Abstract
BACKGROUND
Stereotactic radiosurgery (SRS) is a treatment option for persistent or recurrent acromegaly secondary to a growth hormone secreting pituitary adenoma, but its efficacy is inadequately defined.
OBJECTIVE
To assess, in a multicenter, retrospective cohort study, the outcomes of SRS for acromegaly and determine predictors.
METHODS
We pooled and analyzed data from 10 participating institutions of the International Gamma Knife Research Foundation for patients with acromegaly who underwent SRS with endocrine follow-up of ≥6 mo.
RESULTS
The study cohort comprised 371 patients with a mean endocrine follow-up of 79 mo. IGF-1 lowering medications were held in 56% of patients who were on pre-SRS medical therapy. The mean SRS treatment volume and margin dose were 3.0 cm3 and 24.2 Gy, respectively. The actuarial rates of initial and durable endocrine remission at 10 yr were 69% and 59%, respectively. The mean time to durable remission after SRS was 38 mo. Biochemical relapse after initial remission occurred in 9%, with a mean time to recurrence of 17 mo. Cessation of IGF-1 lowering medication prior to SRS was the only independent predictor of durable remission (P = .01). Adverse radiation effects included the development of ≥1 new endocrinopathy in 26% and ≥1 cranial neuropathy in 4%.
CONCLUSION
SRS is a definitive treatment option for patients with persistent or recurrent acromegaly after surgical resection. There appears to be a statistical association between the cessation of IGF-1 lowering medications prior to SRS and durable remission.
Keywords: Acromegaly, Gamma Knife, Growth hormone, Insulin-like growth factor-1, Pituitary adenoma, radiosurgery
ABBREVIATIONS
- AACE
American Association of Clinical Endocrinologists
- ARE
adverse radiation effects
- CN
cranial nerve
- CI
confidence intervals
- EBRT
external beam radiation therapy
- GH
growth hormone
- HR
hazard ratio
- IGKRF
International Gamma Knife Research Foundation
- IRB
institutional review board
- MRI
magnetic resonance imaging
- SD
standard deviation
- SRS
stereotactic radiosurgery
Acromegaly results from the excessive secretion of growth hormone (GH) by a pituitary adenoma.1 Endoscopic or microscopic transsphenoidal resection of GH-secreting pituitary adenomas is the first-line treatment for acromegaly.2,3 However, approximately 20% to 40% of surgically treated patients fail to achieve endocrine remission.2,4,5 Although medical therapy can be prescribed after failed surgery for acromegaly, lifetime management with IGF-1 lowering medication(s) is required, is not always effective, and is quite expensive.6 Stereotactic radiosurgery (SRS) has an important role in the postoperative management of acromegaly patients with residual or recurrent tumor.3,7,8 However, the literature regarding SRS for acromegaly remains limited to single-center studies, and variations in the success rates are considerable.7,9-14 Furthermore, we currently lack an adequate understanding of the factors associated with endocrine remission and SRS-induced complications. Therefore, the aims of this multicenter, retrospective cohort study are to (1) evaluate the outcomes of SRS for acromegaly and (2) determine the predictors of endocrine remission and complications in patients with acromegaly treated with SRS.
METHODS
Patient Selection
Data from patients with acromegaly who underwent SRS from 1990 to 2016 at 10 institutions participating in the International Gamma Knife Research Foundation (IGKRF) were deidentified and pooled for analysis. Each center, with approval from its respective institutional review board (IRB), retrospectively collected SRS data for these patients. This study was approved by the IGKRF protocol review committee (protocol R-16-11). Since these data for this study were obtained retrospectively, patient consent was not required by the IRB.
All SRS procedures were performed in a single session using the Gamma Knife (Elekta AB, Stockholm, Sweden), the fundamental technique for which has been previously described.15 The inclusion criteria for the study were (1) radiological and endocrine diagnosis of acromegaly and/or histopathological diagnosis of a GH-secreting pituitary adenoma, (2) data regarding endocrine outcome after SRS, and (3) follow-up duration of ≥6 mo after SRS. The diagnosis of acromegaly was made, in consultation by an endocrinologist, according to current guidelines by the American Association of Clinical Endocrinologists (AACE) or those in place at the time of treatment.2 The exclusion criteria for the study were (1) insufficient data regarding post-SRS endocrine outcomes and (2) endocrine follow-up <6 mo.
Baseline Data and Variables
Baseline data included patient features, tumor, and SRS variables. The patient variables were age, gender, pre-SRS endocrinopathy, pre-SRS visual deficit, pre-SRS medical therapy, cessation of medical therapy prior to SRS, pre-SRS random serum GH level, pre-SRS serum IGF-1 level, and duration of endocrine follow-up. Cessation of IGF-1 lowering medication (eg, long-acting somatostatin analogs, dopamine agonists, and pegvisomant) was defined as the withdrawal of medical therapy 4 to 8 wk prior to SRS.
The tumor variables were prior surgical resection, interval between last resection and SRS, prior fractionated external beam radiation therapy (EBRT), SRS indication (primary treatment, residual tumor, or recurrent tumor), and tumor volume. The SRS variables were treatment volume, treatment location (targeting of cavernous sinus, suprasellar component, and/or whole sella), margin dose, maximum dose, isodose line, number of isocenters, and maximum point dose to the optic apparatus.
Follow-up and Outcomes Data
Patients generally underwent serial neuroimaging with a contrasted magnetic resonance imaging (MRI) and serial biochemical testing, including serum random GH and IGF-1 levels, every 6 mo for the first 2 yr following SRS, and then annually thereafter. The primary outcome was endocrine remission, defined as normalization of the serum IGF-1 level, as compared with age- and gender-matched controls, off of all medications affecting GH or IGF-1 production for a period of 4 to 8 wk. The number of patients who suffered biochemical recurrence after initial endocrine remission were noted. Radiologically, tumor progression was defined as an increase in tumor volume ≥20%, whereas tumor regression was defined as a reduction in tumor volume ≥20%, as compared with pre-SRS volume.16 Adverse radiation effects (ARE) were categorized as optic apparatus injury, nonoptic cranial nerve (CN) palsy, new or worsened endocrinopathy unrelated to excess GH or IGF-1 production. New endocrinopathy was defined as the onset of one or more pituitary hormone deficiencies after SRS, as determined by measuring target hormone levels (eg, cortisol, free T4, testosterone, and/or cessation of menses in premenopausal women).
Statistical Analysis
All statistical analyses were performed using R (R version 3.11, The R Foundation for Statistical Computing, Vienna, Austria) and Prism (Graphpad, La Jolla, California). Continuous variables were reported as mean and standard deviation (SD), and categorical variables were reported as frequency and percentage. Kaplan–Meier analyses were performed to calculate the actuarial rates of initial endocrine remission, durable endocrine remission, and recurrence-free survival. Univariate Cox proportional hazards regression analyses, using Breslow's method, were performed to determine the associations between the patient, tumor, and SRS variables listed above and initial emission, durable remission, and biochemical recurrence after initial remission. Multivariate analyses were performed if >10 events were recorded. Covariates with a P-value ≤ .10 in the univariate analysis were entered into a multivariate Cox proportional hazards regression analysis to identify independent predictors of each respective outcome. Hazard ratio (HR) and 95% confidence intervals (CI) were reported for each independent predictor. Logistic regression and Fisher's exact test were performed to determine the associations between continuous and binary patient, tumor, and SRS variables, respectively, and each ARE listed above. All statistical tests were two-sided. A P-value < .05 was considered statistically significant.
RESULTS
Baseline and SRS Treatment Characteristics
The study cohort was comprised of 371 patients with acromegaly who were treated with SRS (Figure 1). Table 1 details the baseline patient and tumor characteristics of the study cohort. Of the 132 patients who were on medical therapy prior to SRS (36% of the study cohort), 77 were taking octreotide (58%), 16 were taking lanreotide (12%), 22 were taking pegvisomant (17%), 34 were taking bromocriptine (26%), and 5 were taking cabergoline (4%); 22 of these patients were taking two IGF-1 lowering medications (17%), while the remaining 110 were each taking one IGF-1 lowering medication (83%). Medical therapy was intentionally held in the time period around the SRS procedure in 74 patients (56%). Table 2 details the SRS treatment characteristics of the study cohort.
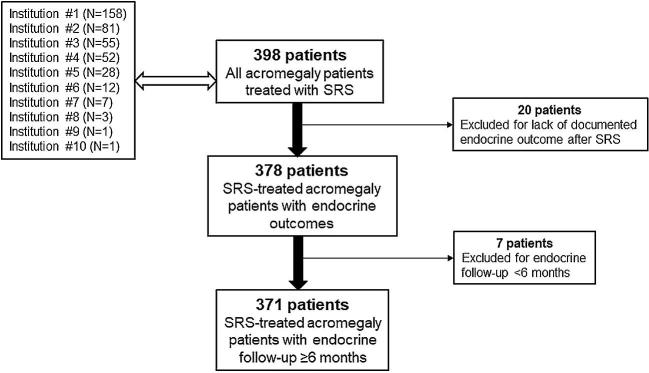
FIGURE 1.
Selection process for the study cohort. A total of 398 patients were treated with SRS at 10 participating institutions of the IGKRF. After excluding 20 patients for a lack of documented endocrine outcome after SRS and 7 patients for endocrine follow-up <6 mo, the study cohort was comprised of 371 SRS-treated acromegaly patients with ≥6 mo endocrine follow-up.
TABLE 1.
Baseline Characteristics of the Study Cohort of 371 Acromegaly Patients Treated With SRS
| Factor | Total (%) |
|---|---|
| Age at SRS (mean ± SD [range], years) | 46.0 ± 13.9 (13.6-92) |
| Gender | |
| Male | 169 (45.6%) |
| Female | 202 (54.4%) |
| Prior surgical resection | 345 (93.0%) |
| Time interval from last resection to SRS (mean ± SD [range], months) | 30.7 ± 42.2 (1-300) |
| Prior EBRT | 20 (5.4%) |
| SRS indication | |
| Primary treatment | 26 (7.0%) |
| Residual tumor | 326 (87.9%) |
| Recurrent tumor | 19 (5.1%) |
| Endocrinopathy prior to SRS | 81 (21.8%) |
| Hypothyroid | 29 (7.8%) |
| Testosterone/Estrogen deficiency | 40 (10.8%) |
| Hypocortisolemia | 8 (2.2%) |
| Diabetes insipidus | 4 (1.1%) |
| Visual deficits prior to SRS | 47 (12.7%) |
| Visual field deficit | 42 (11.3%) |
| Diplopia | 5 (1.3%) |
| Medical therapy prior to SRS | 132 (35.6%) |
| Medical therapy held prior to SRS | 74 (56.1%)a |
| No visible tumor on MRI prior to SRS | 27 (7.3%) |
| Tumor volume (mean ± SD [range], cm3) | 2.5 ± 2.9 (0.1-21.1)b |
| Random serum GH prior to SRS (mean ± SD [range], ng/mL) | 12.6 ± 22.9 (0-173) |
| Serum IGF-1 prior to SRS (mean ± SD [range], ng/mL) | 699.0 ± 365.6 (65.3-2915) |
| Endocrine follow-up duration (mean ± SD [range], months) | 78.9 ± 52.2 (6.0-315) |
SD, standard deviation; SRS, stereotactic radiosurgery; EBRT, fractionated external beam radiation therapy; GH, growth hormone; IGF-1, insulin-like growth factor-1
aProportion of patients who were on medical therapy prior to SRS (n = 132).
bBased on data from 344 patients with visible tumor on pre-SRS MRI.
TABLE 2.
SRS Treatment Characteristics of the Study Cohort of 371 Acromegaly Patients
| Factor | Total (%) |
|---|---|
| Treatment volume (mean ± SD [range], cm3) | 3.0 ± 3.1 (0.1-22.9) |
| Cavernous sinus targeted | 177 (47.7%) |
| Suprasellar component targeted | 40 (10.8%) |
| Whole sella targeted | 83 (22.4%) |
| Margin dose (mean ± SD [range], Gy) | 24.2 ± 6.4 (8.8-40) |
| Maximum dose (mean ± SD [range], Gy) | 48.1 ± 12.3 (20-83.3) |
| Isodose line (mean ± SD [range], %) | 51.2 ± 7.1 (25-90) |
| Isocenters (mean ± SD [range]) | 8.0 ± 5.7 (1-33) |
| Maximum point dose to optic apparatusa (mean ± SD [range], Gy) | 6.3 ± 3.3 (0-20) |
SD, standard deviation
aOptic apparatus includes optic nerve, chiasm and tract.
Tumor Imaging Response, Additional Treatments, and Mortality
The mean duration of radiological follow-up after SRS was 64.5 ± 43.6 mo (range 3-229 mo). Of the 344 patients with visible tumor on pre-SRS MRI, radiological tumor progression and regression were observed in 4 (1.2%) and 224 (65%) patients, respectively. Additional treatments after SRS included initiation of new medical therapy in 156 (42%), repeat SRS in 21 (6%), and surgical resection in 2 (0.5%).
Following SRS, 12 patients died (mortality rate 3.2%), with a median time interval from SRS to death of 105.5 mo (range 19-157 mo). The causes of death included cardiomyopathy, myocardial infarction due to severe coronary artery disease, occipital lobe glioblastoma, and non-Hodgkin's lymphoma each in 1 patient; the causes of death in the remaining 8 patients were unknown. The mortality rate during the study period for patients with durable remission was 1.8% (3/166 patients), compared to 4.3% (9/205 patients) for those without durable remission (P = .27)
Endocrine Outcomes
Initial endocrine remission was achieved in 199 patients (54%), based only on normalization of serum IGF-1 levels off of IGF-1 lowering medications, as compared with age-matched controls. The actuarial rates of normalization of serum IGF-1 at 5, 10, and 15 yr were 51%, 69%, and 74%, respectively (Figure 2A). Of these patients, the serum random GH level was <1 ng/mL off of IGF-1 lowering medications after SRS in 164, yielding a crude initial remission rate of 44% based on the 2010 consensus criteria.4
FIGURE 2.
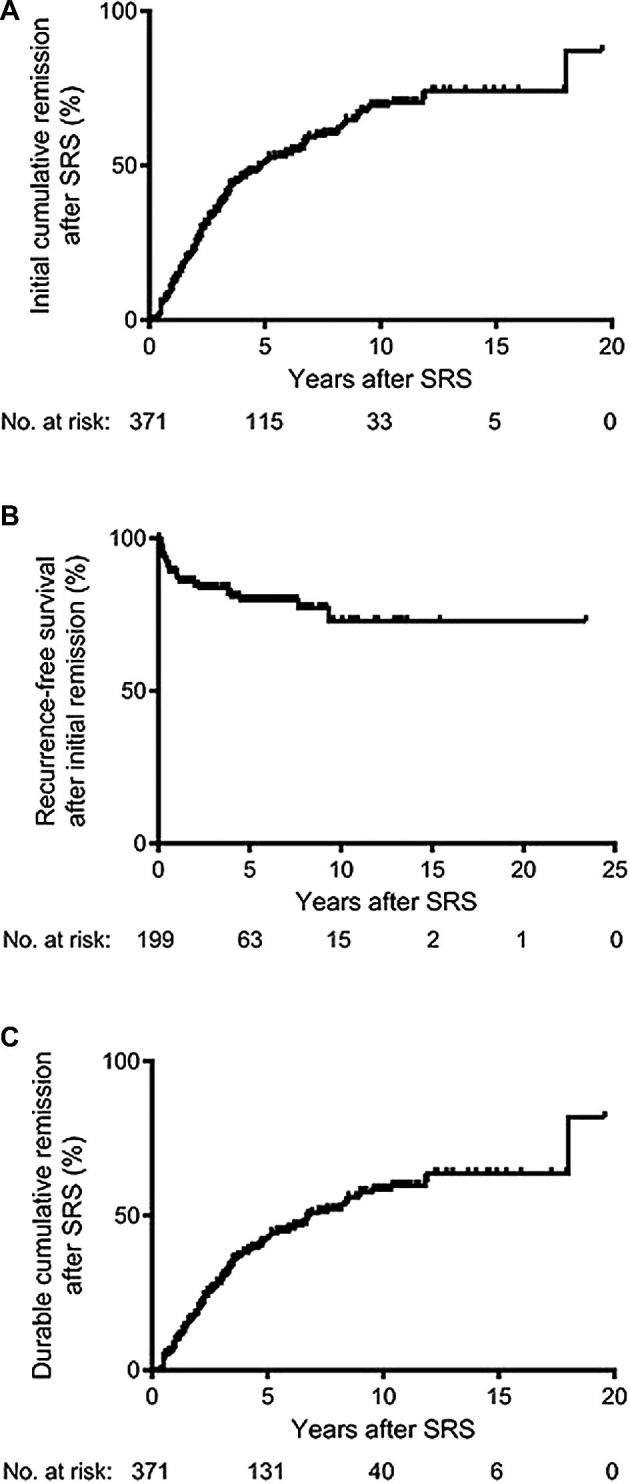
Kaplan–Meier analyses for initial endocrine remission, biochemical recurrence after initial remission, and durable endocrine remission after SRS. A, The actuarial rates of initial remission after SRS in the study cohort (n = 371) at 5, 10, and 15 yr were 51%, 69%, and 74%, respectively. B, The actuarial rates of recurrence-free survival in patients who achieved initial endocrine remission (n = 199) were 80% at 5 yr and 73% each at 10, 15, and 20 yr. C, The actuarial rates of durable endocrine remission after SRS in the study cohort (n = 371) at 5, 10, and 15 yr were 43%, 59%, and 64%, respectively.
Biochemical recurrence after initial remission based upon IGF-1 normalization occurred in 33 patients (9%). The mean time interval from initial remission to recurrence was 17.3 ± 18.4 mo (range 1-72 mo). The actuarial rates of recurrence-free survival were 80% at 5 yr and 73% each at 10, 15, and 20 yr (Figure 2B).
Durable endocrine remission was achieved in 166 patients (45%), based only on normalization of serum IGF-1 levels off of IGF-1 lowering medications. The mean time interval from SRS to serum IGF-1 normalization off IGF-1 lowering medications was 37.7 ± 32.5 mo (range 1-216 mo). The actuarial rates of durable remission at 5, 10, and 15 yr were 43%, 59%, and 64%, respectively (Figure 2C). Of these patients, the serum random GH level was <1 ng/mL off of IGF-1 lowering medications after SRS in 143, yielding a crude durable remission rate of 39% based on the 2010 consensus criteria.4 The mean time interval from SRS to serum random GH < 1 ng/mL off of IGF-1 lowering medications for those with durable remission based on the 2010 consensus criteria was 38.3 ± 30.9 mo (range 1-142 mo).
Subgroup analyses of the endocrine outcomes are provided in the Supplemental Digital Content.
Predictors of Endocrine Remission and Recurrence
Table 3 details the univariate analyses for factors associated with initial endocrine remission, durable endocrine remission, and biochemical recurrence after initial remission. In the multivariate analysis for initial remission, pre-SRS serum IGF-1 level (HR = 0.999, 95% CI: 0.998-1.00; P = .03) and temporary cessation of IGF-1 lowering medication prior to SRS (HR = 2.73, 95% CI: 1.41-5.31; P = .003) were found to be independent predictors. In the multivariate analysis for durable remission, only temporary cessation of IGF-1 lowering medication prior to SRS (HR = 2.49, 95% CI: 1.21-5.11; P = .01) was found to be an independent predictor. In the multivariate analysis for recurrence after initial remission, prior resection (HR = 0.21, 95% CI: 0.06-0.74; P = .01) and maximum dose (HR = 0.92, 95% CI: 0.87-0.99; P = .01) were found to be independent predictors.
TABLE 3.
Univariate Analyses for Factors Associated Initial Endocrine Remission, Durable Endocrine Remission, and Postremission Biochemical Recurrence After SRS for Acromegaly
| Factor | Initial remission P-value | Durable remission P-value | Recurrence after remission P-value |
|---|---|---|---|
| Age | .59 | .89 | .59 |
| Gender | .12 | .26 | .93 |
| Prior surgical resection | .03 | .02 | .10 |
| Random serum GH level prior to SRS | .0004 | .0004 | .07 |
| Serum IGF-1 level prior to SRS | <.0001 | <.0001 | .57 |
| Cessation of medication prior to SRS | .04 | .04 | .54 |
| Tumor volume | .09 | .07 | .24 |
| Whole sella targeted by SRS | .02 | .049 | .80 |
| SRS margin dose | .01 | .0007 | .0005 |
| SRS maximum dose | .0006 | <.0001 | <.0001 |
GH, growth hormone; IGF-1, insulin-like growth factor-1. Bold values are statistically significant (P < .05).
Adverse Radiation Effects
Table 4 details the AREs after SRS. A total of 97 patients developed 1 or more new pituitary hormone deficiencies (26%). Multiple new endocrinopathies were noted in 41 patients (11%), including 12 with 3 endocrinopathies (hypothyroidism, testosterone or estrogen deficiency, and hypocortisolism) and 29 with 2 endocrinopathies (hypothyroidism and testosterone or estrogen deficiency in 12, hypothyroidism and hypocortisolism in 9, and testosterone or estrogen deficiency in hypocortisolism in 8). No patient developed new onset diabetes insipidus. Only SRS targeting of the whole sella was significantly associated with new post-SRS endocrinopathy in the univariate analysis (P = .02); neither margin dose (P = .56), maximum dose (P = .53), prior surgical resection (P = .25), nor prior EBRT (P = .61) were significantly related to new post-SRS endocrinopathy.
TABLE 4.
ARE in 371 Acromegaly Patients Treated With SRS
| Variable | Total (%) |
|---|---|
| New endocrinopathya | 97 (26.1%) |
| Hypothyroidism | 62 (16.7%) |
| Testosterone/Estrogen deficiency | 49 (13.2%) |
| Hypocortisolism | 39 (10.5%) |
| New cranial neuropathyb | 16 (4.3%) |
| CN II | 13 (3.5%) |
| CN III | 1 (0.3%) |
| CN IV | 1 (0.3%) |
| CN V | 3 (0.8%) |
| CN VI | 1 (0.3%) |
CN, cranial nerve
a41 patients had multiple new endocrinopathies.
bTwo patients had multiple new cranial neuropathies.
A total of 16 patients had suffered new cranial neuropathies (4.3%). Two patients suffered multiple new cranial neuropathies, including 1 with 3 (CN III, V, and VI injuries) and another with 2 (CN II and IV). Only SRS targeting of suprasellar tumor was significantly associated with optic apparatus injury in the univariate analysis (P = .02); neither margin dose (P = .23), maximum dose (P = .29), maximum point dose to the optic apparatus (P = .38), nor prior EBRT (P = .99) were significantly related to optic apparatus injury. No factors were found to be significantly associated with SRS-induced nonoptic CN injury in the univariate analysis, including SRS targeting of the cavernous sinus (P = .11), margin dose (P = .43), maximum dose (P = .60), and prior EBRT (P = .99). None of the patients who developed a new cranial neuropathy had radiological evidence of tumor growth.
DISCUSSION
Outcomes after SRS for Acromegaly
The reported outcomes after SRS for acromegaly vary widely in the literature.13 This may be due, in part, to the relatively small cohort sizes of earlier studies, limited endocrine follow-up, and differences in radiosurgical techniques, including the margin dose. In order to overcome the limitations in interpreting the currently available literature, we compiled and analyzed a large, multicenter cohort of 371 SRS-treated acromegaly patients with long-term endocrine follow-up (mean duration 6.6 yr). The actuarial durable remission rate was 59% at 10 yr, which is within the range of the published literature and is indicative of the overall efficacy of SRS for acromegaly.13 Our findings serve to validate the efficacy of SRS for the management of acromegaly and confirm the results of previous single-center series with fewer patients.
Notably, temporary cessation of IGF-1 lowering medications around the time of SRS was an independent predictor of both initial (P = .003) and durable (P = .01) remission. IGF-1 lowering medications were temporarily held before SRS in 56% of patients on pre-SRS medical therapy. Acromegaly patients who halted medical therapy prior to SRS were 2.5 times more likely to achieve durable remission than those who continued medical therapy throughout the SRS procedure. This potential reduction in radiosensitivity induced by IGF-1 lowering medications has been proposed previously.17 However, results from the literature regarding the benefit of holding IGF-1 lowering medications prior to SRS are conflicting. Castinetti et al12 found, in contrast to our study, that pre-SRS serum GH and IGF-1 levels negatively correlated with remission, but cessation of pre-SRS medical therapy did not affect outcomes. Landolt et al17 noted that patients with acromegaly taking octreotide required a significantly longer time to achieve normal levels of GH and IGF-1 after SRS than those who did not receive this medication at the time of SRS.
The temporary cessation of medical therapy prior to SRS is not endorsed by recent guidelines from the AACE and the Endocrine Society.6 However, the biological principle of treating an active tumor (ie, in a patient who is not taking medical therapy) seemed valid, which was the basis for withdrawing medical therapy for a period of 4 to 8 wk before the SRS procedure. Based the findings from this and previous studies, we recommend withholding IGF-1 lowering medications (eg, long-acting somatostatin analogs and dopamine agonists) for a period of 6 to 8 wk prior to SRS, provided there is no medical contraindication to the temporary cessation of these agents.15 Medical therapy for acromegaly can be restarted 4 to 8 wk after the SRS procedure. Dopamine agonists may only need to be withheld for 2, rather than 6 to 8, wk before SRS to have the desired effect of improved tumor radiosensitivity, based on the biochemical and biological half-life of these drugs compared to somatostatin analogs. Additionally, due to its mechanisms of action and lack of antitumor effect, withdrawal of pegvisomant may not substantially enhance the response to SRS. Further studies are necessary to discern the differential effects of halting specific IGF-lowering medications on outcomes after SRS for acromegaly.
Hypopituitarism of one or more axes is the most common complication after SRS, and it occurred in 26% of patients in this study, including 11% with multiple new pituitary hormone deficiencies. In a previously study of all types of pituitary adenoma patients treated with SRS, Xu et al18 reported new hypopituitarism in 30%; higher SRS margin dose and suprasellar tumor extension were significant predictors of new pituitary deficiencies. In contrast, we did not identify a relationship between radiation dose and hypopituitarism. Rather, SRS targeting of the whole sella was significantly associated with development of a new hormone loss after SRS (P = .02). Generally, whole sellar irradiation is only used when residual or recurrent tumor cannot be distinguished on neuroimaging, and it represents the radiosurgical equivalent of a total hypophysectomy.19 Whole sellar radiation with SRS was performed in 22% of our patients. Patients for whom this SRS approach is employed may warrant more rigorous endocrine monitoring for new or worsening endocrinopathies.
Cranial neuropathies were relatively uncommon after SRS, occurring in 4.3% of patients. Visual deficits from injury of the optic apparatus represented the majority of new cranial neuropathies (3.5% of patients). Tumor extension into the suprasellar region was significantly associated with new or worsening visual deficits (P = .02), which is consistent with the intimate anatomical relationship between suprasellar tumors and the optic apparatus. The use of hypofractionated SRS may be an alternative to single-session SRS for reducing the radiation dose to the optic apparatus in the treatment of suprasellar lesions, although data regarding its efficacy for functioning pituitary adenomas are sparse.20,21 Cifarelli et al22 found prior radiation with either SRS or EBRT to be a risk factor for cranial neuropathy in a study of both functioning and nonfunctioning pituitary adenomas treated with SRS. The same negative relationship between prior EBRT and cranial neuropathies was not identified in our cohort of SRS-treated patients with acromegaly.
Role of SRS in the Management of Acromegaly
Because of the systemic effects and potentially life-threatening sequelae of acromegaly, surgical resection remains the preferred first-line treatment for these patients, since it offers the possibility of immediate lowering of GH and IGF-1 to normal levels, as well as improvement of symptoms and signs.1,23 Therefore, the primary role of SRS in the contemporary management of acromegaly is for the treatment of residual or recurrent GH-secreting pituitary adenomas. A prior study from our group demonstrated that a smaller adenoma volume predicts a greater probability of endocrine remission.15 Thus, the merits of an initial resection yielding a chance for remission, histological confirmation, and reduction in target volume are evident in our study, as 93% of patients underwent prior resection. However, because of the potential surgical morbidity of aggressively pursuing a pituitary adenoma that has extended into the cavernous sinus, it is reasonable to leave residual tumor in the cavernous sinus for subsequent treatment with SRS, particularly in cases where complete surgical resection cannot be achieved.23
Since SRS achieves remission more rapidly for functioning pituitary adenomas than EBRT, with a lower risk of complications such as hypopituitarism, it has generally supplanted EBRT as the secondary therapy of choice after failed surgical resection.24 Although the mean interval from treatment to durable remission was 3.1 yr in this study, SRS remains an attractive alternative to lifelong medical therapy if remission can be achieved. Primary treatment with SRS, which comprised 7% of our cases, may be used for appropriately selected patients who are medically unfit for surgical intervention and for whom medical management is either ineffective or not preferred. However, prior resection was a negative independent predictor of biochemical recurrence after initial remission (P = .01), which supports the current role of surgery as the preferred first-line intervention for patients with acromegaly. Residual tumor in the cavernous sinus noted on postoperative imaging should warrant early consideration for SRS, followed by administration of appropriate suppression medication while waiting for IGF-1 levels to normalize.
Some guidelines do not recommend SRS as a second-line treatment for acromegaly after initial surgical resection.6 However, there is currently no medical therapy that yields durable endocrine remission in patients with acromegaly. Additionally, IGF-1 lowering medications are very expensive, and the costs of medications and hospitalizations related to incompletely controlled acromegaly with medical management alone are substantial.25,26 SRS affords the possibility of a cure off of medications after a single procedure, and should therefore be considered for patients with persistent acromegaly after surgical resection as an alternative to lifelong medical therapy.
Study Limitations
This study is limited by its single-arm, retrospective design, which subjects it to the selection, treatment, and referral biases of the treating institutions and physicians, although pooling data from multiple centers mitigates some of these biases. All centers participating in the study were ones with years of experience and relatively high SRS volumes. However, it is possible that selection biases at individual centers could have impacted the patient volume and outcomes at participating sites. Future analyses from nationwide, prospective registries may provide a better assessment of SRS outcomes for acromegaly, but the rarity of this disease and the long-term follow-up necessary to sufficiently determine the efficacy of SRS make it unlikely that such a study will be performed within the next few years.27 Therefore, we believe that this study of a large, multicenter cohort represents the best available attempt to define the outcomes of SRS for acromegaly.
Since all of the patients with acromegaly in this study were treated with SRS alone, a comparison with medical therapy alone, repeat surgical resection, EBRT, or hypofractionated SRS could not be performed. We acknowledge that the evaluation of endocrine outcome was dependent on serum levels of GH and IGF-1, and that during the long study period, assays for GH and IGF-1 were likely to have varied. Additionally, MRI technology has improved over the course of the study period, and this may have influenced the observed radiological outcomes. Since each of the contributing centers is a tertiary referral center for pituitary SRS, detailed clinical follow-up was not available for some patients. Specifically, we are unable to provide an analysis of the time interval between SRS and the development of new pituitary hormone deficiencies, or a more detailed assessment of new optic neuropathies after SRS. Furthermore, the details for some patients who developed new endocrinopathies regarding their serum laboratory values and changes to medication regimens were not available. Given that there is some degree of variability among IGF-1 assays, we would ideally reanalyze all of the patient samples using a single assay. Unfortunately, this is not possible, due to the multicenter and longitudinal nature of this study. Additionally, we are unable to determine the rate of decline in serum random GH levels after SRS over time.
We also acknowledge that there is no definitive data available to validate how long an IGF-1 lowering medication should be withheld prior to SRS. Our recommendation to halt medical therapy for a period of 6 to 8 wk prior to SRS is based primarily on the duration of action of long-acting somatostatin analogs (eg, octreotide and lanreotide), which can have an effect beyond 4 wk. Finally, the remission criteria for acromegaly were revised in 2010, and they provided more stringent parameters (ie, normalization of both IGF-1 and GH, rather than IGF-1 only) for defining endocrine remission after intervention.4 When we analyzed our outcomes based on the 2010 consensus criteria, the crude rates of both initial (44% vs 53%) and durable (39% vs 45%) remission were lower. Therefore, the endocrine remission rates may be overestimated by our analysis.
CONCLUSION
SRS affords durable endocrine remission for approximately half of patients with acromegaly, with a reasonable safety profile in which approximately one-quarter of patients will develop at least 1 new pituitary hormone deficiency. There appears to be a statistical association between the cessation of IGF-1 lowering medications prior to SRS and durable remission, although the management of medical therapy should be governed by an endocrinologist. Currently, SRS remains an important and efficacious adjunctive therapy for acromegaly patients who have failed initial surgical resection. However, since biochemical recurrence occurs in a modest number of patients with initial remission, long-term endocrine follow-up is critical after SRS for the treatment of acromegaly.
Disclosures
Dr Grills receives research funding from Elekta, which is unrelated to this study. Dr Liscak is a consultant for Elekta. Dr Grills is a stockholder and serves on the Board of Directors of Greater Michigan Gamma Knife. Dr Lunsford is a consultant and stockholder in Elekta. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors appreciate the assistance of Ms Linda Baxendell with the coordination of data for the International Gamma Knife Research Consortium.
Neurosurgery Speaks! Audio abstracts available for this article at www.neurosurgery-online.com.
COMMENTS
We would like to congratulate the authors for a very important publication.
Now that acromegaly has frequently become a chronic disease requiring long-term normal hormone values to avoid sequelae,1 the therapeutic discussions are usually based on the optimal antisecretory treatment to use after failed transsphenoidal surgery. One should always keep in mind, however, that only 3 treatments can actually lead to cure of the disease, namely surgery, radiosurgery, and radiotherapy. This is why the results of the large multicenter study reported in this paper on Gamma Knife (Elekta AB) radiosurgery is of major interest for clinicians dealing with acromegaly. Briefly, the main result based on 371 patients followed for a mean endocrine follow-up of 79 months is that radiosurgery can be effective in 44% of the patients when stringent criteria of remission (IGF-1 and GH) are used. The delay to remission is shorter than anticipated (38 months), but still means that an effective antisecretory treatment will have to be maintained during this period of time. Interestingly, as previously shown for Cushing's disease, about 10% of the patients will present recurrence after a mean time of 17 months. This is a new concept in comparison with conformal radiotherapy for which recurrences are very rare, or due to aggressive pituitary neuroendocrine tumors. Classical side-effects, as previously reported, include pituitary deficiencies in 26% of cases, and cranial neuropathy in 4%.
As a summary, the results shown in this study are similar to the ones reported in previous studies based on a smaller number of patients.2,3,4,5 It thus confirms the efficacy of the technique, and emphasizes a still yet controversial point on the detrimental effects of somatostatin analogs given at the time of radiosurgery. Since the first study reported by Landolt et al5 suggesting that tumors on somatostatin analogs at the time of Gamma Knife had a lower turn-over that made Gamma Knife less effective, several studies were reported on the efficacy of Gamma Knife, and all showed contradictory results on this specific point. The usually accepted management of such patients was to withdraw the drug 3 months before to try to get the higher chance of efficacy. In the study shown here, withdrawal of somatostatin analogs before surgery was considered an independent positive predictor of remission.
Now that the efficacy data are well known and based on a sufficient number of patients, the question of extra-pituitary side effects of radiosurgery is still raised by some clinicians. They usually try to extrapolate on these side effects (memory loss, altered quality of life, stroke, etc) based on the side effects that were reported up to 20 years after fractionated radiotherapy. Future studies should try to focus on this point: even if the technique of radiosurgery, presumably safer that radiotherapy, should not lead theoretically to such extra-pituitary side effects, only a prospective comparative study would theoretically allow to determine whether Gamma Knife radiosurgery is as safe as anticipated. This is now still the major burden for increasing the indications of this effective and presumably safe technique, which can allow for cure in about half of cases.
Frederic Castinetti
Jean Régis
Marseille, France
References
- 1. Rochette C, Graillon T, Albarel F, et al Increased Risk of Persistent Glucose Disorders After Control of Acromegaly. J Endocr Soc. 2017;1(12):1531-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castinetti F, Nagai M, Morange I, et al Long-term results of stereotactic radiosurgery in secretory pituitary adenomas. J Clin Endocrinol Metab 2009;94(9):3400-7. [DOI] [PubMed] [Google Scholar]
- 3. Mehta GU, Ding D, Patibandla MR, et al Stereotactic Radiosurgery for Cushing Disease: Results of an International, Multicenter Study. J Clin Endocrinol Metab. 2017;102(11):4284-4291. [DOI] [PubMed] [Google Scholar]
- 4. Pollock BE, Jacob JT, Brown PD, Nippoldt TB. Radiosurgery of growth hormone-producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106(5):833-8. [DOI] [PubMed] [Google Scholar]
- 5. Landolt AM, Haller D, Lomax N, et al Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85(3):1287-9. [DOI] [PubMed] [Google Scholar]
In this large multi-center study it was found that stereotactic radiosurgery is an effective and safe adjuvant therapy for patients with persisting acromegaly after pituitary surgery. Furthermore, it is recommended that IGF-1 lowering medication is discontinued prior to radiosurgery. This is important information to the clinicians taking care of these patients.
Charlotte Hoybye
Stockholm, Sweden
REFERENCES
- 1. Melmed S. Acromegaly. N Engl J Med. 2006;355(24):2558-2573. [DOI] [PubMed] [Google Scholar]
- 2. Katznelson L, Atkinson JL, Cook DM et al.. American Association of Clinical Endocrinologists Medical guidelines for clinical practice for the diagnosis and treatment of acromegaly-2011 update. Endocr Pract. 2011;17(suppl 4):1-44. [DOI] [PubMed] [Google Scholar]
- 3. Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017;19(6):762-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giustina A, Chanson P, Bronstein MD et al.. A consensus on criteria for cure of acromegaly. J Clin Endocrinol Metab. 2010;95(7):3141-3148. [DOI] [PubMed] [Google Scholar]
- 5. Phan K, Xu J, Reddy R, Kalakoti P, Nanda A, Fairhall J. Endoscopic endonasal versus microsurgical transsphenoidal approach for growth hormone-secreting pituitary adenomas-systematic review and meta-analysis. World Neurosurg. 2017;97:398-406. [DOI] [PubMed] [Google Scholar]
- 6. Katznelson L, Laws ER Jr, Melmed S et al.. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933-3951. [DOI] [PubMed] [Google Scholar]
- 7. Lee CC, Vance ML, Xu Z et al.. Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab. 2014;99(4):1273-1281. [DOI] [PubMed] [Google Scholar]
- 8. Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab. 2011;96(7):1992-2003. [DOI] [PubMed] [Google Scholar]
- 9. Franzin A, Spatola G, Losa M, Picozzi P, Mortini P. Results of gamma knife radiosurgery in acromegaly. Int J Endocrinol. 2012;2012:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu X, Kano H, Kondziolka D et al.. Gamma knife radiosurgery for clinically persistent acromegaly. J Neurooncol. 2012;109(1):71-79. [DOI] [PubMed] [Google Scholar]
- 11. Losa M, Gioia L, Picozzi P et al.. The role of stereotactic radiotherapy in patients with growth hormone-secreting pituitary adenoma. J Clin Endocrinol Metab. 2008;93(7):2546-2552. [DOI] [PubMed] [Google Scholar]
- 12. Castinetti F, Taieb D, Kuhn JM et al.. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90(8):4483-4488. [DOI] [PubMed] [Google Scholar]
- 13. Ding D, Starke RM, Sheehan JP. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J Neurooncol. 2014;117(3):445-457. [DOI] [PubMed] [Google Scholar]
- 14. Ding D, Yen CP, Starke RM, Lee CC, Sheehan JP. Unyielding progress: recent advances in the treatment of central nervous system neoplasms with radiosurgery and radiation therapy. J Neurooncol. 2014;119(3):513-529. [DOI] [PubMed] [Google Scholar]
- 15. Sheehan JP, Pouratian N, Steiner L, Laws ER, Vance ML. Gamma Knife surgery for pituitary adenomas: factors related to radiological and endocrine outcomes. J Neurosurg. 2011;114(2):303-309. [DOI] [PubMed] [Google Scholar]
- 16. Snell JW, Sheehan J, Stroila M, Steiner L. Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. J Neurosurg. 2006;104(1):157-162. [DOI] [PubMed] [Google Scholar]
- 17. Landolt AM, Haller D, Lomax N et al.. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85(3):1287-1289. [DOI] [PubMed] [Google Scholar]
- 18. Xu Z, Lee Vance M, Schlesinger D, Sheehan JP. Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery. 2013;72(4):630-637; 636-637. [DOI] [PubMed] [Google Scholar]
- 19. Lee CC, Chen CJ, Yen CP et al.. Whole-sellar stereotactic radiosurgery for functioning pituitary adenomas. Neurosurgery. 2014;75(3):227-237; discussion 237. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen JH, Chen CJ, Lee CC et al.. Multisession gamma knife radiosurgery: a preliminary experience with a noninvasive, relocatable frame. World Neurosurg. 2014;82(6):1256-1263. [DOI] [PubMed] [Google Scholar]
- 21. Iwata H, Sato K, Tatewaki K et al.. Hypofractionated stereotactic radiotherapy with CyberKnife for nonfunctioning pituitary adenoma: high local control with low toxicity. Neuro-oncol. 2011;13(8):916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cifarelli CP, Schlesinger DJ, Sheehan JP. Cranial nerve dysfunction following Gamma Knife surgery for pituitary adenomas: long-term incidence and risk factors. J Neurosurg. 2012;116(6):1304-1310. [DOI] [PubMed] [Google Scholar]
- 23. Starke RM, Raper DM, Payne SC, Vance ML, Oldfield EH, Jane JA Jr.. Endoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remission. J Clin Endocrinol Metab. 2013;98(8):3190-3198. [DOI] [PubMed] [Google Scholar]
- 24. Abu Dabrh AM, Asi N, Farah WH et al.. Radiotherapy versus radiosurgery in treating patients with acromegaly: a systematic review and meta-analysis. Endocr Pract. 2015;21(8):943-956. [DOI] [PubMed] [Google Scholar]
- 25. Placzek H, Xu Y, Mu Y, Begelman SM, Fisher M. Clinical and economic burden of commercially insured patients with acromegaly in the united states: a retrospective analysis. J Manag Care Spec Pharm. 2015;21(12):1106-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Broder MS, Neary MP, Chang E, Cherepanov D, Katznelson L. Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary. 2014;17(4):333-341. [DOI] [PubMed] [Google Scholar]
- 27. Sheehan JP, Kavanagh BD, Asher A, Harbaugh RE. Inception of a national multidisciplinary registry for stereotactic radiosurgery. J Neurosurg. 2016;124(1):155-162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.