Abstract
STUDY QUESTION
What is the peripubertal outcome of recombinant human FSH (r-hFSH) treatment during minipuberty in boys with congenital hypogonadotropic hypogonadism (CHH)?
SUMMARY ANSWER
Sertoli-cell response to r-hFSH, given during the minipuberty of infancy, appears insufficient to maintain Sertoli cell function throughout childhood, as evaluated by inhibin B measurements.
WHAT IS KNOWN ALREADY
Severe CHH in boys can be diagnosed during the minipuberty of infancy. Combined gonadotropin treatment at that age is suggested to improve testicular endocrine function and future fertility, yet long-term evidence is lacking.
STUDY DESIGN, SIZE, DURATION
In this retrospective cohort study, we describe five CHH boys treated with r-hFSH in Helsinki University Hospital or Kuopio University Hospital between 2004 and 2018. Immediate follow-up data (0.1–1.4 months after cessation of the gonadotropin therapy) was available for four boys and long-term observations (at the age of 10.0–12.8 years) was available for three boys. As a retrospective control cohort, we provide inhibin B values of eight untreated CHH boys at the age of 12.7–17.8 years.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Four patients had combined pituitary hormone deficiency, and one had CHARGE syndrome due to a CHD7 mutation. The patients were treated at the age of 0.7–4.2 months with r-hFSH (3.4 IU/kg–7.5 IU/kg per week in 2 or 3 s.c. doses for 3–4.5 months) combined with T (25 mg i.m. monthly for three months for the treatment of micropenis). Inhibin B was chosen as the primary outcome measure.
MAIN RESULTS AND THE ROLE OF CHANCE
During the r-hFSH + T treatment, inhibin B increased from 76 ± 18 ng/l to 176 ± 80 ng/l (P = 0.04) and penile length increased by 81 ± 50% (P = 0.04). Unexpectedly, two boys with robust inhibin B responses in infancy demonstrated low inhibin B values in peripuberty: declining from 290 ng/l (4 months) to 16 ng/l (12.4 years), and from 207 ng/l (6 months) to 21 ng/l (12.8 years). All boys underwent orchiopexy at 2.0 ± 0.7 years of age. Inhibin B values in long-term follow-up, available for the three boys, did not significantly differ from the untreated CHH controls.
LIMITATIONS, REASONS FOR CAUTION
Limitations of this retrospective study are the small number and heterogeneity of the patients and their treatment schemes.
WIDER IMPLICATIONS OF THE FINDINGS
We describe the first long-term follow-up data on CHH boys treated with r-hFSH and T as infants. The results from this small patient series suggest that the effects of infant r-hFSH treatment may be transient, and further longitudinal studies are required to determine the efficacy of this treatment approach to optimise the fertility potential in this patient population.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by the Finnish foundation for Pediatric Research, the Academy of Finland and the Emil Aaltonen Foundation. The authors have no competing interests.
TRIAL REGISTRATION NUMBER
Non-applicable.
Keywords: CHH, combined pituitary hormone deficiency, infant, testosterone, FSH, inhibin b, micropenis
Introduction
Congenital hypogonadotropic hypogonadism (CHH), a rare cause of delayed puberty and male infertility, is seldom diagnosed in infancy (Boehm et al., 2015). However, in any newborn the presence of micropenis, and bilaterally undescended testes should prompt the evaluation of the anterior pituitary function (Grumbach, 2005; Wiygul and Palmer, 2011). If anterior pituitary hormone deficiencies are diagnosed after a thorough work-up (including hormonal testing and MRI imaging), the treatment is replacement of the missing hormone (GH) or the missing end-organ hormones (thyroxine, cortisol) (Higham et al., 2016; Pierce and Madison, 2016). However, controversy exists about the early treatment of CHH (Bouvattier et al., 2011; Boehm et al., 2015; Dwyer et al., 2016). The hypothalamic-pituitary-gonadal (HPG) axis is operative already in utero, and becomes quiescent towards term (Bouvattier et al., 2011). During the first 24 hours of life, there is a brief luteinising hormone (LH) surge (Corbier et al., 1990), whereas a comprehensive re-activation of the HPG axis ensues after the first week of life and lasts for approximately 6 months (Andersson et al., 1998; Grumbach, 2005). During this period in life, also known as the minipuberty of infancy, serum gonadotropin and testicular hormone levels (Leydig cell markers: testosterone and INSL3; Sertoli cell markers: inhibin B and AMH) increase, and penile length and testicular volume grow in size (Andersson et al., 1998; Main et al., 2006; Kuiri-Hänninen et al., 2014). The latter is probably a surrogate of the increase in the number of immature Sertoli cells (Cortes et al., 1987; Bouvattier et al., 2011), which proliferate in response to FSH (Orth et al., 1988; Arslan et al., 1993; Meachem et al., 1996). Since these cells express androgen receptor only weakly in infants, LH-induced endogenous testosterone does not mature the Sertoli cells or activate spermatogenesis (Chemes et al., 2008; Rey et al., 2009). Given that the number of Sertoli cells correlates with sperm-producing capacity later in life (Johnson et al., 1984; Orth et al., 1988), the minipuberty of infancy may serve as a mechanism to ensure future reproductive capacity (Sharpe et al., 2003; Dwyer et al., 2016). This is therefore conceivably the strongest argument favouring replacement therapy for the missing gonadotropin stimulus in boys with severe congenital HH (Bouvattier et al., 2011). Only few reports, however, describe the outcome of gonadotropin treatment in boys with isolated CHH or CHH as a part of combined pituitary hormone deficiency (CPHD) during the first year of life (Table I) (Main et al., 2002; Bougnères et al., 2008; Sarfati et al., 2015; Lambert and Bougneres, 2016; Stoupa et al., 2017). Although the results from these studies show that gonadotropins increase testicular volume and probably stimulate Sertoli cell proliferation, there are currently no long-term follow-up data on these markers.
Table I.
Previous studies on gonadotropin treatment during minipuberty
| Reference | Treatment | Patient | Diagnosis | Crypt-orchidism | Micro-penis | Age (mo) | Treatment duration (mo) | Before treatment | During/after treatment (highest value) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inh-B (ng/L) | TV (mL) | Inh-B (ng/L) | TV (mL) | ||||||||
| Main et al. (2002) | FSH 21.3 IU x2/week, LH 20–40 IU x2/week, T suppositories 1 mg/day | 1 | CHH | No | Yes | 7.9 | FSH 6, LH 3.5, T 1.5 | 121 | 0.3 | 268a | 0.8 |
| Bougnères et al. (2008) | FSH 67–125 IU daily, LH 50–56 IU daily | 1 | CPHD | No | Yes | 1.9 | 4 | 167 (32)b | 0.6 | 701 (284)b | 2.1 |
| 2 | CHH | No | Yes | 4.7 | 7 | 48 (9)b | 0.5 | 426 (189)b | 2.1 | ||
| Sarfati et al. (2015) | FSH 75 IU daily, LH 75 IU daily | 1 | CHH | No | Yes | 1 | 6 | 24 | 0.3 | NA | 2.3 |
| Lambert and Bougneres (2016)c | FSH 75–150 daily, LH 50 daily | 1 | CPHD | Yes | Yes | 6 | 6 | 5 | NA | 55 | 0.6 |
| 2 | CPHD | Yes | Yes | 11 | 6 | 100 | NA | 505 | 2.0 | ||
| 3 | CPHD | Yes | NA | 10 | 6 | 155 | NA | 544 | 2.1 | ||
| 4 | CHH | Yes | Yes | 4.5 | 6.5 | 91 | NA | 111 | 0.8 | ||
| 5 | CHH | Yes | Yes | 2.5 | 6.5 | 73 | NA | 401 | 0.7 | ||
| 6 | CHH | Yes | Yes | 9 | 5 | 5 | NA | 287 | 1.2 | ||
| 7 | CHH | Yes | NA | 5 | 5 | 64 | NA | 514 | 0.9 | ||
| 8 | CHH | Yes | Yes | 0.25 | 6 | 14 | NA | 530 | 2.0 | ||
| Stoupa et al. (2017) | FSH 75 daily, LH 75–150 dailyd | 1 | CHH | Yes | Yes | 4.5 | 6 | 95 (75)e | 0.7 (SD not reported)e | 469 (283)e | 2.2 (SD not reported)e |
| 2 | CPHD | No | Yes | 5.5 | 3 | ||||||
| 3 | CHH | Yes | Yes | 4.5 | 4 | ||||||
| 4 | CHH | Yes | Yes | 3 | 3 | ||||||
| 5 | CHH | Yes | Yes | 3.5 | 5 | ||||||
Inh-B Inhibin B, TV testicular volume, T testosterone, CHH congenital hypogonadotropic hypogonadism, CPHD combined pituitary hormone deficiency.
aMeasured during FSH + LH treatment, further increase as LH was replaced with T.
bMean (SD) for pretreatment and treatment periods.
cMicropenis is reported in this table based on penile lenght provided in the original article, if unequivocal (reference values: Boas et al., 2006).
dPatient #1, only moderately responding to LH/FSH therapy, received im. T 100 mg/m2/every two weeks for 2 months.
eNo individual data, only mean (SD) for patient series available.
Herein, we report five CHH patients treated with recombinant human FSH (r-hFSH) and testosterone (T) during the first year of life and describe the long-term treatment responses in three of them. Our hypothesis was that short-term neonatal treatment with r-hFSH would result in higher inhibin B values in peripuberty compared to the untreated CHH patients. To test this, we present a retrospective control group of eight adolescent CHH boys with no prior gonadotropin treatment.
Materials and Methods
Patients
We reviewed the electronic patient records of five CHH boys, who were treated in two Finnish pediatric tertiary centres (Helsinki University Hospital and Kuopio University Hospital) between 2004 and 2018. We describe the clinical characteristics of these patients in detail in Table II. All five boys were born at term and had normal birth weight and birth length. CHH was diagnosed due to an undervirilised phenotype (penile length 2.5 SD below average in all boys (Boas et al., 2006)) and low testosterone and gonadotropin levels during minipuberty before the age of three months (Grumbach, 2005). One boy had CHH as a part of CHARGE syndrome and the remaining four boys had it as a part of CPHD. The four CPHD patients had ACTH, growth hormone and TSH deficiencies, which were adequately substituted with hydrocortisone, L-thyroxine and growth hormone (12–15 mg/m2, 3–12 μg/kg and 20–34 μg/kg/d, respectively).
Table II.
Clinical characteristics of five CHH boys treated with r-hFSH and T during minipuberty.
| Hormonal | Cryptorchidism | Diagnostic hormonal measurements for CHH | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Diagnosis | deficiencies | Micropenis | at birth | Brain MRI finding | FSH | LH | T |
| #1 | CHH; CHARGE | FSH, LH | Yes | No | hypolastic olfactory bulbs, rudimentary vestibular organs, deviancies in median line structures | 0.2 | <0.1 | <0.2 |
| #2 | CPHD | FSH, LH, ACTH, TSH, GH | Yes | bilateral | selective hypothalamus-pituitary developmental disorder | <0.1 | <0.1 | 0.3 |
| #3 | CPHD | FSH, LH, ACTH, TSH, GH | Yes | bilateral | ectopic posterior pituitary, pituitary hypoplacia | <0.1 | <0.1 | <0.2 |
| #4 | CPHD | FSH, LH, ACTH, TSH, GH | Yes | No | partially ectopic posterior pituitary, optic infundibular dysplasia, pituitary stalk interruption | <0.1 | 0.2 | <0.35 |
| #5 | CPHD; SOD | FSH, LH, ACTH, TSH, GH | Yes | No | ectopic posterior pituitary, hypoplastic optic nerves | 0.9 | NA | 0.1 |
| Normal values median (range)a | 1.79 (0.09–2.93) | 1.74 (0.90–2.64) | 4.02 (1.83–6.54) | |||||
CHH congenital hypogonadotropic hypogonadism, CHARGE syndrome (acronym from coloboma, heart defects, atresia of the choanae, retarded growth and development, genital hypoplasia and ear abnormalities), CPHD combined pituitary hormone deficiency, SOD septo-optic dysplasia.
aReference values (Andersson et al., 1998).
In all patients, serum inhibin B was measured before (range, 3 days to 2.1 months) and during the treatment, and the maximal value during the treatment was recorded. Follow-up data on inhibin B were available from four boys 0.7–1.4 months after the treatment, and long-term data were available from three boys at the age of 10–12.8 years. Testicular ultrasound was performed repeatedly (one to four times) for all patients. At clinical visits, genital status was determined by palpation, testis dimensions were measured with a ruler and testis volume was calculated with the formula [length × width2 × 0.52]. Penile length was measured from the pubo-penile skin junction to the tip of the glans.
Treatment
Table III depicts the individual treatment schedules of the five patients. Recombinant hFSH, combined with T for the treatment of micropenis, was initiated at the age of 2.5 ± 1.3 months (range from 0.7 months to 4.2 months). Patients received subcutaneous injections of r-hFSH for 3 to 4.5 months (Puregon®) (dose, 3.4 IU/kg to 7.5 IU/kg per week in two or three 7.5 IU to 16.7 IU doses) and intramuscular injections of testosterone enanthate for three months (Testoviron®, Sustanon®) (25 mg once a month). For patient #4, T was introduced to the treatment 0.7 months after the initiation of r-hFSH-treatment.
Table III.
Treatment schemes of five infant CHH boys.
| Age at onset of | Treatment | ||
|---|---|---|---|
| Patient | treatment (mo) | FSH | Testosterone |
| #1 | 4.2 | 16.6 IU x2/week for 3 mo | 25 mg x1/mo for 3mo |
| #2 | 0.7 | 8.3 IU x2/week for 3 mo | 25 mg x1/mo for 3mo |
| #3 | 3.1 | 8.3 IU x2/week for 3 mo | 25 mg x1/mo for 3mo |
| #4 | 1.3 | 7.5 IU x3/week for 3.8 mo | 25 mg x1/mo for 3mo |
| #5 | 3.3 | 16.7 IU x2/week for 4.5 mo | 25 mg x1/mo for 3mo |
Currently, no dose finding studies exist on gonadotropin treatment in infancy. The r-hFSH dose used in the this study was guided by our prior work, in which prepubertal boys were given r-hFSH 4.5 IU/kg per week divided in three doses, leading to testis growth and increasing inhibin B levels (Raivio et al., 1997).
Control group and background data
To examine whether CHH boys treated with r-hFSH in infancy would have higher inhibin B levels in long-term follow-up than CHH boys with absent minipuberty and no prior gonadotropin treatment, we assembled a retrospective control group of CHH boys, whose inhibin B was measured between the ages of 12.7 to 17.8 years. Their testis volume was below 2.5 mL or their testis length was less than 1 cm. Patients in the control group are described in detail in Table IV. The control patients were treated in Helsinki University Hospital, Kuopio University Hospital or Turku University Hospital. One of the patients (C8) was diagnosed with CHH as a part of CPHD at the age of one month due to low gonadotropin and testosterone levels. The remaining seven boys had no hormonal measurements in infancy available, but they presented other signs of absent minipuberty: six had bilateral cryptorchidism and/or micropenis, and one was diagnosed with biallelic loss-of-function GNRHR mutation (C2) with completely absent LH response in a GnRH stimulation test (Kohva et al. 2018). We have earlier described induction of puberty with gonadotropin treatment in five of these boys (patients C1 to C5) (Kohva et al., 2018) and the pubertal status of two of the boys (patients C6 and C7) (Varimo et al., 2017).
Table IV.
Control group of eight adolescent CHH boys with no prior gonadotropin treatment.
| Patienta | Diagnosis (mutation) | Cryptorchidism | Micro-penis | Inhibin B (ng/l) | Age at inhibin B measurement (yr) |
|---|---|---|---|---|---|
| C1 | KS (ANOS1 c.571 C > T (p.R191X)) | Bilateral | No | 11 | 14.6 |
| C2 | nCHH (biallelic GNRHR (p.R139H)) | No | No | 14 | 15.1 |
| C3 | KS (2 biallelec PROKR2 c.701 G > A (p.G234D) and c.802 C > T (p.R268C)) | No | Yes | 16 | 17.8 |
| C4 | KS (FGFR1 (p.Gly687Arg)) | Unilateral | Yes | 24 | 14.6 |
| C5 | KS (ANOS1 c.571 C > T (p.R191*)) | Unilateral | Yes | 11 | 12.7 |
| C6 | KS (FGFR1 c.1305_1306dupAT(p.S436YfsX3)) | Bilateral | No | 44 | 13.7 |
| C7 | nCHH, CHARGE | Bilateral | No | 13 | 14.3 |
| C8 | CPHD, SOD | Unilateral | No | 17 | 14.4 |
KS Kallmann syndrome, nCHH normosmic congenital hypogonadotropic hypogonadism, CPHD combined pituitary hormone deficiency SOD septo-optic dysplasia, CHARGE syndrome (acronym from coloboma, heart defects, atresia of the choanae, retarded growth and development, genital hypoplasia and ear abnormalities)
aPatients C1 to C5 are described previously by Kohva et al. (2018) and patients C6 to C7 by Varimo et al. (2017).
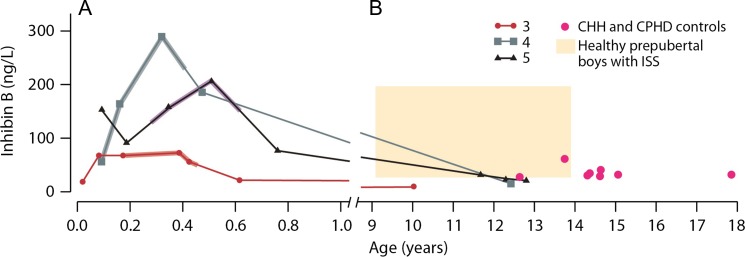
In addition, we present background data of 27 boys with idiopathic short stature (ISS) aged between 9.1 and 13.9 years in stage G1 with testicular volume below 2 mL and treated in our clinic (Hero et al., 2005). In this group, serving here as a reference of inhibin B in healthy prepubertal boys, the mean Inhibin B was 88 ± 34 ng/L (range, 38 to 196 ng/L) (Fig. 1). No abnormalities of the HPG-axis were found in any of the ISS boys at the time of inhibin B measurement (Hero et al., 2005) or in the long-term (Varimo T, unpublished results).
Figure 1.
Infant r-hFSH treatment responses and long-term outcomes in three boys with congenital hypogonadotropic hypogonadism (CHH). The lines represent individual inhibin B levels in three patients with CHH (patients #3 to #5 in Tables II, III and V). Recombinant hFSH [r-hFSH] treatment, shown in thickened lines for each patient, was initiated at the age of 0.2 ± 0.1 years: r-hFSH was administered at 3.4 IU/kg to 7.5 IU/kg s.c. per week in two to three doses for 3–4.5 months and testosterone was administered at 25 mg i.m. once a month for three months. Panel A describes the first year of life of these three treated boys, and panel B the development of their inhibin B values after the age of 9 years. The red dots in panel B mark inhibin B values of eight boys in an untreated CHH and CPHD control group (Table IV, Varimo et al., 2017; Kohva et al., 2018). The beige box in panel B represents, as a reference, the range of inhibin B in 29 healthy prepubertal boys with idiopathic short stature aged between 9.1 and 13.9 years in stage G1 with testicular volume below 2 mL (Hero et al., 2005)
Hormonal assays
Testosterone at the time of diagnosis was measured with radioimmunoassay (Lipidex-5000) until 2005, and with tandem mass spectrometer thereafter (API 2000 and API 3000 (AB Sciex, Concord, ON, Canada)). Gonadotropins were measured with AutoDELFIA (Wallac, Turku, Finland) until 2011, when an electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) was introduced. In the latter, the detection limit of the LH assay was 0.1 IU/l and inter-assay CV was less than 3%, whereas in the FSH assay, the corresponding numbers were 0.1 IU/l and less than 5%. Inhibin B levels were determined with OBI INHB ELISA (MCA1312KZZ, OBI-DSL, Upper Heyford, UK), replaced with INHB Gen II ELISA (A81303) and INHB Gen II Calibrators and Controls (A81304) (Beckman Coulter, Inc., CA, USA) in 2010. The current method for measuring inhibin B has intra- and inter-assay coefficients of variation of 3.8% and 5.6% and the laboratory of Helsinki University Hospital reports values exceeding 10 ng/L. When compared, the current Inhibin B assay showed only 7% lower inhibin B levels than the OBI ELISA.
Statistics
Changes in inhibin B and penile length during r-hFSH + T treatment were assessed with Wilcoxon signed-rank test. Differences in prepubertal inhibin B values of patients treated in infancy (n = 3) and patients of the CHH and CPHD control group (n = 8) were assessed with Mann-Whitney U-test. Inhibin B levels in cryptorchid patients treated with r-hFSH in infancy (current study, n = 3) were compared to patients with cryptorchidism in the control group (n = 6) with Mann-Whitney U-test. The data are presented with a mean (standard deviation) unless otherwise stated. P < 0.05 was accepted to indicate statistical significance. Statistical analyses were performed with SPSS statistical software for Windows, version 22.0 (SPSS, Chicago, IL, USA).
Ethics
The Ethics Committee of the Helsinki University Hospital has approved this electronic, patient-record-based retrospective study, and the research permits were granted by the Helsinki University Hospital and Kuopio University Hospital.
Results
Hormonal levels and genital development before, during and after r-hFSH and T treatment in the five patients are listed in Table V.
Table V.
Clinical characteristics and serum concentration of hormones in five CHH boys treated with r-hFSH + T in infancy.
| Before treatment | During treatment | After treatmenta | Follow-upb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Inhibin B (ng/l) | FSH (IU/l) | Testis size | Penile length (mm) | Max inhibin B (ng/l) | Max FSH (IU/l) | Inhibin B (ng/l) | Testis size | Age at orchiopexy (mo) | Penile length (mm) | Inhibin B (ng/l) |
| #1 | 66 | 0.2 | 0.12 ml | 14 | 169 | 3.5 | NA | 0.1 ml | 16 | 31 | NA |
| #2 | 101 | <0.1 | NA | 10 | 139 | NA | 62 | 10 mm la | 22 | 24 | NA |
| #3 | 68 | <0.1 | 10 mm la | 15 | 74 | 0.4 | 22 | 4 mm la | 37 | 24 | 10 |
| #4 | 57 | <0.1 | 0.15 ml | 24 | 290 | 3.4 | 186 | 0.4 ml | 26 | 35 | 16 |
| #5 | 90 | 0.9 | NA | 23 | 207 | 2.1 | 77 | 13 mm l.sin | 17 | 30 | 21 |
aWithin 2 months after cessation of treatment.
bAfter a 9.5–12.2 year interim period.
Penile length increased from 17 ± 6 mm to 29 ± 5 mm (81 ± 50%, P = 0.04). After 3 months of T-treatment, none of the boys required further testosterone treatment for micropenis. Of the five boys treated with r-hFSH and T, two had bilateral cryptorchidism at birth (patients #2 and #3). The remaining three boys had scrotal testes at birth and exhibited ascent of the testes at the age of 13 ± 10 months, one of them (patient #1) before and two (patients #4 and #5) after r-hFSH treatment. All five boys underwent bilateral orchiopexy at the age of 2.0 ± 0.7 years.
The r-hFSH treatment markedly increased inhibin B levels from 76 ± 18 ng/l to 176 ± 80 ng/l (P = 0.04), and at 1.2 ± 0.4 months after cessation of therapy the levels had decreased to 87 ± 70 ng/l (data unavailable for patient #1). Long-term follow-up (11.2 ± 1.5 years) of inhibin B levels was available for three boys. Interestingly, the rise in inhibin B levels in response to r-hFSH given in infancy appeared to subside completely (Fig. 1). Two of the boys with a marked inhibin B response in infancy demonstrated declined values from 290 ng/l to 16 ng/l (patient #4; age 12.4 years) and from 207 ng/l to 21 ng/l (patient #5; age 12.8 years). The third boy (patient #3) showed a decline of inhibin B from 74 ng/l during the treatment to 10 ng/l by the age of 5.6 years. At that time, 2.6 years after bilateral orchiopexy, the ultrasound detected no vascular network in his testicular structures. Although his inhibin B levels remained at the detection limit of 10 ng/l in follow-up, AMH levels were measurable (0.33 to 0.6 μg/l at a lower detection limit of 0.03 μg/l), suggesting some persistent Sertoli cell activity. We compared the long-term follow-up inhibin B values of patients #3 to #5 to a cohort of prepubertal CHH and CPHD boys (n = 8, Table IV), who had not received r-hFSH treatment during their minipuberty. Their mean inhibin B level was 19 ± 11 ng/l (range from 11 ng/l to 44 ng/l) at the age of 14.7 ± 1.5 years. The inhibin B values in the three r-FSH-treated patients (inhibin B 16 ± 6 ng/l) did not differ from inhibin B values in the untreated control group (Fig. 1). As all the boys treated in infancy had cryptorchidism, we limited the comparison to the cryptorchid patients in the control group (n = 6, inhibin B 20 ± 13 ng/l) to account for the possible confounding effect, but there still was no significant difference between the two groups.
Discussion
The current study is, to our knowledge, the first long-term follow-up report on boys with CHH treated with recombinant FSH in infancy. Treatment responses to gonadotropins administered in infancy in terms of testicular growth and Sertoli cell markers had been reported in only 17 patients, summarised in Table I (Main et al., 2002; Bougnères et al., 2008; Sarfati et al., 2015; Lambert and Bougneres, 2016; Stoupa et al., 2017). In 2002, Main et al. described a 7.9-month-old CHH male treated with a moderate dose of gonadotropins (FSH 21.3 IU and LH 20 IU) twice a week for six months with a good response in inhibin B and testis size. Thereafter, several studies with larger daily FSH and LH doses (FSH 67–150 IU and LH 50–150 IU) have shown further promising results in CHH and CPHD patients (Bougnères et al., 2008; Sarfati et al., 2015; Lambert and Bougneres, 2016; Stoupa et al., 2017). Thus, these studies reporting the immediate outcomes of infant FSH treatment suggest that mimicking the physiologic gonadotropin secretion of minipuberty holds promises for improvement of fertility and testicular endocrine function (Bouvattier et al., 2011).
It has been unknown, however, whether FSH treatment during infancy provides long-lasting advantages in terms of adult male reproductive health (Bouvattier et al., 2011). In this study we chose inhibin B as the primary outcome since: (i) it has been used in previous studies describing short-term FSH responses in infants with CHH (Table I); (ii) it has been shown to reflect FSH action of immature Sertoli cells (Anawalt et al., 1996; Raivio et al., 1997; Grinspon et al., 2018); (iii) the physiological changes in inhibin B during the first few years of life and in peripuberty have been described (Andersson et al., 1997, 1998; Hero et al., 2012); and (iv) inhibin B was the clinicians’ choice more than 10 years ago (i.e. at the beginning of this study period) and currently (Kohva et al. 2018) to monitor their patients on gonadotropin treatment. The goal of gonadotropin treatment in infancy is to mimic the physiological actions of minipuberty, where inhibin B first reaches values comparable to those of adult males and then decreases, but persists at measurable levels (Andersson et al., 1998). Low inhibin B is one of the acceptable markers to discriminate CHH boys from peers with constitutional delay of growth and puberty (Varimo et al., 2017). We thus hypothesised that r-hFSH treatment in infant CHH boys would result in higher, more physiological levels of inhibin B in childhood compared to untreated CHH patients, but the current data did not support this hypothesis.
Three boys in the current study treated with r-hFSH and T in infancy had very low inhibin B values in the long-term follow-up. Two of them had a particularly good inhibin B response to r-hFSH but very low levels in peripuberty. This suggests that the treatment response in inhibin B, indicating increase in Sertoli cell number and activity, might not be persistent over a time-period of more than 10 years. The data on patient #3 with a low inhibin B response to r-hFSH as an infant, bilateral cryptorchidism, extremely small testis size of 4 mm measured after treatment during orchiopexy and low inhibin B in the long-term follow-up do not support long-lasting effects of infant r-hFSH treatment either. It has to be noted, however, that we do not know whether inhibin B reflects the assets of the infant gonadotropin treatment sufficiently. More longitudinal data on other parameters, such as sperm count after induction of puberty, are needed.
It is unclear what kind of treatment scheme should be followed after the infant gonadotropin treatment towards induction of puberty in these patients. For example, we speculate that continuous low-dose FSH stimulus during childhood should be considered to sustain both Sertoli cell function and number. The rationale behind this approach is that FSH secretion in healthy prepubertal boys is not unmeasurably low (Dunkel et al., 1990; Wu et al., 1991) and Sertoli cells appear responsive for FSH throughout the childhood hiatus of low gonadotropin secretion in primates (Rey et al., 1993) and humans (Raivio et al., 1997). Indeed, Countant et al. were able to associate continuous activation of G protein-coupled receptors (likely including those of FSHR) in McCune-Albright syndrome with blunted FSH response to GnRH stimulation, elevated inhibin B and macro-orchidism (Coutant et al., 2001), all consistent with active proliferation of immature Sertoli cells.
Limitations of this study arise from heterogeneity of the patients, variation in their treatment schemes, two different measurement techniques (ruler and ultrasound) for testicular size determination and the lack of testis measurements in some of the patients. Dose-finding studies on this topic are currently lacking: we do not know whether a larger r-hFSH dose would result in more notable changes in inhibin B or testis volume. It is also important to acknowledge that it is not known whether increasing the intratesticular testosterone with LH or hCG would produce more sustainable results in terms of Sertoli cell function. Furthermore, controversy exists on whether hormonal intervention (GnRH, hCG or LH) should be considered for bilateral cryptorchidism in CHH patients, although the Nordic consensus recommends surgical treatment (Ritzén et al., 2007; Bu et al., 2016; Lambert and Bougneres, 2016). All of the five patients in this series treated with r-hFSH and T required bilateral orchiopexy, three of them due to ascent of the testes. Given the small number of the patients, the results of this retrospective study should be interpreted with appropriate caution.
In summary, all five CHH boys responded to r-hFSH and T treatment with increased inhibin B levels and penile length. According to these data, exogenous T does not inhibit the Sertoli cell activity. However, the long-term data suggest that the effects of infant r-hFSH treatment on Sertoli cell function may be transient. More longitudinal studies are clearly required to address the effect of this treatment approach on future fertility and to optimise the hormonal supplementation therapy of infant patients with CHH, small testis size and cryptorchidism, as such patients are at the greatest risk of infertility (Liu et al., 2009; Rastrelli et al., 2014).
Acknowledgements
We thank Professor Jorma Toppari for commenting the manuscript.
Authors’ roles
EK, PJM and TR designed the study concept. EK, HH and JH attained the data while MH, PJM and TR provided input into the interpretation of the data. All authors contributed in drafting and revising the article and approved the final version to be published.
Funding
This work was supported by the Finnish foundation for Pediatric Research, the Academy of Finland and the Emil Aaltonen Foundation.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Anawalt BD, Bebb RA, Matsumoto AM, Groome NP, Illingworth PJ, McNeilly AS, Bremner WJ. Serum inhibin B levels reflect Sertoli cell function in normal men and men with testicular dysfunction. J Clin Endocrinol Metab 1996;81:3341–3345. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Juul A, Petersen JH, Müller J, Groome NP, Skakkebaek NE. Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone, and estradiol levels. J Clin Endocrinol Metab 1997;82:3976–3981. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Toppari J, Haavisto AM, Petersen JH, Simell T, Simell O, Skakkebaek NE. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab 1998;83:675–681. [DOI] [PubMed] [Google Scholar]
- Arslan M, Weinbauer GF, Schlatt S, Shahab M, Nieschlag E. FSH and testosterone, alone or in combination, initiate testicular growth and increase the number of spermatogonia and Sertoli cells in a juvenile non-human primate (Macaca mulatta). J Endocrinol 1993;136:235–243. [DOI] [PubMed] [Google Scholar]
- Boas M, Boisen KA, Virtanen HE, Kaleva M, Suomi A-M, Schmidt IM, Damgaard IN, Kai CM, Chellakooty M, Skakkebaek NE et al. . Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1962 normal boys. Eur J Endocrinol 2006;154:125–129. [DOI] [PubMed] [Google Scholar]
- Boehm U, Bouloux P-M, Dattani MT, Roux N, de, Dodé C, Dunkel L, Dwyer AA, Giacobini P, Hardelin J-P, Juul A et al. . Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism—pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 2015;11:547–564. [DOI] [PubMed] [Google Scholar]
- Bougnères P, François M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J Clin Endocrinol Metab 2008;93:2202–2205. [DOI] [PubMed] [Google Scholar]
- Bouvattier C, Maione L, Bouligand J, Dodé C, Guiochon-Mantel A, Young J. Neonatal gonadotropin therapy in male congenital hypogonadotropic hypogonadism. Nat Rev Endocrinol 2011;8:172–182. [DOI] [PubMed] [Google Scholar]
- Bu Q, Pan Z, Jiang S, Wang A, Cheng H. The effectiveness of hCG and LHRH in boys with cryptorchidism: a meta-analysis of randomized controlled trials. Horm Metab Res 2016;48:318–324. [DOI] [PubMed] [Google Scholar]
- Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, González-Peramato P, Serrano A. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. J Clin Endocrinol Metab 2008;93:4408–4412. [DOI] [PubMed] [Google Scholar]
- Corbier P, Dehennin L, Castanier M, Mebazaa A, Edwards DA, Roffi J. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab 1990;71:1344–1348. [DOI] [PubMed] [Google Scholar]
- Cortes D, Müller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl 1987;10:589–596. [DOI] [PubMed] [Google Scholar]
- Coutant R, Lumbroso S, Rey R, Lahlou N, Venara M, Rouleau S, Sultan C, Limal JM. Macroorchidism due to autonomous hyperfunction of Sertoli cells and G(s)alpha gene mutation: an unusual expression of McCune-Albright syndrome in a prepubertal boy. J Clin Endocrinol Metab 2001;86:1778–1781. [DOI] [PubMed] [Google Scholar]
- Dunkel L, Alfthan H, Stenman U-H, Perheentupa J. Gonadal control of pulsatile secretion of luteinizing hormone and follicle-stimulating hormone in prepubertal boys evaluated by ultrasensitive time-resolved immunofluorometric assays. J Clin Endocrinol Metab 1990;70:107–114. [DOI] [PubMed] [Google Scholar]
- Dwyer AA, Jayasena CN, Quinton R. Congenital hypogonadotropic hypogonadism: implications of absent mini-puberty. Minerva Endocrinol 2016;41:188–195. [PubMed] [Google Scholar]
- Grinspon RP, Urrutia M, Rey RA. Male central hypogonadism in paediatrics—the relevance of follicle-stimulating hormone and sertoli cell markers. Eur Endocrinol 2018;14:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab 2005;90:3122–3127. [DOI] [PubMed] [Google Scholar]
- Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. J Clin Endocrinol Metab 2005;90:6396–6402. [DOI] [PubMed] [Google Scholar]
- Hero M, Tommiska J, Vaaralahti K, Laitinen E-M, Sipilä I, Puhakka L, Dunkel L, Raivio T. Circulating antimüllerian hormone levels in boys decline during early puberty and correlate with inhibin B. Fertil Steril 2012;97:1242–1247. [DOI] [PubMed] [Google Scholar]
- Higham CE, Johannsson G, Shalet SM. Hypopituitarism. Lancet 2016;388:2403–2415. [DOI] [PubMed] [Google Scholar]
- Johnson L, Zane RS, Petty CS, Neaves WB. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol Reprod 1984;31:785–795. [DOI] [PubMed] [Google Scholar]
- Kohva E, Huopio H, Hero M, Miettinen PJ, Vaaralahti K, Sidoroff V, Toppari J, Raivio T. Recombinant human FSH treatment outcomes in five boys with severe congenital hypogonadotropic hypogonadism. J Endocr Soc 2018;2:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr 2014;82:73–80. [DOI] [PubMed] [Google Scholar]
- Lambert A-S, Bougneres P. Growth and descent of the testes in infants with hypogonadotropic hypogonadism receiving subcutaneous gonadotropin infusion. Int J Pediatr Endocrinol 2016;2016:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PY, Baker HWG, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab 2009;94:801–808. [DOI] [PubMed] [Google Scholar]
- Main KM, Schmidt IM, Toppari J, Skakkebaek NE. Early postnatal treatment of hypogonadotropic hypogonadism with recombinant human FSH and LH. Eur J Endocrinol 2002;146:75–79. [DOI] [PubMed] [Google Scholar]
- Main KM, Toppari J, Suomi A-M, Kaleva M, Chellakooty M, Schmidt IM, Virtanen HE, Boisen KA, Kai CM, Damgaard IN et al. . Larger testes and higher inhibin B levels in Finnish than in Danish newborn boys. J Clin Endocrinol Metab 2006;91:2732–2737. [DOI] [PubMed] [Google Scholar]
- Meachem SJ, McLachlan RI, Kretser DM, de, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod 1996;54:36–44. [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 1988;122:787–794. [DOI] [PubMed] [Google Scholar]
- Pierce M, Madison L. Evaluation and initial management of hypopituitarism. Pediatr Rev 2016;37:370–376. [DOI] [PubMed] [Google Scholar]
- Raivio T, Toppari J, Perheentupa A, McNeilly AS, Dunkel L. Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet 1997;350:263–264. [DOI] [PubMed] [Google Scholar]
- Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology 2014;2:794–808. [DOI] [PubMed] [Google Scholar]
- Rey RA, Campo SM, Bedecarrás P, Nagle CA, Chemes HE. Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the cebus monkey from birth to the end of puberty. J Clin Endocrinol Metab 1993;76:1325–1331. [DOI] [PubMed] [Google Scholar]
- Rey RA, Musse M, Venara M, Chemes HE. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc Res Tech 2009;72:787–795. [DOI] [PubMed] [Google Scholar]
- Ritzén EM, Bergh A, Bjerknes R, Christiansen P, Cortes D, Haugen SE, Jörgensen N, Kollin C, Lindahl S, Läckgren G et al. . Nordic consensus on treatment of undescended testes. Acta Paediatr 2007;96:638–643. [DOI] [PubMed] [Google Scholar]
- Sarfati J, Bouvattier C, Bry-Gauillard H, Cartes A, Bouligand J, Young J. Kallmann syndrome with FGFR1 and KAL1 mutations detected during fetal life. Orphanet J Rare Dis 2015;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003;125:769–784. [DOI] [PubMed] [Google Scholar]
- Stoupa A, Samara-Boustani D, Flechtner I, Pinto G, Jourdon I, González-Briceño L, Bidet M, Laborde K, Chevenne D, Millischer A-E et al. . Efficacy and safety of continuous subcutaneous infusion of recombinant human gonadotropins for congenital micropenis during early infancy. Horm Res Paediatr 2017;87:103–110. [DOI] [PubMed] [Google Scholar]
- Varimo T, Miettinen PJ, Känsäkoski J, Raivio T, Hero M. Congenital hypogonadotropic hypogonadism, functional hypogonadotropism or constitutional delay of growth and puberty? An analysis of a large patient series from a single tertiary center. Hum Reprod 2017;32:147–153. [DOI] [PubMed] [Google Scholar]
- Wiygul J, Palmer LS. Micropenis. ScientificWorldJournal 2011;11:1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann’s syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab 1991;72:1229–1237. [DOI] [PubMed] [Google Scholar]