Google Scholar]
- 70. Moncho D, Poca MA, Minoves T, Ferre A, Canas V, Sahuquillo J. Are evoked potentials clinically useful in the study of patients with Chiari malformation type 1? J Neurosurg. 2017;126(2):606–619. [DOI] [PubMed] [Google Scholar]
- 71. Brewis M, Poskanzer DC, Rolland C, Miller H. Neurological disease in an English city. Acta Neurol Scand. 1966;42(S24):1–89. [PubMed] [Google Scholar]
- 72. Brickell KL, Anderson NE, Charleston AJ, Hope JK, Bok AP, Barber PA. Ethnic differences in syringomyelia in New Zealand. J Neurol Neurosurg Psychiatry. 2006;77(8):989–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sakushima K, Tsuboi S, Yabe I et al.. Nationwide survey on the epidemiology of syringomyelia in Japan. J Neurol Sci. 2012;313(1-2):147–152. [DOI] [PubMed] [Google Scholar]
- 74. Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92(6):920–926. [DOI] [PubMed] [Google Scholar]
- 75. Vernooij MW, Ikram MA, Tanghe HL et al.. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 76. Hertel G, Ricker K. A geomedical study on the distribution of syringomyelia in Germany. In: Hartog JWA, Bruyn GW, Heijstee APJ, eds. Neurology: Proceeding of the 11th World Congress of Neurology. Vol 434 Amsterdam: Excerpta Medica; 1978:363–365. [Google Scholar]
- 77. da Silva JA, dos Santos AA Jr, Melo LR, de Araujo AF, Regueira GP. Posterior fossa decompression with tonsillectomy in 104 cases of basilar impression, Chiari malformation and/or syringomyelia. Arq Neuropsiquiatr. 2011;69(5):817–823. [DOI] [PubMed] [Google Scholar]
- 78. Goel A. Basilar invagination, Chiari malformation, syringomyelia: a review. Neurol India. 2009;57(3):235–246. [DOI] [PubMed] [Google Scholar]
- 79. Abbott D, Brockmeyer D, Neklason DW, Teerlink C, Cannon-Albright LA. Population-based description of familial clustering of Chiari malformation type I. J Neurosurg. 2018;128(2);460–465. [DOI] [PubMed] [Google Scholar]