Abstract
Puerto Rico detected the first confirmed case of chikungunya virus (CHIKV) in May 2014 and the virus rapidly spread throughout the island. The invasion of CHIKV allowed us to observe Aedes aegypti (L.) densities, infection rates, and impact of vector control in urban areas using CDC Autocidal Gravid Ovitraps (AGO traps) for mosquito control over several years. Because local mosquitoes can only get the virus from infectious residents, detecting the presence of virus in mosquitoes functions as a proxy for the presence of virus in people. We monitored the incidence of CHIKV in gravid females of Ae. aegypti in four neighborhoods; two with three AGO traps per home in most homes and two nearby neighborhoods without AGO mosquito control traps. Monitoring of mosquito density took place weekly using sentinel AGO traps from June to December 2014. 1334 pools of female Ae. aegypti (23,329 individuals) were processed by RT-PCR to identify CHIKV and DENV RNA. Density of Ae. aegypti females was 10.5 times lower (91%) in the two areas with AGO control traps during the study. Ten times (90.9%) more CHIKV positive pools were identified in the non-intervention areas (50/55 pools) than in intervention areas (5/55). We found a significant linear relationship between the number of positive pools and both density of Ae. aegypti and vector index (average number of expected infected mosquitoes/trap/week). Temporal and spatial patterns of positive CHIKV pools suggested limited virus circulation in areas with AGO traps.
Keywords: Mosquito control, Aedes aegypti, Arboviral Transmission, Invasive Species, Vector-Borne Pathogens
Although chikungunya (CHIKV), dengue (DENV-1, DENV-2, DENV-3, DENV-4), yellow fever (YFV) and Zika (ZIKV) viruses originally circulate between non-human primates and forest mosquitoes in natural areas (Gubler 2002, Weaver and Reisen 2010), over time they have established independent transmission cycles between humans and domestic mosquitoes in urban areas (Musso and Gubler 2016). Currently, YFV outbreaks are mostly limited to areas where there is movement of the virus from enzootic foci to urbanized areas, whereas the sources of epidemics for the rest of these arboviruses primarily originate from other infected urban areas (Monath and Vasconcelos 2015). Lack of vaccines against CHIK, DENV, and ZIKV determines that vector control is the only available approach for the prevention and control of chikungunya, dengue, and Zika fevers.
Current approaches for the control of container Aedes (Stegomyia) mosquitoes involved in human to human transmission of these arboviruses, such as Ae. aegypti and Ae. albopictus are elimination or modification of containers where mosquitoes develop, application of larvicides to containers, and spatial spraying of insecticides against adult mosquitoes. Residual insecticide applications are basically not being used against these vectors, with the exceptions of some limited use for focal control (around cases) in some countries (Ritchie et al. 2002). Widespread insecticide resistance against organophosphate and pyrethroid insecticides has been reported in Ae. aegypti and to a lesser extent in Ae. albopictus (Vontas et al. 2012). Interestingly, a variety of larvicides are effective against the immature stages of these mosquitoes, including bio-rational pesticides such as Bacillus thuringiensis israelensis, spinosad, juvenile hormone mimics, chitin synthesis inhibitors, and biodegradable oils (Barrera 2015).
The ongoing, unprecedented geographical expansion of CHIKV, DENV, and ZIKV (Bhatt et al. 2013, Weaver and Forrester 2015, Higgs 2016) would indicate that vector control is not being effectively achieved or practiced. Current vector control approaches are based on a “seek and control” strategy to deliver vector control agents to places where it is thought that mosquitoes are located, such as by visiting houses to conduct source reduction, larviciding, and fumigation. The main limitations to that approach are: relatively short-lived action of control measures (2-3 weeks) necessitating reapplication of control measures at a frequency that most Vector Control Programs cannot afford, finding that a large fraction of houses cannot be treated because residents are not present or refuse treatment, the increasingly common finding that a large fraction of the mosquito population derives from cryptic aquatic habitats, and insecticide resistance (Barrera 2015).
Other approaches to vector suppression include luring mosquitoes to devices that result in passive or active killing (lure and control strategy) or by means of releasing modified mosquitoes making contact with local individuals of their own species to deliver a control agent (auto-dissemination strategy). Examples of this latter approach are the release of males carrying lethal genes, sterilized by irradiation, infected with Wolbachia bacteria or entomo-pathogenic fungi, or contaminated with pyriproxyfen (Scholte et al. 2004, O’Connor et al. 2012, Alphey et al. 2013, Bellini et al. 2013, Mains et al. 2015). The advantage of auto-dissemination approaches is that once the residents have given their consent, investing time and human resources asking for permission to enter and treat individual houses is unnecessary. Another promising approach not based on population suppression is to permanently replace a vector mosquito population with individuals that cannot transmit a particular arbovirus (Hoffmann et al. 2011, Aliota et al. 2016). These novel approaches are currently in field trials, but none have yet reached the stage of evaluating their impact on human disease.
Insect traps successfully suppress agricultural insect pest populations (Day and Sjogren 1994) and tsetse flies (Lindh et al. 2009), but their use as control tools against Ae. aegypti has been limited to some Vector Control Programs (Rapley et al. 2009). Several traps have been tested as control tools, including BG-Sentinel traps (Degener et al. 2014) and a variety of ovitraps targeting the eggs (Regis et al. 2013) or gravid females (Sithiprasasna et al. 2003, Kittayapong et al. 2008, Ritchie et al. 2008, Barrera et al. 2014a, Degener et al. 2015). Traps targeting ovipositing females eliminate those mosquitoes already fed on blood and possibly infected with arboviruses. A disadvantage of using traps as control tools in urban areas is the need to place traps in protected areas on private properties, thus requiring the consent and acceptance of individual residents. Another logistical factor is the need to deploy enough traps per residence in most of the houses to achieve area-wide population suppression (Degener et al. 2015). As with the other container-Aedes control tools, testing whether mosquito traps are useful for the prevention and control of arbovirus infections and human disease is necessary. Ideally, such studies use epidemiological and clinical data to evaluate the impact of the vector control measure, with adequate experimental methods like the cluster randomized design (Wolbers et al. 2012). However, such a study would require significant resources and conducting smaller studies, such as investigating the incidence of virus in mosquitoes in areas with and without control measures to gather preliminary evidence of efficacy that can reduce costs (Lambrechts et al. 2015).
This investigation used that approach to explore if use of CDC autocidal gravid ovitraps for control (AGO traps; Mackay et al. 2013) could result in significant differences in the incidence of CHIKV in gravid females of Ae. aegypti between neighborhoods with and without traps. Local Ae. aegypti mosquitoes may get the virus from infectious residents or may be born, a sudden increase and persistence of infected local mosquitoes is an indirect indicator of ongoing virus transmission among residents. AGO traps have been tested for their effectiveness at controlling populations of Ae. aegypti in two isolated neighborhoods in southern Puerto Rico; at one site since 2011 and at the other one since 2013 (Barrera et al. 2014a, b). The results of this ongoing, longitudinal entomological study have shown that the populations of Ae. aegypti are being kept 60-80% below expected levels, without presenting the frequent mosquito outbreaks observed in two nearby neighborhoods without control traps. After the first detection of CHIKV cases in Puerto Rico in May 2014 (Sharp et al. 2014), we used our weekly collections of mosquitoes to compare CHIKV virus incidence in Ae. aegypti in areas with and without AGO control traps to test the hypothesis that the presence of control traps limited local outbreaks of CHIKV. Given the observed significant reduction of virus incidence in mosquitoes in areas with traps, we propose values of Ae. aegypti density thresholds that reduced local CHIKV transmission in a non-immune human population. A subsequent study of the prevalence of antibodies against CHIKV in residents of these communities showed significantly lower prevalence (50%) in areas with traps (Lorenzi et al. 2016).
Materials and Methods
The study took place from June to December 2014 in four neighborhoods in southern Puerto Rico. La Margarita (Intervention area I) was a relatively isolated community (17° 58’ 18” N; 66° 18’ 10” W; 3 m elevation) with 327 buildings (18 Ha) where 3 AGO control traps were deployed per home in 85% - 87% of homes (793 traps) since December 2011. Villodas was the other intervention area (Intervention area II) that was also relatively isolated from nearby communities (17° 58’ 13” N; 66° 10’ 48” W; 20 m EL). Villodas had 241 houses (11 Ha) and we deployed 3 AGO control traps per home in 83% - 87% of homes (570 traps). Villodas served as a non-intervention area from December 2011 to February 2013 when control AGO traps were deployed as a partial cross-over intervention (Barrera et al. 2014b). Stationary sentinel AGO traps (SAGO) were uniformly distributed across La Margarita (44 traps) and Villodas (27 traps) to monitor local Ae. aegypti populations weekly.
As the study design did not require isolated control areas, the two non-intervention or reference areas, Arboleda and Playa were part of larger neighborhoods. We deployed 30 SAGO traps in Arboleda (Non-intervention area I) in an area having 398 houses (17° 58’ 46” N; 66° 17’ 23” W; 10 m EL; 21 Ha), whereas 28 SAGO traps were deployed in a sector of Playa (Reference area II) that had 269 houses (17° 57’ 59” N; 66° 18’ 10” W; 1 m EL; 17 Ha). We serviced both control and sentinel AGO traps every two months and examined sentinel traps every week to collect and account for number of adult mosquitoes, species, and sex. Most of the Ae. aegypti females collected every week from SAGO traps in each of the four study sites were pooled (1-20 specimens per pool per site per week) and preserved at −80ºC until they were processed by RT-PCR to identify viral RNA of DENV and CHIKV.
Air temperature, relative humidity and rainfall were recorded using meteorological stations (HOBO Data Loggers, Onset Computer Corporation, Boume, MA) located in the center of La Margarita, Villodas, and Arboleda. Because Playa and La Margarita were adjacent neighborhoods (200 m apart), we used the same meteorological data for both communities. We conducted the study during the warmer and wetter season part of the year. Additional details of the study areas are available (Barrera et al. 2014a, b).
Detecting DENV and CHIKV in mosquitoes by RT-PCR.
Mosquito pools were homogenized using six 2.8mm ceramic grinding beads (VWR, Radnor, PA) in a Qiagen TissueLyser (Qiagen, Germantown, MD) at 25 cycles/second for five minutes with 1% bovine serum albumin (pH 7.0), 1.5mL of BA1 Diluent (2mM L-glutamine, 1x M199-Hank’s salts, 0.05M Tris buffer; pH 7.5), 0.35 mg sodium bicarbonate, 100 units of penicillin, 100μg of streptomycin, 1μg of Amphotericin B per ml, and. The homogenate was centrifuged (three minutes at 8,000 rpm) and the supernatant removed and aliquoted into one tube for virus testing and another one for storage. RNA was extracted using a Qiagen M48 automated extractor and Qiagen MagAttract Virus Mini M48 kits. The presence of CHIKV and DENV ribonucleic acid (RNA) was detected by real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR).
RT-PCR for chikungunya was adapted from (Lanciotti et al. 2007), where each reaction contained 12.5μl of 2x reaction mix, 6.35μl nuclease free water, 0.25μl of each primer (forward and reverse) at a concentration of 100μM, 0.15μl of a FAM labeled probe at a concentration of 25μM, and 0.5μl SuperScript® III RT/Platinum® Taq polymerase. Each reaction contained 5.0μl of RNA template and amplified in 96 well plated on an Applied Biosystems 75000 Fast DX Real Time PCR Instrument. Dengue virus RNA was detected in multiplex using Invitrogen’s Superscript® III Platinum® One-Step quantitative RT-PCR system (Santiago et al. 2013). Briefly, each DENV RT-PCR reaction contained 5.57μL of nuclease free water, 12.5μl 2X reaction mix, and 0.25μl of SuperScript® III RT/Platinum® Taq polymerase. Primers were prepared at a solution of 100μM, of which 0.25μl of DENV type 1 and 3 primers, and 0.125μl of DENV type 2 and 4 primers were added to the master mix. Thermocycling conditions consisted of three stages, 1) 30 minutes at 50˚C, 2) 2 minutes at 95˚C, and 3) 15 seconds at 95˚C and 1 minute at 60˚C. Data were collected at the second step of stage 3, and samples with a Ct value of 37 or below were considered positive for the presence of virus.
Statistical analyses.
We investigated if the number of females of Ae. aegypti captured per trap per week was significantly different in areas with and without traps using a generalized linear mixed model analysis (GLMM). Rainfall (accumulated during the third and second week before each mosquito sampling), temperature (average of current and two weeks before sampling) and relative humidity (average of current and two weeks before sampling) were included as covariates. We used a negative binomial distribution model with log link and a first-order autoregressive function for the covariance structure of the repeated measures. Study site and trap ID were included as random factors to account for trap variability. Additionally, a generalized linear model (GLM) was employed to determine if the number of positive pools identified per site per week could be explained by the presence of AGO control traps (intervention vs. non-intervention sites) using the following covariates: average number of female Ae. aegypti per trap per week, rainfall, temperature, and relative humidity. The null hypothesis was that the number of positive pools detected every week was not statistically different in areas with and without control traps. The distribution probability function of the dependent variable was a Poisson with log link. Statistical analyses were performed using IBM SPSS Statistics 20 software (IBM Corporation, Armonk, NY). Maximum likelihood minimum infection rates of mosquitoes and two sample tests were calculated using PooledInfRate version 4.0 (Biggerstaff 2016). The Vector Index (VI), an indicator of the expected number of infected mosquitoes per trap per week, was calculated as the proportion of infected mosquitoes times the average number of mosquitoes captured per trap per week (Jones et al. 2011).
Results
Mosquito dynamics.
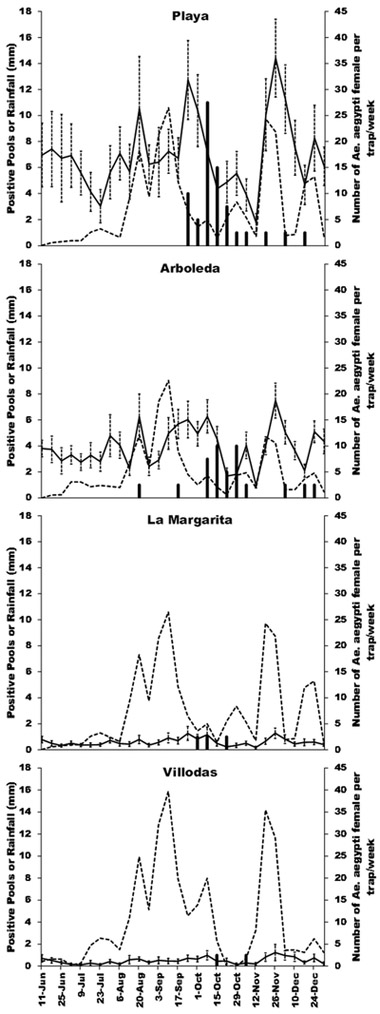
Totals of 26,251 females and 3,649 males of Ae. aegypti were captured from 3,859 traps × weeks between June 11 and December 31, 2014 in the four study areas. Most Ae. aegypti females (55.2%) and males (68%) were captured in Playa, followed by Arboleda (33.5% females, 24.3% males), La Margarita (7.5%, 4.8%), and Villodas (3.8%, 2.9%; Table 1). Aedes mediovittatus (Coquillett) was captured in AGO traps but at very low densities (Table 1). Culex quinquefasciatus Say was also commonly captured but their numbers were not recorded in this study. The GLMM analysis comparing number of Ae. aegypti per trap per week was significant (F4,3814 = 142.6, P< 0.001), with significant effects of the presence of AGO control traps (t= 20.4, P< 0.001), accumulated rainfall (t= 11.4, P< 0.001), and temperature (t= −4.8, P< 0. 01). Means estimated by the model were 11.6 and 1.1 females of Ae. aegypti per trap per week in non-intervention and intervention areas, respectively (fixed predictors: rainfall= 30.4 mm; temperature= 27.7 ºC, relative humidity= 75.7%). Thus, as an average, there were 10.5 times more Ae. aegypti females (91%) in areas without AGO control traps. The coefficient for accumulated rainfall indicated an average increase in the number of female Ae. aegypti per trap per week of one specimen per mm of rainfall. It can be observed that the numbers of adult females increased following corresponding increases in rainfall, particularly in the study sites without control traps (Arboleda, Playa) (Fig. 1).
Table 1.
Number of Ae. aegypti and Ae. mediovittatus mosquitoes captured in SAGO traps and metrological variables (mean ± SE) of the study sites registered from June to December 2014 in southern Puerto Rico.
| Study site |
Ae. aegypti females |
Ae. aegypti males |
Ae. mediovittatus females |
Ae. mediovittatus males |
Average temperature (3 previous weeks; °C) |
Accumulated rainfall (2nd and 3rd weeks before sampling; mm) |
Average relative humidity (3 previous weeks; %) |
|---|---|---|---|---|---|---|---|
| La_Margarita | 1963 | 173 | 13 | 1 | 28.0 ± 0.2* | 30 ± 6* | 75.3 ± 0.5* |
| Villodas | 993 | 102 | 1 | 0 | 27.6 ± 0.2 | 41 ± 8 | 77.1 ± 0.6 |
| Arboleda | 8807 | 887 | 3 | 0 | 27.1 ± 0.2 | 20 ± 4 | 75.4 ± 0.5 |
| Playa | 14488 | 2487 | 9 | 0 | 28.0 ± 0.2* | 30 ± 6* | 75.3 ± 0.5* |
| Total | 26251 | 3649 | 26 | 1 | 27.7± 0.1 | 30 ± 3 | 75.8 ± 0.3 |
Data came from the same meteorological station because these communities are adjacent
Figure 1.
Average number of female Aedes aegypti per sentinel AGO trap, CHIKV-positive mosquito pools, and accumulated rainfall (third and second week before sampling) per week in non-intervention (Playa, Arboleda) and intervention areas (La Margarita, Villodas) during the second half of 2014 in Puerto Rico.
Detection of DENV and CHIKV in Ae. aegypti.
A total of 1,334 pools of Ae. aegypti females was collected and analyzed by RT-PCR to identify DENV and CHIKV RNA in the four study sites. Mosquito pools could not be collected in four out of the 30 weeks of the study because of a shortage of personnel (July 1 and 7, August 5 and 26). None of the pools were positive for DENV. A total of 55 pools tested positive for CHIKV, for an overall infection rate of 2.41 mosquitoes per thousand (1.83 – 3.11 95% CI; Table 2). The first positive pool was registered on August 19 and the last on December 24. Using data only from August to December, the overall infection rate would be 3.24 (2.46 – 4.18; 905 pools, 17500 specimens). The resulting number of pools and mosquitoes processed varied per site according to their local abundance (Table 2). Ten times more CHIKV positive pools were identified in the non-intervention areas (50/55 pools or 91%; Playa, Arboleda) than in intervention areas (5/55 or 9%; La Margarita and Villodas; Table 2). The CHIKV minimum infection rates in each of the four sites were similar, with overlapping confidence intervals (Table 2). A two-sample test of the difference in infection rates between sites with the lowest (1.75) and highest (2.40) infection rates was not significant (D = −0.75; −2.47 – 2.32; P> 0.05). None of the 16 pools of Ae. mediovittatus was positive for DENV or CHIKV.
Table 2.
Number of CHIKV positive pools of female Ae. aegypti, infection rates (per thousand mosquitoes), average pool size, number of tested mosquitoes, and average females trapped per week in SAGO traps in each of the study sites in southern Puerto Rico, from June to December 2014.
| Study site / Treatment |
CHIKV Positive pools |
CHIKV infection Rate (x 1000; 95% CI) |
Pools (Avg. mosquitoes / pool) |
Ae. aegypti females in pools |
Average (± SE) density of female Ae. aegypti per trap per week |
Vector Index (Average infected mosquitoes / trap / week) |
|---|---|---|---|---|---|---|
| La_Margarita / Intervention | 3 | 1.75 (0.46 - 4.72) | 104 (17) | 1730 | 1.5 ± 0.1 | 0.003 |
| Villodas / Intervention | 2 | 2.13 (0.38 – 6.98 | 65 (15) | 950 | 1.2 ± 0.1 | 0.003 |
| Arboleda / Non-Intervention | 19 | 2.46 (1.53 - 3.77) | 443 (18) | 7881 | 9.8 ± 0.2 | 0.024 |
| Playa / Non-Intervention | 31 | 2.48 (1.72 - 3.48) | 722 (18) | 12768 | 17.4 ± 0.6 | 0.043 |
| Total | 55 | 2.41 (1.83 – 3.11) | 1334 | 23329 | 6.8 ± 1.0 | 0.016 |
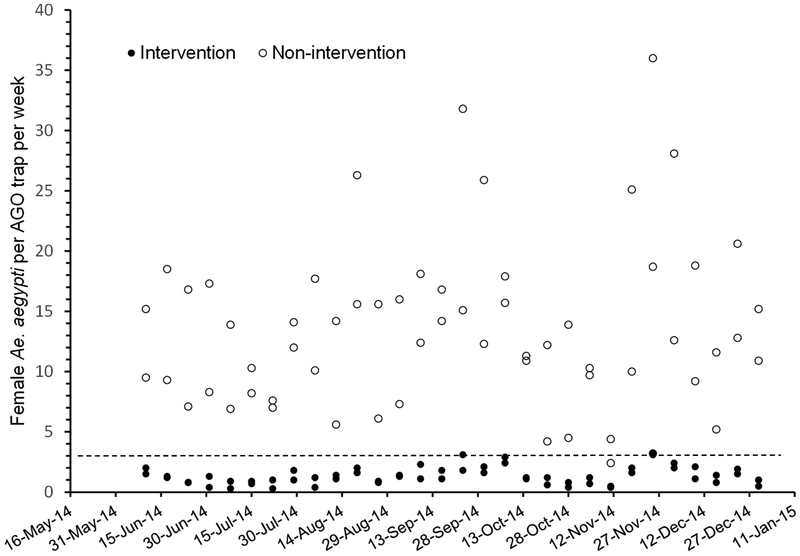
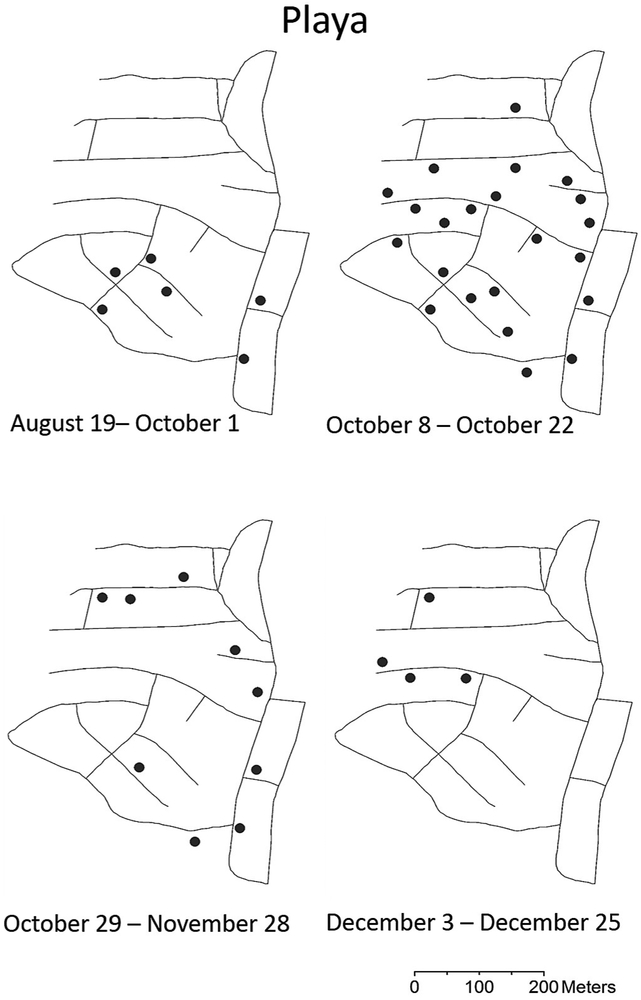
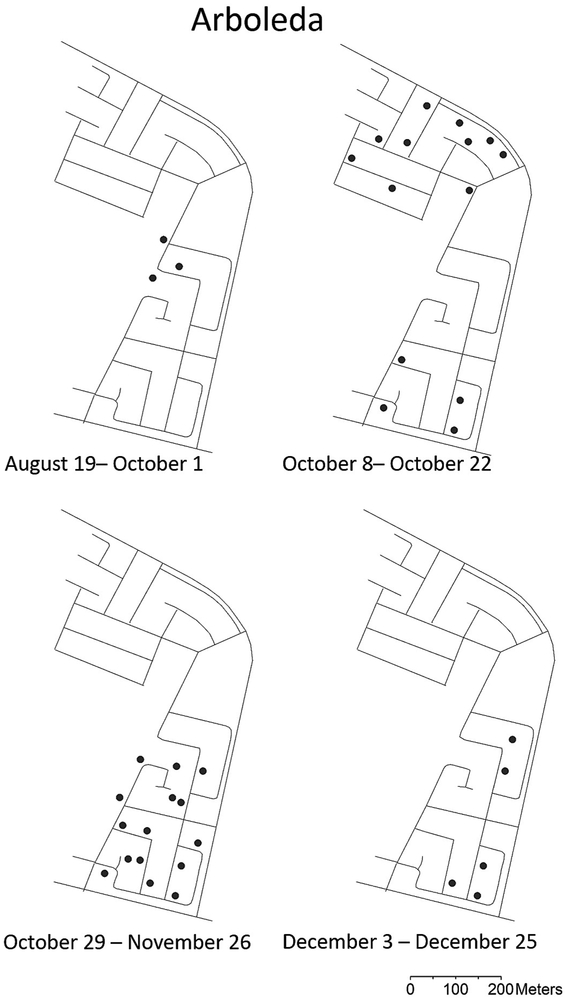
The results of the GLM analysis comparing number of positive pools per site per week between intervention and non-intervention areas was significant (Wald’s χ2 = 24.1, df= 1, P <0.01). Positive pools were detected for seven consecutive weeks out of ten weeks showing positive pools in Playa (September 23 – November 4), five out of ten weeks in Arboleda (October 7 - November 4), two out of three weeks in La Margarita (October 21 – 29), and no consecutive positive pools were detected in Villodas (Fig. 1). Consecutive virus detections in non-intervention areas disappeared when mosquito densities decreased to 2.4 - 4.4 female Ae. aegypti per trap per week on November 12, 2014 (Figs. 1, 2). The density of Ae. aegypti in intervention areas were at or below three females per trap per week (Fig. 2). Most positive pools were registered between October 8-22, and they were scattered across the entire Playa and Arboleda communities (Figs. 3, 4). Maps of the locations of positive pools in the intervention communities were not possible to draw, because given their lower mosquito densities, pools had to be made from specimens collected in many or most of the traps. For example, the total number of female Ae. aegypti mosquitoes collected in Villodas on the week of November 4 in all 27 sentinel AGO traps was 19, so that just one pool was made which was positive for CHIKV.
Figure 2.
Average number of female Aedes aegypti per trap per week in intervention (solid dots) and non-intervention (open dots) areas in Puerto Rico from June to December 2014. The dotted line drawn at 3 females per trap per week separates most average captures between intervention and non-interventions areas.
Figure 3.
Map of Playa community showing streets and locations of traps with CHIKV-positive mosquito pools from June to December 2014 in southern Puerto Rico.
Figure 4.
Map of Arboleda community showing streets and locations of traps with CHIKV-positive mosquito pools from June to December 2014 in southern Puerto Rico.
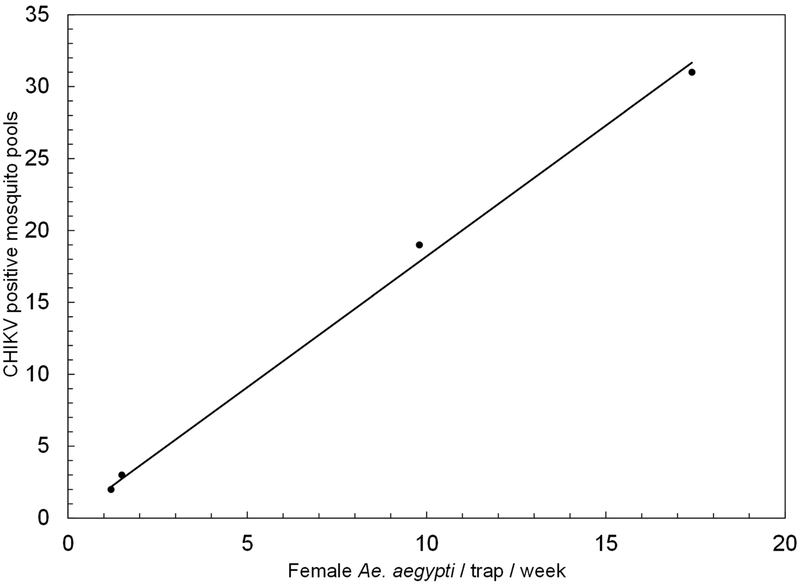
Rainfall peaked twice during the study; the first and larger peak during August – September associated with corresponding increases in Ae. aegypti captures and CHIKV detections, and a second peak in November associated with increases in mosquito densities but scattered virus detections (Fig. 1). The densities of Ae. aegypti in non-intervention areas were well above those observed in intervention areas most of the time (Fig. 2). In spite of the limited rainfall recorded in June and July, the density of Ae. aegypti in non-intervention areas stayed at relatively high levels (8.5 – 14.2). The overall Vector Index, or expected number of infected mosquitoes per trap per week, in each of the two intervention sites (0.003) was eight and 14 times smaller than in the non-interventions sites Arboleda (0.024) and Playa (0.043), respectively (Table 2). The number of positive pools and average density of Ae. aegypti females per trap per week in the study locations had a highly significant linear relationship (Fig. 5; r= 0.998; P< 0.01). The correlation between number of positive pools and the Vector Index was similar (r= 0.998; P< 0.01).
Figure 5.
Relationship between number of CHIKV-positive mosquito pools and average number of female Aedes aegypti per trap per week in all study areas from June to December 2014.
Discussion
We have shown that using three CDC AGO traps per home in most homes (> 85%) per neighborhood caused sustained reductions of Ae. aegypti captures in the order of 60-80% for several years (Barrera et al. 2014a, b). The purpose of the current investigation was to determine if mosquito population reduction in areas with traps was sufficient to prevent or limit the extent of local outbreaks of CHIKV. The hypothesis was that AGO control traps reduce the incidence of CHIKV in mosquitoes as a result of lowered vector densities. Aedes aegypti mosquitoes can only acquire the virus from infected persons, and thus virus detection in Ae. aegypti reflects infections in people living nearby. This approach to monitoring virus circulation is not common because the proportion of mosquitoes infected with arboviruses is generally very low, thus requiring large samples, and Ae. aegypti is typically a low-abundance mosquito (Barrera 2015). An advantage of using gravid traps to monitor infected mosquitoes is that their infection rate should be higher than in samples of adult mosquitoes captured using traps for host-seeking mosquitoes, which include nulliparous non-infectious females.
The results show that the densities of female Ae. aegypti were about ten times lower in neighborhoods with control traps than in two nearby neighborhoods without control traps. Also, increases in mosquito density following rains were limited in intervention sites in comparison with the large increases observed in non-intervention neighborhoods. The total number of CHIKV positive mosquito pools detected was also about ten times larger in non-intervention neighborhoods, showing a significant linear relationship with the density of female Ae. aegypti per trap per week among study sites. Interestingly, the minimum infection rate was similar across study sites. For this reason, the Vector Index, or expected number of infected mosquitoes, showed the same linear relationship with the total number of positive pools as mosquito density. Perhaps the most important observation was the presence of CHIKV positive pools in consecutive weeks in non-intervention areas, interpreted as evidence of sustained local transmission. By contrast, positive pools in intervention areas were very few and scattered, interpreted as lack of sustained transmission or outbreak. Results obtained from a subsequent cross-sectional investigation of the prevalence of IgG CHIKV antibodies in residents of the study sites showed significantly lower prevalence in areas with AGO traps (Lorenzi et al. 2016). These results confirm that studies of the incidence of arboviruses in mosquitoes can be a proxy for human infections. The infection rates observed in this study (1.8 - 4.2 per thousand) were lower than those observed using AGO traps around confirmed CHIKV cases (8 per thousand) in various neighborhoods in Puerto Rico during 2014 (CDC, unpublished). Infection rates found in this study were lower than in other reports, but comparisons are difficult to establish because of the use of different capture and sampling techniques (Sang et al. 2008, Diaz-Gonzalez et al. 2015, Dzul-Manzanilla et al. 2015).
Proving the efficacy of vector control interventions against arboviruses in natural settings is challenging due to the short duration of infections in humans and the transient occurrence of local outbreaks, which result from buildup of life-long immunity and exhaustion of susceptible hosts. This limitation is particularly important in small groups of people, such as those one would use in cluster randomized designs. For these reasons, alternative and more affordable approaches have been suggested as a way to test the effectiveness of vector control tools (Lambrechts et al. 2015). The approach followed in this investigation mirrors an observational cohort study, with one group having a presumed protective intervention and the outcome of exposure to circulating viruses followed over time in mosquito populations rather than people. We anticipated exposure to DENV and CHIKV because dengue viruses have been endemic in Puerto Rico since 1980’s (Barrera 2010) and the first ever detection of local transmission of CHIKV was in May 2014 (Sharp et al. 2014). The current spread of Zika virus in Puerto Rico and the Americas suggest its eventual occurrence in the same urban areas affected by DENV and CHIKV in the past. This methodology is applicable then to testing the effectiveness of vector control measures against all three viruses which share the same transmission cycle.
The results from this investigation suggest that local transmission of CHIKV in the two communities was more likely when the density of Ae. aegypti was larger than three females per trap per week. Even in the non-intervention areas, the presence of virus in gravid mosquitoes decreased and became more sporadic when the density fell to 2.4 - 4.4 females of Ae. aegypti per trap per week in November 2014. Mosquito densities around confirmed CHIKV cases in three neighborhoods with positive pools of female Ae. aegypti in Puerto Rico in 2014 were 5.3 – 20.5 per AGO trap per week and 4.3 in the neighborhood where no positive pools were found (CDC, unpublished). Ritchie et al. (2004) observed that no DENV was observed in mosquitoes and human cases dropped, when the density of gravid Ae. aegypti females fell below 0.5 females per sticky trap per week. Because their trap was smaller than the AGO trap, fewer mosquitoes are expected to signal a possible threshold for transmission. In a previous study, we compared captures in BG-Sentinel and AGO traps in the four study areas, showing a significant, positive non-linear relationship (Barrera et al. 2014a). The equivalent density in BG-Sentinel traps to three females per AGO trap per week is one female per trap per day. We are not aware of any previous studies using BG-Sentinel traps where a threshold for arbovirus transmission has been proposed. Active DENV transmission was reported during the dry and cooler season in San Juan city when the density of Ae. aegypti in BG-Sentinel traps was the lowest but still between 2-3 females per trap per day (Barrera et al. 2011). Additionally, a comparison of captures in AGO traps and paired ovitraps in Puerto Rico was significant, with a positive non-linear relationship (Mackay et al. 2013). The calculated equivalent egg density in paired ovitraps to three females per AGO trap per week is six eggs per day. Mogi et al. (1990) reported the appearance of dengue hemorrhagic fever cases when the density of eggs of Ae. aegypti was larger than three eggs per ovitrap per day, which is similar to our figure of six eggs per pair of ovitraps. Investigators have suggested Ae. aegypti density thresholds for arbovirus transmission using larval indices (Connor and Monroe 1923, Brown 1974). Modeling shows that threshold densities vary with temperature, frequency and amount of virus importation, and herd immunity (Focks et al. 2000). Generally, higher thresholds or more mosquitoes would be required to cause an outbreak at lower temperature, lower frequency of virus importation, and higher levels of herd immunity. Other factors may also come into play affecting threshold densities by reducing vectorial capacity (Newton and Reiter 1992), such as smaller contact rates between mosquitoes and people with the use of screens and other personal protection measures (Waterman et al. 1985). The ability to define minimum numbers of Ae. aegypti females protective against rampant arboviral outbreaks is important so vector control programs can have clearly defined goals; but defining such thresholds requires additional research. The relatively recent availability of practical tools for monitoring the adult Ae. aegypti population will facilitate such a task (Barrera 2016).
This investigation used AGO traps for surveillance and control purposes, thus in treatment areas sentinel traps were surrounded by many control traps. A concern is that mosquito density in surveillance traps may provide an underestimation of the real mosquito density in the presence of control traps, which would then reflect an overestimated reduction in vector density. We addressed that concern in an earlier work (Barrera et al. 2014a) where we compared weekly captures of female Ae. aegypti in sentinel AGO and in modified BG traps (Barrera et al. 2013) in areas with and without control traps for over one year. The results showed significant, non-linear positive relationships between captures in both traps, which were similar in areas with and without AGO control traps. For that reason, we are confident that AGO traps are reliable surveillance tools in the presence of control traps.
Acknowledgements
We thank the residents and Municipalities of Salinas and Guayama and our technical personnel Orlando Gonzalez, Juan Medina, Jose González and Luis Rivera. Funding was provided by the Division of Vector Borne Infectious Diseases, Centers for Disease Control and Prevention. This investigation would not have been possible without the support of Dr. Harold Margolis, Chief of the CDC Dengue Branch.
References Cited
- Agarwal A, Dash PK, Singh AK, Sharma S, Gopalan N, Rao PVL, Parida MM, and Reiter P. 2014. Evidence of experimental vertical transmission of emerging novel ECSA genotype of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 8(7): e2990. doi: 10.1371/journal.pntd.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Walker EC, Uribe Yepes A, Dario Velez I, Christensen BM, and Osorio JE. 2016. The wMel strain of Wolbachia reduces transmission of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis 10: e0004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alphey L, McKemey A, Nimmo D, Neira Oviedo M, Lacroix R, Matzen K, and Beech C. 2013. Genetic control of Aedes mosquitoes. Pathog Glob Health 107: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R 2010. Dinamica del dengue y Aedes aegypti en Puerto Rico. Biomedica 21: 179–195. [Google Scholar]
- Barrera R 2015. Considerations for disrupting dengue virus transmission: Ecology of Aedes aegypti and current (non genetic) methods of control, pp. 103–124. In Adelman ZN (ed.), Genetic control of malaria and dengue. Academic Press, Oxford. [Google Scholar]
- Barrera R 2016. Recomendaciones para el monitoreo de Aedes aegypti. Biomedica 36(3) (published ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, Acevedo V, Hemme RR, and Felix G. 2014a. Sustained, area-wide control of Aedes aegypti using CDC autocidal gravid ovitraps. Amr J Trop Med Hyg 91: 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, Acevedo V, Caban B, Felix G, and Mackay AJ. 2014b. Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J Med Entomol 51: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, MacKay AJ, and Amador M. 2013. An improved trap to capture adult container-inhabiting mosquitoes. J Am Mosq Control Assoc 29: 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini R, Medici A, Puggioli A, Balestrino F, and Carrieri M. 2013. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J Med Entomol 50: 317–325. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, and Hay SI. 2013. The global distribution and burden of dengue. Nature. 496(7446):504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff B 2016. PooledInRate, version 4.0: An Excel® add-in to compute infection rates from pooled data. Centers for Disease Control and Prevention; .http://www.cdc.gov/westnile/resourcepages/mosqsurvsoft.html. Accessed May 8, 2016. [Google Scholar]
- Brown AWA 1974. World wide surveillance of Aedes aegypti. In Proceedings and Papers of the Forty second Annual Conference of the California Mosquito Control Association, Inc. and the Thirtieth Annual Meeting of the American Mosquito Control Association, Inc. [Google Scholar]
- Connor ME, and Monroe WM. 1923. Stegomyia indices and their value in yellow fever control. Am J Trop Med Hyg 3: 9–19. [Google Scholar]
- Day JF, and Sjogren RD. 1994. Vector control by removal trapping. Am J Trop Med Hyg 50: 126–133. [DOI] [PubMed] [Google Scholar]
- Degener CM, Eiras AE, Azara TMF, Roque RA, Rosner S, Codeco CT, Nobre AA, Rocha ESO, Kroon EG, Ohly JJ, and Geier M. 2014. Evaluation of the effectiveness of mass trapping with BG-Sentinel traps for dengue vector control: a cluster randomized controlled trial in Manaus, Brazil. J Med Entomol 51: 408–420. [DOI] [PubMed] [Google Scholar]
- Degener CM, Azara TM, Roque RA, Rosner S, Rocha ES, Kroon EG, Codeco CT, Nobre AA, Ohly JJ, Geier M, and Eiras AE. 2015. Mass trapping with MosquiTRAPs does not reduce Aedes aegypti abundance. Mem Inst Oswaldo Cruz. doi 10.1590/0074-02760140374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Gonzalez EE, Kautz TF, Dorantes-Delgado A, Malo-Garcia IR, Laguna-Aguilar M, Langsjoen RM, Chen R, Auguste DI, Sanchez-Casas RM, Danis-Lozano R, Weaver SC, and Fernandez-Salas I. 2015. First report of Aedes aegypti transmission of chikungunya virus in the Americas. Am J Trop Med Hyg 93:1325–9. doi: 10.4269/ajtmh.15-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzul-Manzanilla F, Martinez NE, Cruz-Nolasco M, Gutierrez-Castro C, Lopez-Damian L, Ibarra-Lopez J, Martini A, Torres-Leyva J, Bibiano-Marin W, Tornez-Benitez C, Ayora-Talavera G, and Manrique-Saide P. 2015. Arbovirus surveillance and first report of chikungunya virus in wild opulations of Aedes aegypti from Guerrero, Mexico. J Am Mosq Control Assoc 31: 275–277. [DOI] [PubMed] [Google Scholar]
- Focks DA, Brenner RJ, Hayes J, and Daniels E. 2000. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg 62: 11–18. [PubMed] [Google Scholar]
- Gubler DJ 2002. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res 33: 330–342. [DOI] [PubMed] [Google Scholar]
- Higgs S 2016. Zika virus: emergence and emergency. Vector Borne Zoonotic Dis 16: 75–76. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, Cook H, Axford J, Callahan AG, Kenny N, Omodei C, McGraw EA, Ryan PA, Ritchie SA, Turelli M, and O’Neill SL. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–459. [DOI] [PubMed] [Google Scholar]
- Jones RC, Weaver KN, Smith S, Blanco C, Flores C, Gibbs K, Markowski D, and Mutebi JP. 2011. Use of the vector index and geographic information system to prospectively inform West Nile virus interventions. J Am Mosq Control Assoc 27: 315–319. [DOI] [PubMed] [Google Scholar]
- Kittayapong P, Yoksan S, Chansang U, Chansang C, and Bhumiratana A. 2008. Suppression of dengue transmission by application of integrated vector control strategies at sero-positive GIS-based foci. Am J Trop Med Hyg 78: 70–76. [PubMed] [Google Scholar]
- Lambrechts L, Ferguson NM, Harris E, Holmes EC, McGraw EA, O’Neill SL, Ooi EE, Ritchie SA, Ryan PA, Scott TW, Simmons CP, and Weaver SC. 2015. Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis 15: 862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, and Campbell GL. 2007. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis 13: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh JM, Torr SJ, Vale GA, and Lehane MJ. 2009. Improving the cost-effectiveness of artificial visual baits for controlling the tsetse fly Glossina fuscipes fuscipes. PLoS Negl Trop Dis 3(7): e474 Doi 10.1371/journal.pntd.0000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi OD, Major C, Acevedo V, Perez-Padilla J, Rivera A, Biggerstaff BJ, Munoz-Jordan J, Waterman S, Barrera R, and Sharp TM. 2016. Reduced incidence of chikungunya virus infection in communities with ongoing Aedes aegypti mosquito trap intervention studies - Salinas and Guayama, Puerto Rico, November 2015-February 2016. MMWR 65: 479–480. [DOI] [PubMed] [Google Scholar]
- Mackay A, Amador M, and Barrera R. 2013. An improved autocidal gravid ovitrap for the control and surveillance of Aedes aegypti. Parasit Vectors 6(1):225. doi: 10.1186/1756-3305-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains JW, Brelsfoard CL, and Dobson SL. 2015. Male mosquitoes as vehicles for insecticide. PLoS Negl Trop Dis 15;9(1):e0003406. doi: 10.1371/journal.pntd.0003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monath TP, and Vasconcelos PF. 2015. Yellow fever. J Clin Virol 64: 160–173. [DOI] [PubMed] [Google Scholar]
- Newton EA, and Reiter P. 1992. A model of the transmission of dengue fever with an evaluation of the impact of ultra-low volume (ULV) insecticide applications on dengue epidemics. Am J Trop Med Hyg 47: 709–720. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Plichart C, Sang AC, Brelsfoard CL, Bossin HC, and Dobson SL. 2012. Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis 6(11):e1797. doi: 10.1371/journal.pntd.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapley LP, Johnson PH, Williams CR, Silcock RM, Larkman M, Long SA, Russell RC, and Ritchie SA. 2009. A lethal ovitrap-based mass trapping scheme for dengue control in Australia: II. Impact on populations of the mosquito Aedes aegypti. Med Vet Entomol 23: 303–316. [DOI] [PubMed] [Google Scholar]
- Regis LN, Acioli RV, Silveira JC Jr., Melo-Santos MA, Souza WV, Ribeiro CM, da Silva JC, Monteiro AM, Oliveira CM, Barbosa RM, Braga C, Rodrigues MA, Silva MG, Ribeiro PJ Jr., Bonat WH, de Castro Medeiros LC, Carvalho MS, and Furtado AF. 2013. Sustained reduction of the dengue vector population resulting from an integrated control strategy applied in two Brazilian cities. PLoS One 8(7):e67682. doi: 10.1371/journal.pone.0067682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, Long SA, McCaffrey N, Key C, Lonergan G, and Williams CR. 2008. A biodegradable lethal ovitrap for control of container-breeding Aedes. J Am Mosq Control Assoc 24: 47–53. [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Hanna JN, Hills SL, Piispanen JP, McBride WJH, Pyke A, and Spark RL. 2002. Dengue control in North Queensland, Australia: case recognition and selective indoor residual spraying. Dengue Bulletin 26: 7–13. [Google Scholar]
- Sang RC, Ahmed O, Faye O, Kelly CL, Yahaya AA, Mmadi I, Toilibou A, Sergon K, Brown J, Agata N, Yakouide A, Ball MD, Breiman RF, Miller BR, and Powers AM. 2008. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am J Trop Med Hyg 78: 77–82. [PubMed] [Google Scholar]
- Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, Medina F, Colon C, Margolis H, and Munoz-Jordan JL. 2013. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis 7(7):e2311. doi: 10.1371/journal.pntd.0002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte EJ, Knols BGJ, Samson RA, and Takken W. 2004. Entomopathogenic fungi for mosquito control: A review. J Insect Sci 4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp TM, Roth NM, Torres J, Ryff KR, Perez Rodriguez NM, Mercado C, Pilar Diaz Padro MD, Ramos M, Phillips R, Lozier M, Arriola CS, Johansson M, Hunsperger E, Munoz-Jordan JL, Margolis HS, and Garcia BR. 2014. Chikungunya cases identified through passive surveillance and household investigations--Puerto Rico, May 5-August 12, 2014. MMWR 63: 1121–1128. [PMC free article] [PubMed] [Google Scholar]
- Sithiprasasna R, Mahapibul P, Noigamol C, Perich MJ, Zeichner BC, Burge B, Norris SL, Jones JW, Schleich SS, and Coleman RE. 2003. Field evaluation of a lethal ovitrap for the control of Aedes aegypti (Diptera: Culicidae) in Thailand. J Med Entomol 40: 455–462. [DOI] [PubMed] [Google Scholar]
- Vontas J, Kioulos E, Pavlidi N, Morou E, della Torre A, and Ranson H. 2012. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pest Biochem Physiol 104: 126–131. [Google Scholar]
- Waterman SH, Novak GE, Sather G,E, Bailey RE, Rios I, and Gubler DJ. 1985. Dengue transmission in two Puerto Rican communities in 1982. Am. J. Trop. Med. Hyg. 34: 625–632. [DOI] [PubMed] [Google Scholar]
- Weaver SC, and Reisen WK. 2010. Present and future arboviral threats. Antiviral Res 85: 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, and Forrester NL. 2015. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res 120: 32–39. [DOI] [PubMed] [Google Scholar]
- Wolbers M, Kleinschmidt I, Simmons CP, and Donnelly CA. 2012. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Negl Trop Dis 6(11):e1937. doi: 10.1371/journal.pntd.0001937 [DOI] [PMC free article] [PubMed] [Google Scholar]