Introduction
Multiple sclerosis (MS) is an autoimmune, inflammatory disease of the central nervous system (CNS) that is mediated by autoreactive B and T cells. The pivotal role of B cells in disease pathogenesis has been reinforced by the successful development of B cell-depleting monoclonal antibodies that target the CD20 surface antigen on cells of the B cell lineage (1,2). The recently reported phase 3 trials of ocrelizumab, a humanized monoclonal anti-CD20 antibody (3,4), revealed profound suppression of inflammation in relapsing MS (RMS) patients and a partial reduction of disability progression in primary progressive MS (PPMS); these results mark a breakthrough in our understanding of disease pathogenesis and, most importantly, in improving the lives of MS patients (2–4). With the subsequent approval of ocrelizumab for RMS and PPMS by the Food and Drug Administration (FDA) in March 2017, representing the first and only agent ever approved for PPMS, and decisions by other regulatory bodies pending, we now appear to be at the dawn of a new era of B cell immunology and therapeutics. However, these treatment successes also raise many unanswered questions about the fundamental role of B cells in RMS, and its contribution to sustained inflammation in the progressive phase of the disease.
The first part of this review summarizes current knowledge of B cell immunology and the principles underlying use of CD20-depleting therapies. The second part explores the possible mechanisms of action of B cell depleting agents in MS, prospects for development of clinically useful biomarkers to monitor treatment response, and the potential role of other B cell-targeting agents.
B cells in MS: a key player in pathogenesis
Historically, rodent T cell mediated acute experimental autoimmune encephalomyelitis (EAE) models have shaped a T cell-centric view of human MS (5). First described more than 85 years ago, EAE remains today the most commonly used and versatile model of central nervous system (CNS) autoimmunity in general, and, more specifically, for MS. However, EAE is not a single entity; depending upon the strain or species of animal used, the antigen administered, and even the method of inoculation and the local microbial environment, distinct EAE phenotypes characterized by different immunopathologies, topographical patterns of involvement, and clinical courses (acute or chronic, relapsing or progressive) can result. In general, however, the pure T cell mediated models of EAE lack large sharply demarcated areas of demyelination that are the hallmark of MS (6). Through the development of demyelinating disease models that more closely resemble human MS, and demonstration that this MS-like pattern of tissue damage results from a combined T cell and humoral (e.g. antibody-mediated) pathology (5,7), the experimental basis was set for the clinical trials of anti-CD20 targeted B cell therapeutics (1) leading eventually to the groundbreaking success of ocrelizumab (8).
In MS, the presence of immunoglobulins (Ig) and complement deposition in the majority of acute demyelinating lesions is a well-recognized phenomenon (9,10). Moreover, oligoclonal bands (OCBs), which are intrathecally produced clonally expanded antibodies, have long been recognized as prognostic and diagnostic markers. OCBs are produced by CNS-infiltrating plasmablasts/plasma cells (11,12) and are clonally related to B cell clones that are present in the brain parenchyma, meninges, CSF, and the periphery (11,13–17). Whether a subgroup of those intrathecally produced antibodies is indeed pathogenic (12), or rather targeted against intracellular antigens as suggested by a recent study (18,19) remains unanswered (20). Nevertheless, findings from human T cell receptor (TCR) and B cell receptor (BCR) repertoire studies provide strong evidence for antigen-driven clonal expansion occurring locally in the brain, CSF and meninges (15,21–25). However, both experimental data and clinical observations, including the very rapid onset of efficacy with CD20-depleting therapies in RMS, indicate that the pathogenic role of B cells in MS is likely not restricted to antibody production (5,26).
B cells are likely to influence MS pathology through additional effector functions including antigen presentation and roles in pro-inflammatory and regulatory immune responses (27,28). B cells represent a unique population of antigen-presenting cells (APCs), cells that can bind antigens, and then internalize, process and express antigen fragments on class II molecules of the major histocompatibility complex (MHC). In the context of co-stimulatory molecules, T cells that bear T cell receptors capable of recognizing the specific antigen-MHC complex that is being presented on the B cell surface are then activated. What makes B cells unique among APCs is that they are highly specialized, presenting primarily only those antigens that bind to their clonal B cell receptor or Ig molecule; by contrast, other APCs, such as microglia or dendritic cells, are able to present a broad range of exogenous and endogenous antigens. In a series of elegant experiments, transgenic mice that were selectively deficient in the expression of MHC class II molecules only on B cells were resistant to EAE induction, whereas the absence of secreted antibodies did not alter disease susceptibility (29). These data provide direct evidence that some T-cell mediated autoimmune responses to CNS antigens are absolutely dependent upon B cells acting as APCs.
Moreover, B cells entering the CSF are important in recruiting other inflammatory cells and in promoting their survival (30), and aggregates of B cells are now known to persist as lymphoid follicle-like structures located in the meninges of chronic, and especially secondary progressive, MS patients (31,32). Lastly, B cells from MS patients have been reported to be inherently polarized towards secretion of high levels of proinflammatory molecules including interleukin-6 (IL-6) and granulocyte macrophage-colony stimulating factor (GM-CSF), and lower levels of the regulatory cytokine IL-10 (33,34). This could reflect an influence of genetic factors active on B cells that are involved in MS risk (35), an environmental effect (see microbiome, below), or a combination of the two.
Though the rapid response of B cell depletion therapy on focal inflammation and RMS argues against a primary effect on antibodies as initially hypothesized, the possible elimination of a yet-to-be identified autoantibody in MS cannot be completely excluded. CD20-depletion ablates short-lived plasmablasts, which are a potentially important effector cell subset in MS (36). Also, antibodies of the IgG4 subclass, which are often synthesized by short-lived plasmablasts, show a rapid decline in patients with IgG4-mediated autoimmune disease, which is paralleled by a clinical response (37). The response in RMS could also reflect the rapid depletion of peripheral autoreactive B cells, as functional studies have shown that B cell tolerance is impaired in the periphery but that central tolerance is normal in MS (38), unlike the case in many other autoimmune diseases in which central tolerance is impaired (39).
CD20+ depletion: Targeting the B cell lineage and distinct T cell subsets
Ocrelizumab is a humanized monoclonal antibody of the IgG1 subtype that targets the large extracellular loop of CD20 (3,40), a surface protein that functions as an ion channel. The full humanization as well as the distinct epitope on CD20 differentiate it from the other CD20-depleting agents rituximab and ofatumumab. CD20 is expressed on B cells across different stages of maturation, ranging from pre-B cells in the bone marrow to short-lived plasmablasts. It spares CD20-negative, long-lived plasma cells, which produce antibodies directed against previously encountered pathogens and vaccines, which might explain the favorable safety profile. The B cell-depleting effect of ocrelizumab is mediated through apoptosis, antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Its stronger ADCC than CDC capacity differentiates ocrelizumab from other CD20-targeting agents (rituximab and ofatumumab). Rituximab, a chimeric human-mouse anti-CD20 antibody, was the first B cell-depleting agent used for MS in preliminary clinical trials (1) and as such is the forerunner to ocrelizumab, while ofatumumab, a fully human anti-CD20 antibody that targets a different epitope of CD20, also showed promising efficacy against RMS in a preliminary trial (41) and a pivotal phase III study is in progress.
Treatment with ocrelizumab is administered initially as two paired doses of 300 mg i.v. separated by a two-week interval, and cycles are repeated as single infusions of 600 mg i.v. every 6 months. A single treatment leads to a rapid depletion of CD20+ B cells. Circulating B cells, which represent only about 2% of the total B cell pool, are the compartment most efficiently depleted by CD20-targeting agents. In contrast, that B cell depletion in lymphoid organs and in other tissues including the CNS is limited (42). Rituximab has been found to eliminate B cells in the CSF and in CNS perivascular spaces without any detectable effect on OCBs (43–45). This is most likely explained by the highly effective and sustained depletion of circulating B cells which prevents their recirculation into the target tissue, but persistence of some B cells residing in protected CNS niches. Four to eight months after treatment B cells repopulate in the peripheral blood from progenitors residing in the bone marrow. Repopulating B cells in the peripheral blood are comprised of primarily naïve and immature B cells with fewer memory B cells and plasmablasts (1,16,46,47). Potentially disease-driving memory B cells as well as pro-inflammatory B cells appear to show a prolonged suppression up to 2 years following a single course of B cell depletion (33,46). Due to its sparing of antibody-producing plasma cells and the long half-life of plasma cells, CD20-targeting agents typically do not cause a marked reduction in serum IgG, though modest reductions in IgA and IgM levels can occur (1). However, with long-term use of ocrelizumab antibody levels will most likely decrease, requiring ongoing monitoring of antibody levels.
Though classically recognized as a protein expressed by B cells, CD20+ B cell depletion also leads to depletion of a small population of T cells and significant alteration of T cell function. This effect is mediated through the direct depletion of a small subset of T cells and also through the loss of B cell influenced effects on T cells. Surprisingly, it is now recognized that expression of CD20 is not restricted to B cells but is also expressed on ~5% of CD3+ T cells, albeit at lower levels (CD20dim) (46,48). In MS, and in other neurologic diseases, CD3+CD20dim T cells are present at similar frequencies in cerebrospinal fluid (CSF) and peripheral blood (48) with a possible small increase in MS (46). Phenotypically CD3+CD20dim cells are more prevalent amongst CD8+ than CD4+ T cells (46,48). Functionally, they show a higher frequency of pro-inflammatory cytokine production (INFγ, TNFα, IL-17) (48). CD3+CD20dim T cells are effectively depleted by CD20-targeting therapy but repopulate earlier than CD20+ B cells (46,48). Of note, CD3+CD20dim T cell replenishment has been tentatively associated with clinical relapse (48).
In addition to direct effects on CD3+CD20dim T cells, limited data indicate that a reduction in proliferation and pro-inflammatory cytokine production of other T cell subsets also occurs following B cell depletion (49). In systemic lupus erythematosus (SLE), CD3+CD25+FoxP3+ regulatory T cells increase following CD20-depletion (50). The effects of CD20-depletion on T cells are only beginning to be defined, and additional work is sorely needed to define the biological relevance of CD20+ T cells as well as perturbations in other T cell populations accompanying anti-CD20 therapy. A better understanding of the functional consequences of B cell depletion therapy on T cells could illuminate the mechanism of action of these drugs on MS, and possibly also lead to development of biomarkers for monitoring anti-CD20 therapy.
Outstanding research questions and novel avenues for B cell-depleting therapies
While the selective depletion of CD20+ B cells as monotherapy in MS suppresses disease activity in both RMS and PPMS, the success of this highly selective immune therapy also raises many new questions about the role of B cells in MS as well as the underlying pathophysiology of PPMS.
CNS effects of ocrelizumab
The beneficial effects of ocrelizumab on clinical and MRI indices of progression in PPMS has highlighted the role of B cells in progressive forms of the disease, previously considered to be primarily driven by neurodegeneration. As noted above, there is limited evidence that OCBs can persist in the CSF even after chronic treatment with CD20-depleting therapy, indicating that there is an ongoing humoral immune response within the CNS that is resistant to treatment. In light of evidence for sequestration of some pathogenic immune responses behind an intact blood-brain barrier (BBB), particularly in chronic MS, intrathecal administration of ocrelizumab might improve therapeutic deletion of the relevant B cell subsets. A recent preliminary trial of intrathecal CD20-B cell depletion with rituximab in secondary progressive MS, however, showed no clinical benefit, possibly because insufficient concentrations of rituximab were present in the CSF and perhaps also due to inadequate CDC capacity in the CNS compared to the periphery (51). It is possible that ocrelizumab, which relies on ADCC more than CDC in its mechanism of action, might be more potent than rituximab in mediating CNS B cell depletion via an intrathecal route.
From a practical perspective, intrathecal dosing is not an attractive option for chronic use. More promising are approaches that employ small molecule drugs that cross the BBB effectively, or designer antibodies that can shuttle across the BBB by exploiting endogenous receptor-mediated transporters (52). Recent data from Alzheimer trials employed bispecific antibodies, in which one half of the antibody is designed to exploit an existing transportation system (the transferrin receptor) while the other half targets the protein of interest (amyloid plaque); this approach has shown promise in overcoming the BBB for therapeutic antibody delivery (53). Further studies need to address current safety concerns associated with bispecific antibodies (54) and development of more efficient technologies for enhanced shuttling of therapeutic antibodies across the BBB.
Targeted depletion of selective pathogenic CD20+ B cell subsets
Ocrelizumab leads to highly efficient B cell depletion and demonstrates a favorable safety profile (3,4,8). Infusion reactions and infections are the most frequent adverse effects reported with CD20-depletion, and with ocrelizumab the induction of anti-drug antibodies is very low (0.4%) due to its low immunogenicity (3). Very recently one case of progressive multifocal leukoencephalopathy (PML) was reported in a John Cunningham virus (JCV) positive patient who had recently begun treatment with ocrelizumab; the PML was likely unrelated to ocrelizumab but due to prior treatment with natalizumab, an immunomodulatory agent well known to be associated with PML risk. However, continued vigilance will be important, as many patients are expected to begin treatment with ocrelizumab in the near future. In the clinical trials there was also an imbalance in the number of malignancies, and especially in cases of female breast cancer, present with ocrelizumab compared with interferon (IFN)-β1 or placebo use (3,4). Although additional analyses and longer term follow up during the extension phase of the studies provide some optimism that these statistical imbalances may not represent a true biologic increase in risk, further long-term assessment of the safety profile of ocrelizumab will be required to fully define the risk of these rare adverse events.
Despite this overall favorable safety-profile, an even more targeted treatment of a selective pathogenic CD20+ B cell subset in the future would be desirable. While GM-CSF-producing B cells have been identified more recently as a pro-inflammatory subset that might be potentially pathogenic in MS patients (33), and B cells from MS patients are reported to secrete yet unidentified factors toxic to oligodendrocytes (55) and neurons (56), functional studies are needed to confidently identify disease-driving B cell subsets as well as antigen-specific pathogenic cells.
B cell-related biomarkers to tailor ocrelizumab treatment to the individual needs of every patient
In the Phase III clinical trials, ocrelizumab was administered at fixed intervals of 24 weeks independent of an individual’s B cell reconstitution kinetics. In the real-life clinical setting, the optimal treatment cycle and duration of treatment still needs to be defined. B cell-reconstitution, as determined by CD19+ B cell levels in the peripheral blood, is currently the only readily available specific biomarker of treatment but based on studies in rheumatoid arthritis it is clear that B cell levels should not be used as a guide to the need for retreatment. Among potential future biomarkers, circulating memory B cells, B cell cytokine profiles, shed receptors of activated B cells and plasma cells (such as the B cell maturation antigen (BCMA) (57) and the transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) (58)), CD3+CD20dim T cell levels, and neurofilament light (NfL) levels as a marker for neurodegeneration are all under study. A recently launched, open-label, multi-center biomarker study (OBOE) of RMS and PPMS patients will address some of the issues through collection and immunological assessment of peripheral and CSF B and T cells before and during ocrelizumab treatment.
Effects of B cell depletion on the mucosal B cell compartment
A novel and rapidly evolving field of interest is the composition of the gut microbiome as a potential disease-relevant environmental factor and its impact on adaptive and innate immunity in the context of autoimmunity. Recent evidence from animal models and human MS suggest that the triggers and perpetuating factors of MS might be traced to the gut (59–61). As the differential composition of the gut microbiota in MS patients compared to controls is only beginning to be unraveled, the modifying effect of CD20-depleting therapies on the microbiome as well as the role of B cells and antibodies in the immunologic and neurohumoral communication between the gut and the brain - the so called “gut-brain axis” - in MS remains elusive (62–64).
At the microbiota-mucosal interface in the gut, B cells are important cellular and humoral mediators of immunity. Gut microbiota and microbial products have been shown to directly regulate B cell development, activation and differentiation (65–68). In a complementary fashion, the humoral B cell responses mediate homeostasis of the microbial composition via production of antibodies, in particular IgA. IgA-binding of gut bacteria has been recently been shown to identify potential pro-inflammatory bacteria in the context of autoimmunity (69–71) and, thus, might function as an important regulator between mucosal and systemic immunity. While IgA is the most abundantly produced immunoglobulin in humans, its role in the pathogenesis of MS has been understudied. Interestingly, mucosal IgA plasma cells residing in the gut lamina propria are not depleted by anti-CD20 depleting therapy (72). In the light of clinical trials with CD19-depleting therapies (Inebilizumab, MEDI-551) (73) that additionally target the pro-B cell and plasma cell compartment, the relevance of IgA-plasma cells in the gut and the consequences of potential depletion by tissue-resident B cells in the gut for mucosal homeostasis need to be addressed.
Novel therapeutic strategies for B cell-depletion
As noted above, anti-CD20 therapies eliminate most circulating B cells, leaving B cells in secondary lymphoid organs and other sites, such as meningeal follicle-like structures, unaffected. The resistance of B cell niches to treatment with anti-CD20 therapies likely explains the only partial effect of CD20-depleting agents on PPMS as well as the appearance of secondary progressive MS during CD20-depletion (74). Promising alternate areas of targeting those niche-residing B cells are the development of monoclonal antibodies that target plasma cells as well as small molecules that inhibit critical B cell signaling pathways.
MEDI-551 (Inebilizumab), a humanized, monoclonal antibody, binds with high-affinity to CD19, a surface marker expressed on a broad range of B cells including long-lived plasma cells (73). It is currently being evaluated in a phase II clinical trial in patients with neuromyelitis optica (NMO) spectrum disease. Another possible target antigen for monoclonal antibody therapy is CD38, a surface protein expressed on plasma cells. Daratumumab, an anti-CD38 monoclonal antibody, is approved for treatment of multiple myeloma (75). However, the wide expression of CD38 on other immune cells, including regulatory B cells, could discourage its potential use in MS.
Targeting selective pathways downstream of the BCR signaling with small molecules is another promising strategy. BCR signaling activates phosphoinositide 3-kinase (P13K), a lipid kinase, to produce a second messenger molecule, which recruits other proteins to the membrane. Novel BCR signaling pathway inhibitors include Ibrutinib and Idealisib among others (76–78). Both molecules target the P13K pathway and inhibit downstream BCR signaling thus interfering with B cell activation. Future studies will need to assess their capability to cross the blood-brain barrier.
Conclusions
It seems likely that ocrelizumab will emerge as a vital treatment of RMS and PPMS. Equally important, the remarkable clinical success of CD20-depleting therapies has also fundamentally reshaped our understanding of MS pathogenesis. Initially developed based on experimental data derived from an improved disease model and a goal to eliminate pathogenic antibodies, the profound anti-inflammatory effect of anti-CD20 therapy has stimulated new efforts to elucidate previously unappreciated functional roles of B cells in this complex demyelinating disease. Although many questions remain, it is clear that novel insights into the fundamental cause of MS are likely to be revealed and that a new era of B cell-directed approaches to this disease will emerge.
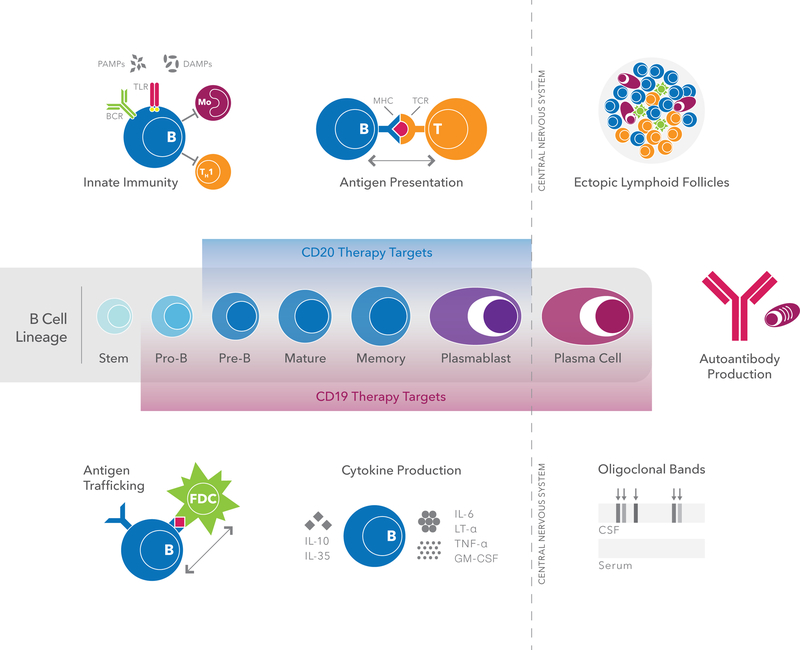
Figure 1: A neurotropic view of B cell trafficking and function in autoimmunity.
Multifunctional B cells evolve through a series of differentiation stages, from stem cells to antibody-producing plasma cells. B cells are potent antigen-presenting cells; participate in antigen transport; produce a range of pro-inflammatory and regulatory cytokines; and secrete antibodies that have undergone affinity maturation in germinal centers of lymphoid structures. In addition to these roles in adaptive immunity, B cells are also important players in innate immunity primarily through signaling via TLRs, a family of receptors that can recognize DAMPs and PAMPs. In chronic MS, memory B cells and plasma cells also accumulate in protected follicle-like structures located in the meninges and perivascular spaces. All of these functions of B cells may contribute to the effects of B cell depletion on disease activity in MS. As shown, B cell-depleting therapies targeted against CD20 and CD19 eliminate distinct B cell subpopulations.
Abbreviations: B = B cell, APC = antigen presenting cell, BCR = B cell receptor, CSF = cerebrospinal fluid, DAMPs = damage-associated molecular pattern molecules, GM-CSF = granulocyte macrophage-colony stimulating factor, IL = interleukin, LT-α = lymphotoxin-alpha, MHC = major histocompatibility complex, P = plasma cell, M = microglia/monocytes, PAMPs = pathogen-associated molecular pattern molecules, T = T cell, TCR = T cell receptor, TLR = toll-like receptor, TNF-α = tumor necrosis factor alpha.
Acknowledgment:
We thank Andrew Barnecut for outstanding assistance with preparation of this manuscript.
Funding:
A.-K.P. is supported by a postdoctoral fellowship from the Swiss National Science Foundation (P2SKP3_164938/1; P300PB_177927). S.L.H. receives funding from the National Institute of Health (NIH) (R01NS026799; R01NS049477), the National Multiple Sclerosis Society (NMSS) (RG2899) and the Conrad N. Hilton Foundation.
Footnotes
Conflict of interest:
A.-K.P. received travel reimbursement from Genzyme, Baxalta and Merck and speaker honoraria from Bayer used for research support S.L.H. serves on the Scientific Advisory Boards of Annexon, Bionure, Symbiotix, and Molecular Stethoscope, and on the Board of Trustees of Neurona; and also reports receiving travel reimbursement and writing assistance from F. Hoffmann-La Roche for CD20-related meetings and presentations.
References
- 1.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, HERMES Trial Group. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, Yin M, Leppert D, Glanzman R, Tinbergen J, Hauser SL. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, OPERA I and OPERA II Clinical Investigators. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 2017;376:221–234. [DOI] [PubMed] [Google Scholar]
- 4.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, De Sèze J, Giovannoni G, Hartung H-P, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, ORATORIO Clinical Investigators. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 2017;376:209–220. [DOI] [PubMed] [Google Scholar]
- 5.Hauser SL. The Charcot Lecture | beating MS: a story of B cells, with twists and turns. Mult Scler 2015;21:8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol 2017;133:223–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genain CP, Hauser SL. Creation of a model for multiple sclerosis in Callithrix jacchus marmosets. J Mol Med 1997;75:187–197. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and Other CD20(+) B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017;133:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol 1998;43:465–471. [DOI] [PubMed] [Google Scholar]
- 10.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 2000;47:707–717. [DOI] [PubMed] [Google Scholar]
- 11.Obermeier B, Mentele R, Malotka J, Kellermann J, Kümpfel T, Wekerle H, Lottspeich F, Hohlfeld R, Dornmair K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med 2008;14:688–693. [DOI] [PubMed] [Google Scholar]
- 12.Büdingen von H-C, Harrer MD, Kuenzle S, Meier M, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol 2008. 38:2014–2023. [DOI] [PubMed] [Google Scholar]
- 13.Bankoti J, Apeltsin L, Hauser SL, Allen S, Albertolle ME, Witkowska HE, Büdingen von H-C. In multiple sclerosis, oligoclonal bands connect to peripheral B-cell responses. Ann Neurol 2014;75:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermeier B, Lovato L, Mentele R, Brück W, Forne I, Imhof A, Lottspeich F, Turk KW, Willis SN, Wekerle H, Hohlfeld R, Hafler DA, O’Connor KC, Dornmair K. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J Neuroimmunol 2011;233:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büdingen von H-C, Kuo TC, Sirota M, van Belle CJ, Apeltsin L, Glanville J, Cree BA, Gourraud P-A, Schwartzburg A, Huerta G, Telman D, Sundar PD, Casey T, Cox DR, Hauser SL. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest 2012;122:4533–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palanichamy A, Apeltsin L, Kuo TC, Sirota M, Wang S, Pitts SJ, Sundar PD, Telman D, Zhao LZ, Derstine M, Abounasr A, Hauser SL, von Büdingen HC. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med 2014; 6:248ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern JNH, Yaari G, Vander Heiden JA, Church G, Donahue WF, Hintzen RQ, Huttner AJ, Laman JD, Nagra RM, Nylander A, Pitt D, Ramanan S, Siddiqui BA, Vigneault F, Kleinstein SH, Hafler DA, O’Connor KC. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 2014;6:248ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brändle SM, Obermeier B, Senel M, Bruder J, Mentele R, Khademi M, Olsson T, Tumani H, Kristoferitsch W, Lottspeich F, Wekerle H, Hohlfeld R, Dornmair K. Distinct oligoclonal band antibodies in multiple sclerosis recognize ubiquitous self-proteins. Proc Natl Acad Sci USA 2016;113:7864–7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winger RC, Zamvil SS. Antibodies in multiple sclerosis oligoclonal bands target debris. Proc Natl Acad Sci USA 2016;113:7696–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirmer L, Srivastava R, Hemmer B. To look for a needle in a haystack: the search for autoantibodies in multiple sclerosis. Mult Scler 2014;20:271–279. [DOI] [PubMed] [Google Scholar]
- 21.Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, Gilden DH. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol 1998;43:236–243. [DOI] [PubMed] [Google Scholar]
- 22.Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, Oksenberg JR. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 1999;163:5133–5144. [PubMed] [Google Scholar]
- 23.Qin Y, Duquette P, Zhang Y, Talbot P, Poole R, Antel J. Clonal expansion and somatic hypermutation of V(H) genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest 1998;102:1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo M, Dono M, Gazzola P, Roncella S, Valetto A, Chiorazzi N, Mancardi GL, Ferrarini M. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol 2000;164:2782–2789. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, Owens GP. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J Immunol 2004;173:649–656. [DOI] [PubMed] [Google Scholar]
- 26.Hohlfeld R, Meinl E. Ocrelizumab in multiple sclerosis: markers and mechanisms. Lancet Neurol 2017;16:259–261. [DOI] [PubMed] [Google Scholar]
- 27.Büdingen von H-C, Palanichamy A, Lehmann-Horn K, Michel BA, Zamvil SS. Update on the autoimmune pathology of multiple sclerosis: B-cells as disease-drivers and therapeutic targets. Eur Neurol 2015;73:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumbholz M, Derfuss T, Hohlfeld R, Meinl E. B cells and antibodies in multiple sclerosis pathogenesis and therapy. Nat Rev Neurol 2012;8:613–623. [DOI] [PubMed] [Google Scholar]
- 29.Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod’homme T, Varrin-Doyer M, Shetty A, Linington C, Slavin AJ, Hidalgo J, Jenne DE, Wekerle H, Sobel RA, Bernard CCA, Shlomchik MJ, Zamvil SS. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J Exp Med 2013;210:2921–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann-Horn K, Sagan SA, Bernard CCA, Sobel RA, Zamvil SS. B-cell very late antigen-4 deficiency reduces leukocyte recruitment and susceptibility to central nervous system autoimmunity. Ann Neurol 2015;77:902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004;14:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130:1089–1104. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, Gommerman JL, Prat A, Fillatreau S, Bar-Or A, Canadian B cells in MS Team. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015; 7:310ra166–310ra166. [DOI] [PubMed] [Google Scholar]
- 34.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 2012;209:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Multiple Sclerosis Genetics Consortium. Network-based multiple sclerosis pathway analysis with GWAS data from 15,000 cases and 30,000 controls. Am J Hum Genet 2013;92:854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, Sommer N, Hartung HP, Hemmer B. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 2005;128:1667–1676. [DOI] [PubMed] [Google Scholar]
- 37.Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum 2010;62:1755–1762. [DOI] [PubMed] [Google Scholar]
- 38.Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, Herold KC, Hafler DA, O’Connor KC, Meffre E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest;2013:123:2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J-Y, Stathopoulos P, Gupta S, Bannock JM, Barohn RJ, Cotzomi E, Dimachkie MM, Jacobson L, Lee CS, Morbach H, Querol L, Shan JL, Vander Heiden JA, Waters P, Vincent A, Nowak RJ, O’Connor KC. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann Clin Transl Neurol 2016;3:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein C, Lammens A, Schäfer W, Georges G, Schwaiger M, Mössner E, Hopfner K-P, Umaña P, Niederfellner G. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. mAbs 2012;5:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, Drulovic J, Filippi M. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 2014;82:573–581. [DOI] [PubMed] [Google Scholar]
- 42.Kamburova EG, Koenen HJPM, Borgman KJE, Berge ten IJ, Joosten I, Hilbrands LB. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 2013; 13:1503–1511. [DOI] [PubMed] [Google Scholar]
- 43.Büdingen von H-C, Bischof A, Eggers EL, Wang S, Bevan CJ, Cree BAC, Henry RG, Hauser SL. Onset of secondary progressive MS after long-term rituximab therapy - a case report. Ann Clin Transl Neurol 2017:4:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin MDP, Cravens PD, Winger R, Kieseier BC, Cepok S, Eagar TN, Zamvil SS, Weber MS, Frohman EM, Kleinschmidt-Demasters BK, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol 2009;66:1016–1020. [DOI] [PubMed] [Google Scholar]
- 45.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons J-A. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol 2006;180:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palanichamy A, Jahn S, Nickles D, Derstine M, Abounasr A, Hauser SL, Baranzini SE, Leppert D, Büdingen von H-C. Rituximab efficiently depletes increased CD20-expressing T cells in multiple sclerosis patients. J Immunol 2014. 193:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007;178:6092–6099. [DOI] [PubMed] [Google Scholar]
- 48.Schuh E, Berer K, Mulazzani M, Feil K, Meinl I, Lahm H, Krane M, Lange R, Pfannes K, Subklewe M, et al. Features of Human CD3+CD20+ T Cells. J Immunol 2016:197:1111–1117. [DOI] [PubMed] [Google Scholar]
- 49.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010;67:452–461. [DOI] [PubMed] [Google Scholar]
- 50.Vallerskog T, Gunnarsson I, Widhe M, Risselada A, Klareskog L, van Vollenhoven R, Malmström V, Trollmo C. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol 2007;122:62–74. [DOI] [PubMed] [Google Scholar]
- 51.Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, Maric D, Kosa P, Wu T, Bielekova B. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol (2016) 3:166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuchero YJY, Chen X, Bien-Ly N, Bumbaca D, Tong RK, Gao X, Zhang S, Hoyte K, Luk W, Huntley MA, et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016;89:70–82. [DOI] [PubMed] [Google Scholar]
- 53.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, Atwal J, Elliott JM, Prabhu S, Watts RJ, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med 2011;3:84ra44–84ra44. [DOI] [PubMed] [Google Scholar]
- 54.Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med 2013;5:183ra57–1–12. [DOI] [PubMed] [Google Scholar]
- 55.Lisak RP, Benjamins JA, Nedelkoska L, Barger JL, Ragheb S, Fan B, Ouamara N, Johnson TA, Rajasekharan S, Bar-Or A. Secretory products of multiple sclerosis B cells are cytotoxic to oligodendroglia in vitro. J Neuroimmunol 2012;246:85–95. [DOI] [PubMed] [Google Scholar]
- 56.Lisak RP, Nedelkoska L, Benjamins JA, Schalk D, Bealmear B, Touil H, Li R, Muirhead G, Bar-Or A. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J Neuroimmunol 2017;309:88–99. [DOI] [PubMed] [Google Scholar]
- 57.Laurent SA, Hoffmann FS, Kuhn P-H, Cheng Q, Chu Y, Schmidt-Supprian M, Hauck SM, Schuh E, Krumbholz M, Rübsamen H, et al. γ-secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015. 6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann FS, Kuhn P-H, Laurent SA, Hauck SM, Berer K, Wendlinger SA, Krumbholz M, Khademi M, Olsson T, Dreyling M, et al. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol 2015;194:542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011;479:538–541. [DOI] [PubMed] [Google Scholar]
- 60.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hauser SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci USA 2017;363:10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci USA (2017) 145:10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colpitts SL, Kasper LH. Influence of the Gut Microbiome on Autoimmunity in the Central Nervous System. J Immunol 2017;198:596–604. [DOI] [PubMed] [Google Scholar]
- 63.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology 2012;10:735–742. [DOI] [PubMed] [Google Scholar]
- 64.Pröbstel AK, Baranzini SE. The Role of the Gut Microbiome in Multiple Sclerosis Risk and Progression: Towards Characterization of the “MS Microbiome”. Neurotherapeutics 2017. Epub ahead of print [DOI] [PMC free article] [PubMed]
- 65.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, Panchakshari RA, Rodig SJ, Kepler TB, Alt FW. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature 2013;501:112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, Harris KA, Jones SA, Klein N, Mauri C. Regulatory B cells are induced by gut microbiota-driven interleukin-1β and interleukin-6 production. Nat Med 2014;20:1334–1339. [DOI] [PubMed] [Google Scholar]
- 67.Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 2010;1:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim M, Kim CH. Regulation of humoral immunity by gut microbial products. Gut Microbes 2017;67:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014;158:1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, Manary MJ, Liu T-C, Stappenbeck TS, Maleta KM, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med 2015;7:276ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A, et al. IgA-coated E. coli enriched in Crohn’s disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med 2017;9:eaaf9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mei HE, Frölich D, Giesecke C, Loddenkemper C, Reiter K, Schmidt S, Feist E, Daridon C, Tony H-P, Radbruch A, et al. Steady-state generation of mucosal IgA+ plasmablasts is not abrogated by B-cell depletion therapy with rituximab. Blood 2010;116:5181–5190. [DOI] [PubMed] [Google Scholar]
- 73.Chen D, Gallagher S, Monson NL, Herbst R, Wang Y. Inebilizumab, a B Cell-Depleting Anti-CD19 Antibody for the Treatment of Autoimmune Neurological Diseases: Insights from Preclinical Studies. J Clin Med 2016; 5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Büdingen von H-C, Bischof A, Eggers EL, Wang S, Bevan CJ, Cree BAC, Henry RG, Hauser SL. Onset of secondary progressive MS after long-term rituximab therapy - a case report. Ann Clin Transl Neurol 2017;4:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med 2015; 373:1207–1219. [DOI] [PubMed] [Google Scholar]
- 76.Puri KD, Di Paolo JA, Gold MR. B-cell receptor signaling inhibitors for treatment of autoimmune inflammatory diseases and B-cell malignancies. Int Rev Immunol 2013;32:397–427. [DOI] [PubMed] [Google Scholar]
- 77.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]