Abstract
Background: Acinetobacter baumannii is an emerging nosocomial pathogen causing serious complications due to the propensity of its multi-drug resistant property. Due to the indiscriminate and wide-spread use of antibiotics, A. baumannii strains emerge as MDR-Ab, XDR-Ab and in recent years pan-DR-Ab strains. Routine therapy incorporates the application of fewer antibiotics and antibiotic surveillance data is not monitored frequently. This study is thus an attempt to screen for the frequency of antibiotic resistance profile against different classes of antibiotics as per CLSI guidelines.
Methods: Phenotypically and genotypically characterized 73 A. baumannii strains were utilized for the antibiogram profile using Group A, B, and U antibiotics as per CLSI recommendations using standard Kirby Bauer disc diffusion method. Interpretations of susceptible, intermediate and resistance were recorded by measuring zone diameter criteria.
Results: Group A antibiogram profile showed highest non-susceptibility (n=73) (100%) to ampicillin-sulbactam, ceftazidime and imipenem followed by 82.19%, 79.45%, 67.12%, 56.16% and 49.31% non-susceptible isolates against ciprofloxacin, gentamicin, meropenem, tobramycin, and levofloxacin respectively. Group B antibiogram profile showed 100% non-susceptibility piperacillin-tazobactam and to amikacin, 91.78% (n=67) resistance against ceftriaxone. Among the cyclines, 19.71% and 6.84% of isolates were resistant to doxycycline and minocycline respectively. Under Group U, 76.71% showed resistance against tetracycline. The frequency of MDR (71.23%) and XDR (39.72%) A. baumannii isolates were detected.
Conclusion: Periodical antibiotic surveillance is essential to curb the menace of the emergence of MDR and XDR A. baumannii in the hospital environment thus improving the patient care by the administration of alternate drug of choice or by combination therapy.
Keywords: MDR, XDR, resistance, A. baumannii
↑ What is “already known” in this topic:
Acinetobacter baumannii is reported as one of the dreadful and emerging nosocomial pathogens by World Health Organization (WHO). In recent years, serious complications are encountered by A. baumannii strains due to its multi-drug resistant property. Routine susceptibility pattern incorporates only few antibiotics for profiling which needs to be elaborated.
→ What this article adds:
The incidence of MDR and XDR strains of PCR confirmed isolates of A. baumannii was high when subjected to antibiotic profiling for the frequency of resistance against different classes of antibiotics as per CLSI guidelines. Further, the study emphasizes the necessity of periodical antibiotic surveillance to curb the menace of drug resistance among A. baumannii strains.
Introduction
Emergence of A. baumannii (Ab) as a major cause of hospital-acquired infections is mainly due to its propensity of accumulating and exhibiting antimicrobial drug re-sistance and thus large outbreaks with great challenges for the treating physicians (1). MDR-Ab refers to strains that exhibit resistance to more than three or more antimicrobi-al drug classes (2). XDR-Ab refers to A. baumannii strains resistant to all but two drug classes (3). Pan-drug re-sistance refers to resistance exhibited by the strains to all drug clas-ses (4), and emergence of pan-resistant A. baumann ii isolates was reported including the isolates that were re-sistant to carbapenems, colistin, and polymyxins (5, 6). This alarming scenario is mainly due to multiple mecha-nisms of drug resistance exhibited by A. baumannii viz., impermeable outer membrane, production of enzymes such as AmpC-β lactamase (7), class D OXA – type β lactamase (8), and class D metallo β lactamase (9) that allows resistance to carbapenems, porin channel altera-tion as well as efflux pumps and other genetic factors that may lead to resistance to fluoroquinolones (10). Lack of routine assessment of A. baumannii incidence and period-ical antibiotic surveillance has transformed A. baumannii as one of the emerging UTI pathogens in south India.
Mounting evidence is available towards the emergence of MDR, XDR and pan-DR A. baumannii strains globally and also in India. In recent decades MDR A. baumannii has been reported in hospitalized older adults with a lengthier hospital stay, especially in patients with invasive devices and/or underlying comorbidities (11). Increasing reports of MDR-Ab globally and its rapid spread is of im-portance in the national scale (12, 13). Information re-garding the prevalence and pattern of resistance in A. baumannii through CLSI based antibiotic surveillance is the need of the hour to combat and control the resistance pattern in A. baumannii (14).
Clinical laboratory and Standards Institute (15) rec-ommends the application of Group A (Routine/primary testing and report), Group B (Optional testing) and Group U (Supplementary for urine samples) for antibiotic surveil-lance testing and reporting in the diagnostic laboratory. Most of the MDR and XDR studies among A. baumannii do not incorporate these criteria and there is a lacuna for the same data in recent years. This present investigation is thus designed to investigate a CLSI based antibiogram profile of A. baumannii characterized from the urine samples of the patients with severe urinary tract infections [UTI], to detect the MDR and XDR A. baumannii.
Methods
Study design
This prospective, single-centric study utilized a total of 73 consecutive, non-repetitive A. baumannii strains for the antibiogram profile analysis which were isolated and collected during a period of 12 months (2014-2015) at a private hospital, from the urine samples of the patients with severe urinary tract infections [UTI] (N=1000). The sample size was estimated using the formula n= (Z1-α)2 (P (1− P)/D2) (16), with an approximate prevalence of 50%, confidence interval 95%, precision 5%, and the power was set as 80%. As an inclusion criteria, patients were selected based on the UTI manifested with one or more of the following symptoms such as frequency and urgency of urination, suprapubic discomfort, dysuria, and flank pain, without prior administration of antibiotics. All the other strains isolated were excluded from the study. Random sampling technique regardless of demographic data like age, clinical data, period of hospital stay etc., was used. Proper ethical clearance and consents were obtained as per standard guidelines (17).
Phenotypic and genotypic identification of the strains
Preliminary identification of the strains was done by colony morphology, gram stain, positive citrate, negative oxidase, and urease tests. All the strains were phenotypically characterized by simplified assays as recommended by Prashanth et al. 2000 (18). The strains were also further genotypically confirmed by extracting the A. baumannii DNA by QIAamp DNA mini kit and by further DNA amplification by PCR as per Elhabibi and Ramzy (2012) (19) using the forward primer as F’-5’- AGAGTTTGATCCTGGCTCAG-3’ and reverse primer as R’-5’-TACCAGGGTATCTAATCCTGTT-3’ [Eurofins Genomic India Pvt Ltd, Bangalore]. The amplicon size was 750 bp.
Antibiogram profiling
Antimicrobial susceptibility testing of the A. baumannii isolates was performed by Kirby Bauer method as recommended by Clinical Laboratory and Standards Institute (CLSI, 2012). The antibiotic discs were obtained from Hi-Media laboratories, Mumbai. As per CLSI, 2012, group A primary testing, reporting and recording was done using the following antibiotics viz., Ampicillin-sulbactam (10µg/10µg), ceftazidime (30 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), Doripenem (10 µg), imipenem (10 µg), meropenem (10 µg), gentamicin (10 µg) and tobramycin (10 µg). As an optional primary test and for the study purpose, group B antibiotics such as amikacin (30 µg), piperacillin-tazobactam (100 µg/10 µg), cefepime (30 µg), cefotaxime (30 µg), ceftriaxone (30 µg), doxycycline (30 µg), minocycline (30 µg), trimethoprim–sulphamethoxazole (1.25 µg/23.75 µg) were also included. Group U supplementary antibiotic tetracycline (30 µg) was also included as the study involves the strains isolated from urine specimens. Colistin and tigecycline were also included for the detection of pan-DR A. baumannii isolates and was assayed by microbroth dilution method.
Briefly, a suspension of A. baumannii isolates was prepared equivalent to 0.5 McFarland standards and was made as lawn cultures onto sterile Mueller Hinton agar plates. Using sterile forceps, the antibiotic discs were placed on the surface of the plates and were incubated at 37°C for 18-24 hrs. Criteria for the interpretation of the zone diameter (rounded to nearest mm) for susceptible, intermediate and resistance was done and recorded as per CLSI guidelines, 2012. The antibiotic potency of the disks was standardized against the reference strains Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923) and Pseudomonas aeruginosa (ATCC 27853). Multidrug-resistant (MDR), extremely drug resistant (XDR) and pan drug--resistant (PDR) group of A. baumannii were defined according to the international expert proposal for interim standard definitions for acquired resistance (20).
Results
Group A antibiogram profile based on CLSI breakpoints of the antimicrobial agents used for the study showed highest non-susceptibility (n=73 (100%)) to ampicillin-sulbactam, ceftazidime and imipenem by A. baumannii isolates (Fig. 1). This is followed by 82.19%, 79.45%, 67.12%, 61.64%, 56.16% and 49.31% non-susceptible isolates against ciprofloxacin, gentamicin, meropenem, doripenem, tobramycin, and levofloxacin respectively. The highest intermediate resistance was recorded by 30.13% (n=22) against levofloxacin. However, highest susceptibility was observed in 30.13% against tobramycin followed by 20.54% against levofloxacin (Table 1).
Fig. 1.
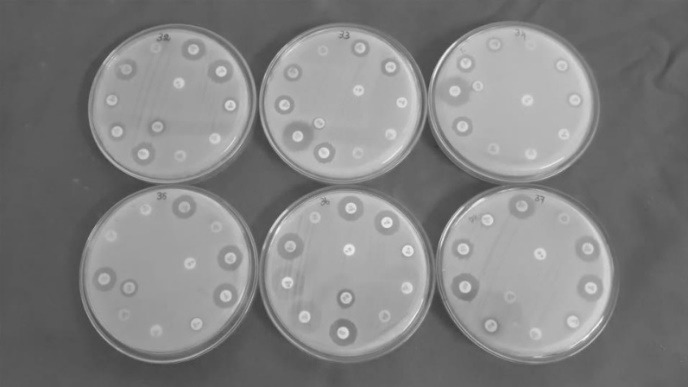
Antibiogram profile of A. baumannii isolated from urine samples showing resistance against most of the Group A and Group B antibiotics as recommended by CLSI, 2012
Table 1. Group A, Group B and Group U antibiogram susceptibility profile of A. baumannii isolated from urine samples [as per CLSI recommendations, 2012] .
| Suggested groupings M02 & M07 [Table 1A, CLSI, 2012] | Antimicrobial agent | Disc con-tent [in µg] | Zone Diameter Interpretive Crite-ria (nearest whole mm) | A. baumannii antibiogram pattern [n=73 (%)] | ||||
| S | I | R | S | I | R | |||
| Group A primary testing and report | Ampicillin-sulbactam | 10/10 | ≥15 | 12-14 | ≤11 | 0 (0) | 0 (0) | 73 (100) |
| Ceftazidime | 30 | ≥18 | 15-17 | ≤14 | 0 (0) | 0 (0) | 73 (100) | |
| Ciprofloxacin | 5 | ≥21 | 16-20 | ≤15 | 5 (6.84) | 8 (10.95) | 60 (82.19) | |
| Levofloxacin | 5 | ≥17 | 14-16 | ≤13 | 15 (20.54) | 22 (30.13) | 36 (49.31) | |
| Doripenem | 10 | ≥18 | 15-17 | ≤14 | 11 (15.06) | 17 (23.28) | 45 (61.64) | |
| Imipenem | 10 | ≥22 | 19-21 | ≤18 | 0 (0) | 0 (0) | 73 (100) | |
| Meropenem | 10 | ≥18 | 15-17 | ≤14 | 10 (13.69) | 14 (19.17) | 49 (67.12) | |
| Gentamicin | 10 | ≥15 | 13-14 | ≤12 | 6 (8.21) | 9 (12.32) | 58 (79.45) | |
| Tobramycin | 10 | ≥15 | 13-14 | ≤12 | 22 (30.13) | 10 (13.69) | 41 (56.16) | |
| Group B optional primary testing | Amikacin | 30 | ≥17 | 15-16 | ≤14 | 0 (0) | 0 (0) | 73 (100) |
| Piperacillin-tazobactam | 100/10 | ≥21 | 18-20 | ≤17 | 0 (0) | 0 (0) | 73 (100) | |
| Cefepime | 30 | ≥18 | 15-17 | ≤14 | 0 (0) | 0 (0) | 73 (100) | |
| Cefotaxime | 30 | ≥23 | 15-22 | ≤14 | 0 (0) | 0 (0) | 73 (100) | |
| Ceftriaxone | 30 | ≥21 | 14-20 | ≤13 | 6 (8.2) | 0 (0) | 67 (91.78) | |
| Doxycycline | 30 | ≥13 | 10-12 | ≤9 | 41 (56.16) | 18 (24.65) | 14 (19.71) | |
| Minocycline | 30 | ≥16 | 13-15 | ≤12 | 56 (76.71) | 12 (16.43) | 5 (6.84) | |
| Trimethoprim – suphamethoxa-zole | 1.25 / 23.75 | ≥16 | 11-15 | ≤10 | 4 (5.47) | 2 (2.73) | 67 (91.78) | |
| Group U supplementary for urine | Tetracycline | 30 | ≥15 | 12-14 | ≤11 | 0 | 17 (23.28) | 56 (76.71) |
Group B antibiogram profile showed 100% non-susceptibility by n=73 isolates of A. baumannii to piperacillin-tazobactam and to amikacin. Similarly, none of them were susceptible to cefepime and cefotaxime, with 91.78% (n=67) of isolates resistant to ceftriaxone with 8.2% (n=6) showing susceptibility for the same. Among the cyclines, 19.71% and 6.84% of isolates were resistant to doxycycline and minocycline respectively. However, 76.71% of the isolates were susceptible to minocycline followed by 56.16% susceptibility against doxycycline (Table 1 & Fig. 1). Group U supplementary antibiotic testing for urine specimens with tetracycline as per CLSI recommendations which was performed in the study recorded 23.28% intermediate and 76.71% of resistance by the tested A. baumannii isolates with n=73 nonsusceptible isolates against the same.
Among the different classes of drugs tested, highest non-susceptibility of 100% was observed against β-lactam inhibitors, followed by cephems [ceftazidime, cefepime, cefotaxime,, and ceftriaxone] and folate pathway inhibitors [Trimethoprim sulphamethoxazole] each group exhibiting 91.78% non-susceptibility. This was followed by aminoglycosides (53.42%), carbapenems (50.68%) and fluoroquinolones (45.20%). The least susceptibility was observed with the cycline group of drugs (Fig. 2).
Fig. 2.
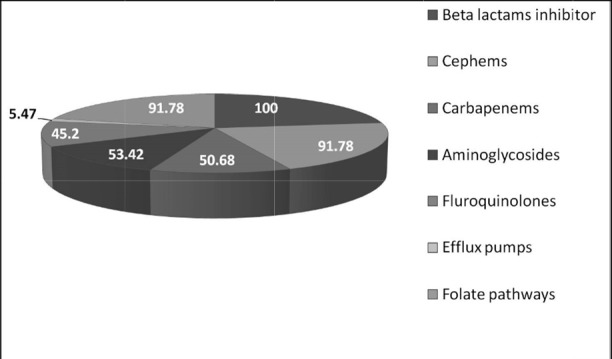
A. baumannii strains showing resistance to drugs in each antibiotic group (values represented in percentage)
According to the interim standard definitions for acquired resistance as recommended by Magiorakos et al. 2011 (20), the present investigation has observed the frequency of MDR-Ab in 71.23% (n=52) of isolates and XDR-Ab in 39.72% (n=29) based on the antibiogram profile against different classes of antibiotics (Fig. 3). None of the strains were categorized as pan-DR A. baumannii as they showed 100% susceptibility to colistin and tigecycline with MIC≤ 2 µg/ml and ≤ 0.1 µg/ml respectively.
Fig. 3.
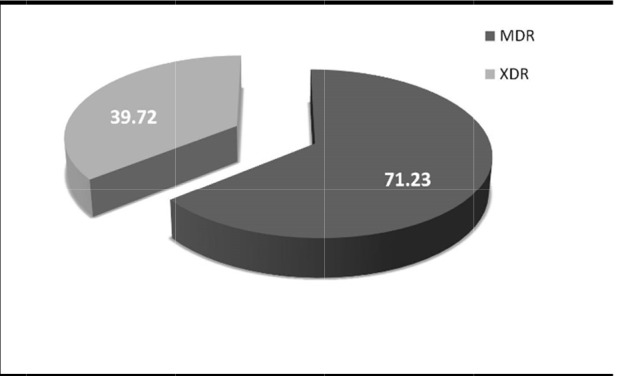
Frequency of MDR and XDR A.baumannii in the present study.
Discussion
A. baumannii is a ubiquitous opportunistic nosocomial pathogen and in recent decades has shown more complications in hospitalized patients. In the present investigation, special concern to confirm all the strains by PCR with an amplicon size of 750 bp was made prior subjecting the strains for antibiogram profiling, as phenotypic characterization is not reliable. In UTI cases, A. baumannii is a serious concern due to the increasing rate of resistance to multiple classes of antibiotics (21) and studies show that this resistance is expanding among hospitalized patients (22). A. baumannii being an explorer of intrinsic resistance by nature and its unusual and unpredictable susceptibility patterns (23) it is of paramount importance to monitor the incidence of MDR-Ab, XDR-Ab, and pan-DR-Ab and to limit its spread among hospitalized patients. Increasing reports of MDR-Ab is not unusual (24). Many reports are available from different parts of the world and appear to be at a startling rate posing a serious threat to the community (25-28). In spite of many available data of MDR-Ab, the prevalence rate of the same in the past two years is still non-convincing. In this view, the present findings recorded the overall relative frequency of the incidence of MDR and XDR A. baumannii among the study population (N=1000) with UTI during the period of 2014-2015.
Among the cephems tested, highest non-susceptibility (100% (n=73)) to ceftazidime, cefotaxime, and cefepime was observed which correlated with the earlier studies (29, 30). In addition, 91.78% non-susceptibility exhibited by the A.baumannii against ceftriaxone in the present investigation is consistent with the earlier studies (9). The resistance against cephalosporins might be due to the presence of plasmid-mediated blaTEM, blaSHV, and blaCTX-M as detected in our earlier studies (31). Carbapenems such as imipenem have recorded 100% non-susceptibility which has correlated with earlier studies (32). This is followed by meropenem and doripenem suggesting that carbapenem-resistant strains were frequently associated with considerable mortality and hospital costs in nosocomial infections (33, 34). Also, in correlation with the present investigation high frequency of carbapenem resistance is recorded among clinical isolates of A.baumannii (35) due to the plasmid-mediated genetic determinants viz., blaIMP, blaVIM and blaGIM (36). However, lower resistance profile against carbapenems was also recorded (37).
High level of aminoglycoside resistance was also recorded in the present study. In the routine treatment, aminoglycosides are often used in combination with broad spectrum β-lactams to treat gram-negative bacterial infections (38). In recent years, resistance to amikacin and gentamicin is considerably higher resulting as a serious problem in combination therapy (39). The present investigation also reports the prevalence of aminoglycoside-resistant strains of A. baumannii among 53.42% of isolates posing an alarming scenario. Amikacin showed 100% non-susceptibility followed by gentamicin (79.45%) and tobramycin (56.16%). Among the fluoroquinolones, ciprofloxacin and levofloxacin resistance exhibited by 82.19% and 49.31% isolates was comparable with the earlier reports (40).
In the present scenario of the emergence of MDR-Ab nosocomial infections, application of older antibiotics like tetracycline and related cyclines (doxycycline and minocycline) has been highly reduced thus lacking improper data against the resistance exhibited by the same. The frequency of tetracycline resistance exhibited by A. baumannii was relatively high (41), which correlates with the 76.71%, 19.71% and 6.84% of resistance against tetracycline, doxycycline, and minocycline respectively observed in the present study and might be due to the plasmid-mediated tetA and tet B genetic determinants (42).
Intrinsic resistance seems to play a significant role in MDR-Ab emergence and particularly with trimethoprim resistance. In the present investigation, as the strains were isolated from urine specimen, the application of trimethoprim – sulphamethoxazole (TMP-SMX) is considered as essential and is routinely included in the therapeutic regimen (43). In an earlier study (44), 70.6% of A. baumannii was reported to exhibit resistance to TMP-SMX which correlates with the high non-susceptibility (91.78%) exhibited by the A. baumannii isolates in the present investigation. 100% of non-susceptibility against the β lactams inhibitors such as ampicillin-sulbactam and piperacillin-tazobactam might be due to intrinsic resistance exhibited by the A. baumannii isolates as reported by Hans et al. 2015 (45).
The categorization of MDR and XDR in the present study was done as per standard interim protocols as done in earlier studies (46). A. baumannii is considered as MDR-Ab when it exhibits resistance to at least three different classes of antimicrobial agents mainly beta-lactams (third-generation cephalosporins), aminoglycosides, fluoroquinolones and more recently carbapenems (47-49). In correlation with this, it was not infrequent in the present investigation 71.23% of the isolates showing multidrug resistance to more than three antimicrobial categories. Similarly, XDR-Ab strains explore resistance to all group of drugs but two. In this concern, the present study has investigated the occurrence of the same in 39.72% of isolates. 4.10% (n=3) was recorded with non-susceptibility against all the tested groups except colistin and tigecycline suggesting the suitability of these drugs in the treatment of A. baumannii nosocomial infections (50).
In addition, the present investigation in an indirect manner portrays about the clinical impact of these routine drugs of choice. 71.23% of MDR-Ab observed in the study suggests the complications of the nosocomial infections that might arise due to these strains. The combination of drugs may not be effective too, as XDR-Ab has also accounted to 39.72% among the tested strains. This emphasizes the need for a vast epidemiological data and also suggests the ecological factors which are responsible for the emergence of these types of resistant strains resulting in complicated urinary tract infections. The study has limited itself to urine samples, but epidemiological studies involving the strains from various clinical samples might give additional information about the prevalence and incidence of resistant strains among A. baumannii and a vivid picture about the clinical impact in the administration of these drugs in the treatment of other nosocomial infections.
Conclusion
A. baumannii strains are posing a serious threat of nosocomial infections, and complications in patient care, the rapid institution of appropriate antimicrobial chemotherapy may be lifesaving. A significant impact on patient care can be achieved only with the rapid assessment and periodical monitoring using antibiotic surveillance as done in the present study. The emergence of MDR and XDR strains of A. baumannii is alarming and choosing the correct antibiotic of choice or application of a combination of antibiotics is essential to curb the menace of emerging resistance pattern by A. baumannii strains in hospital environment.
Acknowledgements
The authors are grateful to Dr.Senthil Pragash Dandapany (Associate Professor, Department of Microbiology, Melmaruvathur Athiparasakthi Medical College and Research Institute, Tamilnadu, for rendering the culture strains for the study.
Conflict of Interests
The authors declare that they have no competing interests.
Cite this article as: Smiline Girija AS, Vijayashree Priyadharsini J. CLSI based antibiogram profile and the detection of MDR and XDR strains of Acinetobacter baumannii isolated from urine samples. Med J Islam Repub Iran. 2019 (8 Feb);33:3. https://doi.org/10.34171/mjiri.33.3
References
- 1.Montefour K, Frieden J, Hurst S, Helmich C, Headley Headley, D D, Martin M. et al. Acinetobacter baumannii: An emerging multidrug-resistant pathogen in critical care. Crit Care Nurse. 2008;28:15–25. [PubMed] [Google Scholar]
- 2.Leski TA, Vora GJ, Barrows BR, Piemental G, House BL, Nicklasson M. et al. Molecular Characterization of Multidrug Resistant Hospital Isolates Using the Antimicrobial Resistance Determinant Microarray. PLoS One. 2013;8:e69507. doi: 10.1371/journal.pone.0069507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10:S122–9. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 4.Lee CM, Lim HK, Liu CP, Tseng HK. Treatment of pan drug resistant Acinetobacter. Scand J Infect Dis. 2005;37:195–9. doi: 10.1080/00365540510026869. [DOI] [PubMed] [Google Scholar]
- 5.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 6.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 7.Beceiro A, Perez A, Fernandez C, Martinez ML, Pascual A, Vila J. et al. Genetic Variability among ampC Genes from Acinetobacter Genomic Species 3. Antimicrob Agents Chemother. 2009:1177–1184. doi: 10.1128/AAC.00485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins PG, Poirel L, Lehmann M, Nordmann P, Siefert H. OXA-143, a novel carbapenem-hydrolyzing class D-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:5035–5038. doi: 10.1128/AAC.00856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singla P, Sikka R, Deep A, Chaudary U. Phenotypic detection and prevalence of metallo lactamases (MBL) in carbapenem resistant isolates of acinetobacter species at a tertiary Care hospital in north India. Int J Pharm Med Bio Sci. 2013;2:2278–5221. [Google Scholar]
- 10.Saroj SD, Clemmer KM, Bonomo RA, Rather PN. Novel mechanism for fluoroquinolone resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2012;56:4955–7. doi: 10.1128/AAC.00739-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Medina T, Carmeli Y. The pivotal role of long-term care facilities in the epidemiology of Acinetobacter baumannii: another brick in the wall. Clin Infect Dis. 2010;50:1617–1618. doi: 10.1086/652760. [DOI] [PubMed] [Google Scholar]
- 12.Wisplinghoff H, Schmitt R, Wöhrmann A, Stefanik D, Siefert H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J Hosp Infect. 2007;66:174–81. doi: 10.1016/j.jhin.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Wroblewska M, Towner K, Marcher H, Luczack M. Emergence and spread of carbapenem-resistant strains of Acinetobacter baumannii in a tertiary-care hospital in Poland. Clin Microbiol Infect. 2007;13:490–6. doi: 10.1111/j.1469-0691.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 14.Halstead DC, Abid J, Dowzicky MJ. Antimicrobial susceptibility among Acinetobacter calcoaceticus-baumannii complex and Enterobacteriaceae collected as part of the Tigecycline evaluation and surveillance trial. J Infect. 2007;55:49–57. doi: 10.1016/j.jinf.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 15. CLSI guidelines: April 2012. Clinical and Laboratory Standards Institute Standards Development Policies and Process.
- 16.Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. ICMR guidelines Indian Council Of Medical Research National Ethical Guidelines for Biomedical and Health Research Involving Human Participants 2012.
- 18.Prashanth K, Badrinath S. Simplified phenotypic tests for the identification of Acinetobacter sp, and their antimicrobial susceptibility status. J Med Microbiol. 2000;49:773–778. doi: 10.1099/0022-1317-49-9-773. [DOI] [PubMed] [Google Scholar]
- 19.Elhabibi T, Ramzy S. Effect of Antibiotic Combinations on the Sensitivity of Carbapenem Resistant Acinetobacter baumannii Strains. J Microb Biochem Technol. 2017;9:132–137. [Google Scholar]
- 20.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 21.Michalopoulos A, Falagas ME. Treatment of Acinetobacter infections. Expert Opin Pharmacother. 2010;11:779–88. doi: 10.1517/14656561003596350. [DOI] [PubMed] [Google Scholar]
- 22.Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23:332–9. doi: 10.1097/QCO.0b013e32833ae38b. [DOI] [PubMed] [Google Scholar]
- 23.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–84. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eliopoulos GM, Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–63. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 25.Shi WF, Jiang JP, Xu N, Huang ZM, Wang YY. Inhibitory effects of reserpine and carbonyl cyanide m-chloro-phenylhydrazone on fluoroquinolone resistance of Acinetobacter baumannii. Chin Med J. 2005;118:340–3. [PubMed] [Google Scholar]
- 26.Srinivasan VB, Rajamohan G, Gebreyes WA. Role of AbeS, a novel efflux pump of the SMR family of transporters, in resistance to antimicrobial agents in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53:5312–6. doi: 10.1128/AAC.00748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunz AN, Brook I. Emerging resistant gram-negative aerobic bacilli in hospital-acquired infections. Chemotherapy. 2010;56:492–500. doi: 10.1159/000321018. [DOI] [PubMed] [Google Scholar]
- 28.Adams-Haduch JM, Paterson DL, Sidjabat HE, Pasculle AW, Potoski BA, Muto CA. Genetic basis of multidrug resistance in Acinetobacter baumannii clinical isolates at a tertiary medical center in Pennsylvania. Antimicrob Agents Chemother. 2008;52:3837–43. doi: 10.1128/AAC.00570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doi Y, Murray G, Peleg A. Acinetobacter baumannii: Evolution of Antimicrobial Resistance-Treatment Options. Semin Respir Crit Care Med. 2015;36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shamsizadeh Z, Nikaeen M, Esfahani BN, Mirhoseini SH, Hatemzadeh M, Hassanzadeh A. Detection of antibiotic resistant Acinetobacter baumannii in various hospital environments: potential sources for transmission of Acinetobacter infections. Environ Health Preven Med. 2017;22:44. doi: 10.1186/s12199-017-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiline Girija AS, Vijayashree Priyadhasini J, Paramasivam A. Molecular characterization of plasmid encoded blaTEM, blaSHV and blaCTX-M among extended spectrum β-lactamases [ESBL’s] producing Acinetobacter baumannii. Br J Biomed Sci. 2018;75:200–202. doi: 10.1080/09674845.2018.1492207. [DOI] [PubMed] [Google Scholar]
- 32.Karthika RU, Rao RS, Sahoo S, Shashikala P, Kanungo R, Jayachandran S. et al. Phenotypic and genotypic assays for detecting the prevalence of metallo-b-lactamases in clinical isolates of Acinetobacter baumannii from a South Indian tertiary care hospital. J Med Microbiol. 2009;58:430–435. doi: 10.1099/jmm.0.002105-0. [DOI] [PubMed] [Google Scholar]
- 33.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J. et al. Epidemiology and impact of imipenem resistance in Acinetobacter baumannii. Infect Control. 2009;30:1186–92. doi: 10.1086/648450. [DOI] [PubMed] [Google Scholar]
- 34.Lemos E, de la Hoz F, Einarson T, McGhan WF, Quevedo E, Castaneda C. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20:416–23. doi: 10.1111/1469-0691.12363. [DOI] [PubMed] [Google Scholar]
- 35.Custovic A, Smajlovic J, Tihic N, Hadzic S, Ahmetagic S, Hadzagic H. Epidemiological monitoring of nosocomial infections caused by Acinetobacter baumannii. Med Arch. 2014;68:402–6. doi: 10.5455/medarh.2014.68.402-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smiline Girija AS, Vijayashree Priyadhasini J, Paramasivam A. Prevalence of VIM and GIM producing Acinetobacter baumannii from patients with severe UTI. Acta Microbiol Immunol Hung. 2018;16:1–12. doi: 10.1556/030.65.2018.038. [DOI] [PubMed] [Google Scholar]
- 37.D'Arezzo S, Principe L, Capone A, Petrosilio N, Petrucci A, Visca P. Changing carbapenemase gene pattern in an epidemic multidrug-resistant Acinetobacter baumannii lineage causing multiple outbreaks in central Italy. J Antimicrob Chemother. 2011;66:54–61. doi: 10.1093/jac/dkq407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Song W, Gu B, Mei YN, Tang JP, Meng L. et al. Correlation between carbapenem consumption and antimicrobial resistance rates of Acinetobacter baumannii in a university-affiliated hospital in China. J Clin Pharmacol. 2013;53:96–102. doi: 10.1177/0091270011435988. [DOI] [PubMed] [Google Scholar]
- 39.Nice L, Yuemeng L, Yuana M, Hu X, Nie T, Yang X. et al. Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China. Acta Pharm Sin B. 2014;4:295–300. doi: 10.1016/j.apsb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hujer KM, Hujer AM, Endimiani A, Thomson JM, Adams MD, Goglin K. et al. Rapid Determination of Quinolone Resistance in Acinetobacter spp. J Clin Microbiol. 2009;47:1436–1442. doi: 10.1128/JCM.02380-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maleki MH, Sekawi Z, Soroush S, Azizi-Jalilian F, Asadollahi K, Mohammadi S. et al. Phenotypic and genotypic characteristics of tetracycline resistant Acinetobacter baumannii isolates from nosocomial infections at Tehran hospitals. Iran J Basic Med Sci. 2014;17:21–26. [PMC free article] [PubMed] [Google Scholar]
- 42.Smiline Girija AS, Vijayashree Priyadhasini J, Paramasivam A. Plasmid encoded tet-A and tet-B mediated tetracycline, doxycycline and minocycline resistance among Acinetobacter baumannii isolated from urine samples. Roum Arch Microbiol Immunol. 2017;76(3-4):134–140. [Google Scholar]
- 43.Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clin Infect Dis. 2001;32:1608–1614. doi: 10.1086/320532. [DOI] [PubMed] [Google Scholar]
- 44.Shin HW, Lim J, Kim S, Kim J, Kwon GC, Koo SH. Characterization of Trimethoprim-Sulfamethoxazole Resistance Genes and Their Relatedness to Class 1 Integron and Insertion Sequence Common Region in Gram-Negative Bacilli. Microbiol Biotechnol. 2015;25:137–142. doi: 10.4014/jmb.1409.09041. [DOI] [PubMed] [Google Scholar]
- 45.Hans R, Bisht D, Agarwal R, Irfan M. Phenotypic detection of MBL, AmpC beta-lactamase and carbapenemases in Multi drug resistant isolates of Acinetobacter baumannii. Int J Med Res Health Sci. 2015;4:311–316. [Google Scholar]
- 46.Dedeic LA, Granov D, Hukic M. Emergence of extensive drug resistant Acinetobacter baumannii in the clinical Center University of Sarajevo, Bosnia and Herzegovina. Med Glas (Zenica) 2015;12:169–76. doi: 10.17392/809-15. [DOI] [PubMed] [Google Scholar]
- 47.Mak JK, Kim MJ, Pham J, Tapsall J, White PA. Antibiotic resistance determinants in nosocomial strains of multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2009;63:47–54. doi: 10.1093/jac/dkn454. [DOI] [PubMed] [Google Scholar]
- 48.Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY. et al. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect. 2008;14:1010–19. doi: 10.1111/j.1469-0691.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 49.Sedighi M, Vaez H, Moghoofeie M, Hadifar S, Oryan G, Faghri J. Molecular detection of metallo-β-lactamase gene blaVIM-1 in imipenem-resistant Pseudomonas aeruginosa strains isolated from hospitalized patients in the hospitals of Isfahan. Adv Biomed Res. 2015;4:57. doi: 10.4103/2277-9175.151872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hornsey M, Wareham DW. In vivo efficacy of glycopeptide colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Antimicrobial Agents Chemother. 2011;55:3534–3537. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


