Abstract
Enhanced recovery after surgery (ERAS) is a multimodal perioperative care pathway designed to achieve early recovery for patients undergoing major surgical procedures. Meta-analyses, randomized controlled trials, and large prospective cohort studies were reviewed. Each item of the perioperative treatment pathway in the English language was examined and reviewed. Enhanced recovery after surgery items that were the strongest predictors for a shorter hospital stay and lower morbidity were absence of a nasogastric tube, early mobilization, early oral nutrition, early removal of the epidural, early removal of the urinary catheter, and utilization of nonopioid analgesia. Based on evidence available for each element of the perioperative care pathway, ERAS provides a protocol for perioperative care. This protocol allows for further evidence-based studies that are adequately powered between institutions.
There are over 234 million major surgical procedures performed worldwide annually (Feldheiser, et al., 2016). Enhanced recovery after surgery (ERAS) clinical pathways have been developed to improve the quality of perioperative care with the goal of minimizing loss of functional capacity and enhancing the recovery process. Reduction of perioperative morbidity is the overall goal, with decreased length of stay an effect of ERAS protocols (Joliat et al., 2015; Nussbaum et al., 2015).
The ERAS Society, an international group, was formed in 2010 to construct comprehensive and evidence-based frameworks for the best perioperative care for various surgeries. Data were retrieved from databases and reviewed for strength of evidence to create these protocols (Lassen et al., 2013). The American Nurses Credentialing Center in 2015 challenged hospitals to focus on delivering quality care while integrating evidence-based practices (Bohnenkamp, Pelton, Rishel, & Kurtin, 2014). Enhanced recovery after surgery clinical pathways are an example of the integration of evidence-based practices to improve perioperative care.
METHODS
A literature search was conducted to identify a comprehensive range of relevant publications. Databases searched included the Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, Scopus, Google Books, and ProQuest Dissertations & Theses (PQDT). The following key words and medical subject heading (MeSH) phrases were used: enhanced recovery after surgery, fast track surgery, early feeding after surgery, postoperative care, and clinical pathways after surgery. The search was limited to accessible full-text research articles, review articles, books, and expert content (textbooks) in English. The search dates were limited to between January 2010 and March 2016. Disciplines outside of nursing were sought. Inclusion criteria included mention of a clinical pathway or enhanced recovery after surgery and gastrointestinal surgical procedures (colorectal, gastric, and pancreaticoduodenectomy). Exclusion criteria included mention of pediatric surgical cases or pediatric surgery. Data ranges were not used as exclusion criteria, and all relevant literature available in the above-mentioned databases was reviewed.
RESULTS
Databases were searched (CINAHL, PubMed, Scopus, Google Books, PQDT), resulting in 2,160 titles and abstracts. After removal of duplicates and nonrelevant titles, 100 articles remained and were read in their entirety. Of these, 76 papers were then excluded based on the inclusion or exclusion criteria specified above or a lack of focus on enhanced recovery after surgery. Of those remaining, 10 focused on early feeding after surgery, 1 discussed facilitating early recovery of bowel motility, 1 discussed the nurse’s role in implementation of an enhanced recovery pathway, 2 concentrated on consensus statements or guidelines of professional organizations regarding enhanced recovery after surgery, 9 focused on the implementation of an enhanced recovery after surgery protocol and improved outcomes, and 1 was related to a cost-benefit analysis of an enhanced recovery protocol.
DISCUSSION
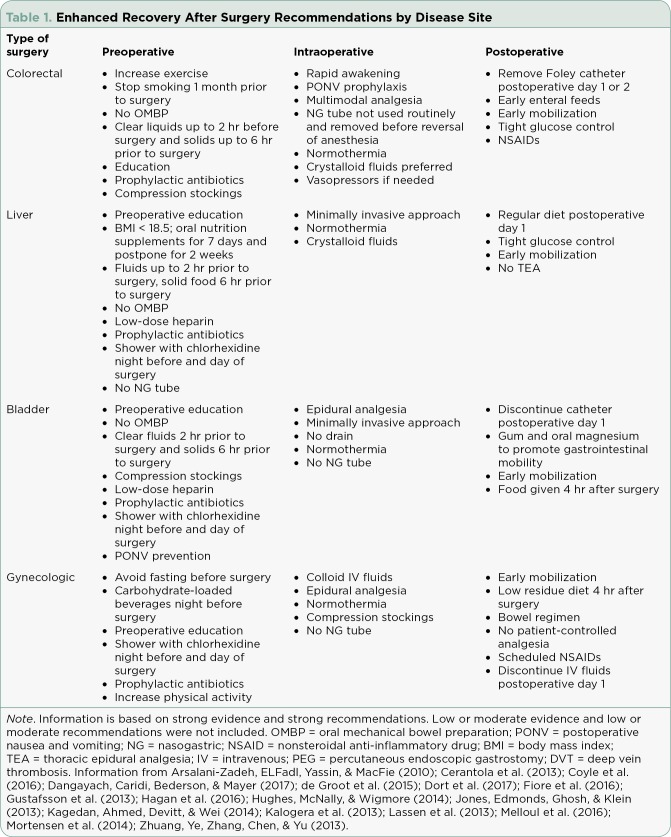
Enhanced recovery after surgery protocols consist of multimodality perioperative management to improve postoperative recovery. Enhanced recovery after surgery pathways encompass the interval prior to surgery to the day of discharge. These standardized multidisciplinary guidelines address a specific diagnosis or procedure. Enhanced recovery after surgery protocols are a quality improvement tool to reduce postoperative morbidity (Nussbaum et al., 2015). These protocols have been shown to decrease length of stay and cost through improved postoperative pain control, better nausea control, integration of preoperative, intraoperative, and postoperative care, and education for the patient and family to participate in care (Pędziwiatr et al., 2015). The clinical pathways are detailed treatment strategies based on best practice guidelines. These pathways are only utilized for uncomplicated postoperative courses. If a patient requires fluid resuscitation or vasopressor support, the patient is not a candidate for an ERAS pathway. (See Table 1 illustrating different areas of oncology with published ERAS protocols.)
Table 1.
Enhanced Recovery After Surgery Recommendations by Disease Site
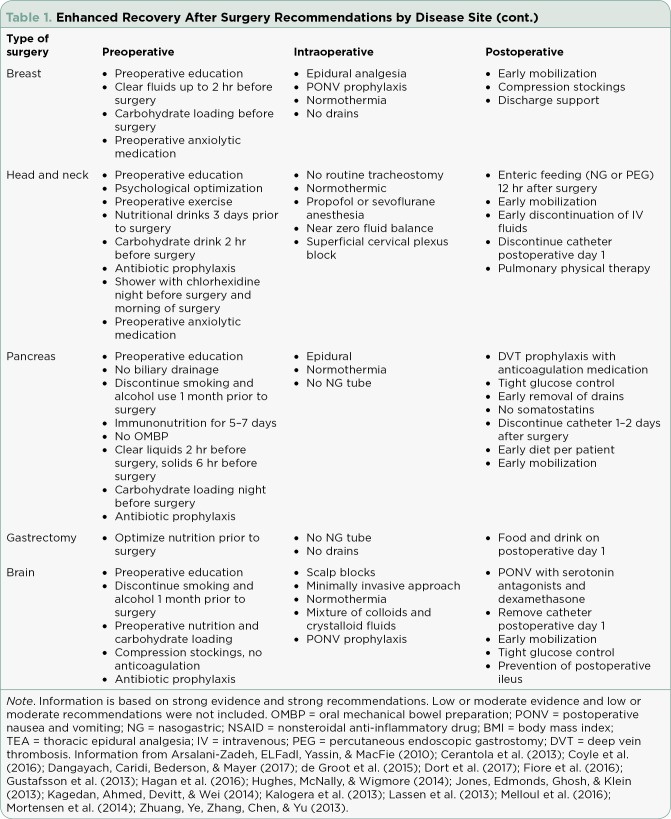
Table 1.
Enhanced Recovery After Surgery Recommendations by Disease Site (cont.)
Preexisting Health Conditions
The goal of preoperative ERAS protocols is to optimize preexisting health conditions such as alcohol use, tobacco use, anemia, and anxiety. Patients with a moderate alcohol use (defined by the World Health Organization as 3 drinks/day) have an increased risk for bleeding and wound infection (Feldheiser et al., 2016). Alcohol impairs the metabolic stress response and the body’s immune function. A minimum of 4 weeks’ abstinence from alcohol is needed to reduce these risks. Patients may need 8 to 12 weeks to return to a normal baseline.
Smokers also have an increased risk of poor wound and tissue healing. Smoking cessation for 4 weeks prior to surgery improves wound healing. Nicotine replacement therapy and counseling can be used for preoperative smoking cessation.
Anemia is a predictor of postoperative complications and mortality. Transfusions should take place prior to surgery to bring the hemoglobin to a baseline level. Iron, folate, vitamin B12 supplements and/or erythropoietin should be planned 3 to 4 weeks prior to surgery in order for the supplementation to take effect.
Other medical conditions, such as those that are cardiac or pulmonary, should also be optimized with preoperative evaluation and clearance by a health-care provider. Anxiety is a predictor for postoperative pain intensity. Education and counseling with preoperative analgesia and anxiolytics should be addressed preoperatively. Short-term benzodiazepines should be avoided in those older than 60 years of age. Long-acting sedatives and opioids should be avoided due to impairment of mobility resulting in increased length of stay.
Oral Intake
One of the foci of ERAS is early oral intake of fluid and food (Dummigan, 2014; Fujii et al., 2014). Supplements high in protein are recommended in order to prevent catabolism (Brady, Keller, & Delaney, 2015). Traditionally, on the day of surgery patients are allowed nothing by mouth (npo) at midnight, although guidelines support the safety of allowing clear liquids up to 2 hours and solid food up to 6 hours before the induction of anesthesia (Feldheiser et al., 2016). Complete gastric emptying takes place in 90 minutes (Feldheiser et al., 2016). There is not enough evidence to support that by ensuring an empty stomach, the aspiration risk is less. Studies have shown that fasting after midnight increases insulin resistance, patient discomfort, and decreases intravascular volume (Conchin, Muirhead, Ferrie, & Carey, 2013).
Preoperative recommendations are to give the patient oral complex carbohydrate supplements at approximately 800 mL the night before surgery and another 400 mL of carbohydrate supplements 2 to 3 hours before the induction of anesthesia (Feldheiser et al., 2016). This reduces the catabolic state caused by fasting and surgery. Fasting before surgery inhibits insulin secretion and promotes the release of catabolic hormones, such as glucagon and cortisol (Sugisawa et al., 2015). Increasing insulin levels preoperatively with oral carbohydrates reduces postoperative insulin resistance, maintains glucagon reserves, decreases protein breakdown, and improves muscle strength (Lassen et al., 2013). Barriers to implementing this evidence-based recommendation include resistance among surgeons and anesthesiologists.
The stomach and jejunum regain motility 12 to 24 hours after major surgery, while the colon regains motility in 48 to 72 hours (Feldheiser et al., 2016). The small bowel recovers after 4 to 8 hours of surgery (Feldheiser et al., 2016). Prophylactic nasogastric (NG) tubes placed during surgery to evacuate air should be removed prior to the reversal of anesthesia. Fever, oropharyngeal, and pulmonary complications are more frequent in patients with NG tubes. Avoidance of NG decompression is associated with an earlier return of bowel function (Wallström & Frisman, 2013). Even in gastroduodenal and pancreatic surgery, there is no evidence of a beneficial effect of the use of prophylactic NG tubes (Hwang, Jung, Cho, & Yu, 2014). Traditionally, patients are kept npo or only given sips of water (Rohatiner et. al., 2012). The classic sign that a postoperative ileus was resolving was the passage of flatus. Only then would patients be given a diet (Dummigan, 2014). With ERAS protocols in place, the patient is given sips of water or a clear liquid diet the day of surgery (Fujii et al., 2014; Rohatiner et al., 2012). The diet is then rapidly progressed to a regular diet (Gerritsen et al., 2014). Early oral feeding has not been shown to increase postoperative complications, readmission rate, and the incidence of anastomotic leak (Liu et. al., 2014; Mahmoodzadeh, Shoar, Sirati, & Khorgami, 2015). Patients who start early feeding protocols have fewer surgical complications and are less likely to be readmitted (Mahmoodzadeh et al., 2015; Sierzega et al., 2015).
Postoperative Nausea and Vomiting
The incidence of postoperative nausea and vomiting (PONV) is 20% to 30% (Feldheiser et al., 2016). Reduction of postoperative fasting, carbohydrate loading, and adequate hydration may decrease PONV. Serotonin antagonists (ondansetron) or application of a scopolamine patch or dopamine antagonists (droperidol) may be given prior to or at the end of surgery to decrease PONV. Dexamethasone at 4 to 5 mg intravenously after the induction of anesthesia has also been shown to be effective. Intraoperative fluid therapy should have the goal of maintaining a zero fluid balance (Nikfarjam et al., 2013). Crystalloid excess increases the risk of pulmonary complications, prolonged ileus, and delayed recovery.
Analgesia
Thoracic epidural analgesia is the gold standard for postoperative pain control in patients undergoing open abdominal surgery (Teeuwen et al., 2010). Epidural analgesia is associated with a 40% reduction of mortality (Teeuwen et al., 2010). Compared with parenteral opioids, epidural analgesia has been shown to provide a sustained analgesia for 72 hours, accelerate the recovery of gastrointestinal function, reduce insulin resistance, and reduce cardiac and respiratory complications (Teeuwen et al., 2010). Nonsteroidal anti-inflammatory drugs and COX-2 drugs, such as celecoxib, have been shown to improve postoperative analgesia by reducing opioid consumption. (See Table 2 illustrating the perioperative process.)
Table 2.
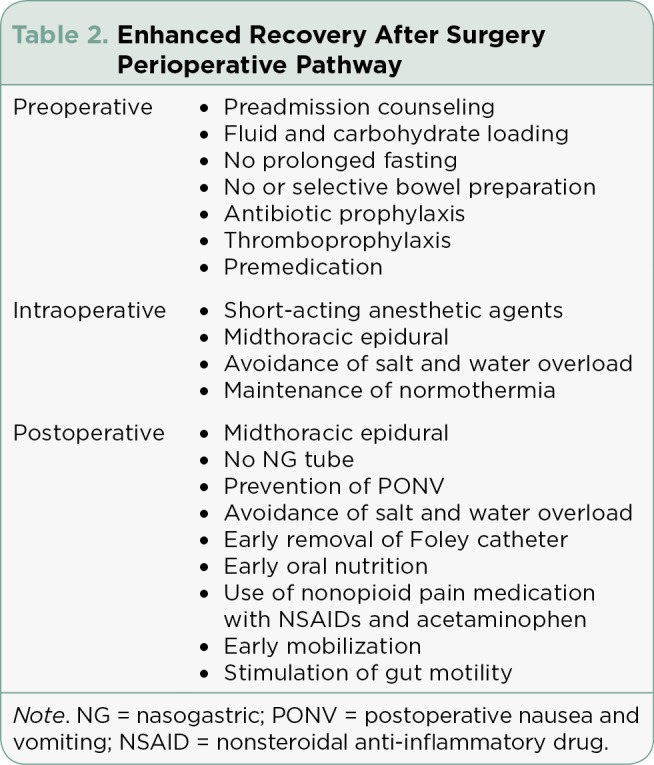
Enhanced Recovery After Surgery Perioperative Pathway
Delirium
Postoperative delirium is common in critical care and in postoperative patients. Factors such as age, prolonged preoperative fluid fasting times, deep anesthesia time, disturbance of the sleep–wake cycle, the use of sedatives and benzodiazepines, a history of excessive alcohol use, low body mass index, low serum albumin levels, intraoperative hypotension, and perioperative blood transfusions predispose the patient to delirium (Scholz, Oldroyd, McCarthy, Quinn, & Hewitt, 2016). Early detection in the postoperative period is important as delayed treatment can increase complications, the hospital length of stay, and mortality. Nonpharmacologic interventions and the use of haloperidol may be necessary to treat postoperative delirium.
Mobilization
Mobilization occurs early in the postoperative course. Instructions detailing daily mobilization goals are given to the patient and family in the outpatient setting prior to surgery. Patients may be given a log for daily activity or instructed on exercises to be done prior to surgery. Patients should also be encouraged to increase their activity in the preoperative phase. These instructions are reinforced with written material and should be brought by the patient to the hospital on the day of surgery. This allows the patient and family to review the written materials and to participate in the patient’s care.
ERAS Protocol Steps
Creating and implementing an ERAS protocol involves many steps. The first step is to form a multidisciplinary team consisting of disciplines that will be involved in the patient’s care. This allows for all disciplines to function as a team in order to provide the best patient care. The next step is for the team to define the patient population or medical/nursing treatment that will be served (Bakker, Cakir, Doodemen, & Houdijk, 2015). Once the population has been identified, a systematic literature search is conducted (Hain & Kear, 2015). Enhanced recovery after surgery protocols have been documented in several populations since 2000. After the literature review, clinical pathways and process maps are designed using evidence-based practice and best standards of care. The next step is to create standardized order sets, which ensures that all necessary documentation becomes the standard of care. (See Table 3 for a sample of order sets.) The final step is to identify individuals responsible for monitoring patient outcomes and collecting data in order to revise and improve the clinical pathways and patient care.
Table 3.
Postoperation Orders for Pancreaticoduodenectomy
CONCLUSION
The development of ERAS protocols using current evidence provides standardized care that improves patient outcomes. The literature has shown that institutions that have developed ERAS protocols have reported decreased costs associated with hospitalization and decreased length of stay. By using current evidence to develop these protocols, institutions can do further research through adequately powered multi-institutional studies.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. The British journal of surgery. 2011;98:181–196. doi: 10.1002/bjs.7331. [DOI] [PubMed] [Google Scholar]
- 2.Bakker Nathalie, Cakir Hamit, Doodeman H J, Houdijk A P J. Eight years of experience with Enhanced Recovery After Surgery in patients with colon cancer: Impact of measures to improve adherence. Surgery. 2015;157:1130–1136. doi: 10.1016/j.surg.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Bohnenkamp Susan, Pelton Nicole, Rishel Cindy J, Kurtin Sandra. Implementing evidence-based practice using an interprofessional team approach. Oncology nursing forum. 2014;41:434–437. doi: 10.1188/14.ONF.434-437. [DOI] [PubMed] [Google Scholar]
- 4.Brady Karen M, Keller Deborah S, Delaney Conor P. Successful Implementation of an Enhanced Recovery Pathway: The Nurse’s Role. AORN journal. 2015;102:469–481. doi: 10.1016/j.aorn.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Cerantola Yannick, Valerio Massimo, Persson Beata, Jichlinski Patrice, Ljungqvist Olle, Hubner Martin, Kassouf Wassim, Muller Stig, Baldini Gabriele, Carli Francesco, Naesheimh Torvind, Ytrebo Lars, Revhaug Arthur, Lassen Kristoffer, Knutsen Tore, Aarsether Erling, Wiklund Peter, Patel Hitendra R H. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clinical nutrition (Edinburgh, Scotland) 2013;32:879–887. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Conchin Simone, Muirhead Ros, Ferrie Suzie, Carey Sharon. Can’t we just let them eat? Defining and addressing under-use of the oral route in a post-surgical ward. Asia Pacific journal of clinical nutrition. 2013;22:200–205. doi: 10.6133/apjcn.2013.22.2.12. [DOI] [PubMed] [Google Scholar]
- 7.Coyle M J, Main B, Hughes C, Craven R, Alexander R, Porter G, Thomas S. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2016;41:118–126. doi: 10.1111/coa.12482. [DOI] [PubMed] [Google Scholar]
- 8.Dangayach Neha S, Caridi John, Bederson Joshua, Mayer Stephan A. Enhanced Recovery After Neurosurgery: Paradigm Shift and Call to Arms. World neurosurgery. 2017;100:683–685. doi: 10.1016/j.wneu.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 9.de Groot Jeanny J A, Ament Stephanie M C, Maessen José M C, Dejong Cornelis H C, Kleijnen Jos M P, Slangen Brigitte F M. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta obstetricia et gynecologica Scandinavica. 2016;95:382–395. doi: 10.1111/aogs.12831. [DOI] [PubMed] [Google Scholar]
- 10.Dort Joseph C, Farwell D Gregory, Findlay Merran, Huber Gerhard F, Kerr Paul, Shea-Budgell Melissa A, Simon Christian, Uppington Jeffrey, Zygun David, Ljungqvist Olle, Harris Jeffrey. Optimal Perioperative Care in Major Head and Neck Cancer Surgery With Free Flap Reconstruction: A Consensus Review and Recommendations From the Enhanced Recovery After Surgery Society. JAMA otolaryngology-- head & neck surgery. 2017;143:292–303. doi: 10.1001/jamaoto.2016.2981. [DOI] [PubMed] [Google Scholar]
- 11.Dummigan C. Early feeding protocol in elective colorectal surgery. Gastrointestinal Nursing. 2014;12(9):15–21. [Google Scholar]
- 12.Feldheiser A, Aziz O, Baldini G, Cox B P B W, Fearon K C H, Feldman L S, Gan T J, Kennedy R H, Ljungqvist O, Lobo D N, Miller T, Radtke F F, Ruiz Garces T, Schricker T, Scott M J, Thacker J K, Ytrebø L M, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta anaesthesiologica Scandinavica. 2016;60:289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore J F, Jr, Bejjani J, Conrad K, Niculiseanu P, Landry T, Lee L, Feldman L S. Systematic review of the influence of enhanced recovery pathways in elective lung resection. Journal of Thoracic and Cardiovascular Surgery. 2016;151(3):708–715. doi: 10.1016/j.jtcvs.2015.09.112. [DOI] [PubMed] [Google Scholar]
- 14.Fujii T, Morita H, Sutoh T, Yajima R, Yamaguchi S, Tsutsumi S, Kuwano H. Benefit of oral feeding as early as one day after elective surgery for colorectal cancer: Oral feeding on first versus second postoperative day. International Surgery. 2014;99(3):211–215. doi: 10.9738/INTSURG-D-13-00146.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerritsen Arja, Wennink Roos A W, Besselink Marc G H, van Santvoort Hjalmar C, Tseng Dorine S J, Steenhagen Elles, Borel Rinkes Inne H M, Molenaar I Quintus. Early oral feeding after pancreatoduodenectomy enhances recovery without increasing morbidity. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2014;16:656–664. doi: 10.1111/hpb.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gustafsson U O, Scott M J, Schwenk W, Demartines N, Roulin D, Francis N, McNaught C E, Macfie J, Liberman A S, Soop M, Hill A, Kennedy R H, Lobo D N, Fearon K, Ljungqvist O. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World journal of surgery. 2013;37:259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 17.Hagan Katherine B, Bhavsar Shreyas, Raza Shaan M, Arnold Benjamin, Arunkumar Radha, Dang Anh, Gottumukkala Vijay, Popat Keyuri, Pratt Greg, Rahlfs Thomas, Cata Juan P. Enhanced recovery after surgery for oncological craniotomies. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2016;24:10–16. doi: 10.1016/j.jocn.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Hain D J, Kear T M. Using evidence-based practice to move beyond doing things the way we have always done them. Nephrology Nursing Journal. 2015;42(1):11–21. [PubMed] [Google Scholar]
- 19.Hughes Michael J, McNally Stephen, Wigmore Stephen J. Enhanced recovery following liver surgery: a systematic review and meta-analysis. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2014;16:699–706. doi: 10.1111/hpb.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang Si Eun, Jung Mi Jin, Cho Baik Hwan, Yu Hee Chul. Clinical feasibility and nutritional effects of early oral feeding after pancreaticoduodenectomy. Korean journal of hepato-biliary-pancreatic surgery. 2014;18:84–89. doi: 10.14701/kjhbps.2014.18.3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joliat G-R, Labgaa I, Petermann D, Hübner M, Griesser A-C, Demartines N, Schäfer M. Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. The British journal of surgery. 2015;102:1676–1683. doi: 10.1002/bjs.9957. [DOI] [PubMed] [Google Scholar]
- 22.Jones N L, Edmonds L, Ghosh S, Klein A A. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia. 2013;68:179–189. doi: 10.1111/anae.12067. [DOI] [PubMed] [Google Scholar]
- 23.Kagedan Daniel J, Ahmed Mahrosh, Devitt Katharine S, Wei Alice C. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2015;17:11–16. doi: 10.1111/hpb.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalogera Eleftheria, Bakkum-Gamez Jamie N, Jankowski Christopher J, Trabuco Emanuel, Lovely Jenna K, Dhanorker Sarah, Grubbs Pamela L, Weaver Amy L, Haas Lindsey R, Borah Bijan J, Bursiek April A, Walsh Michael T, Cliby William A, Dowdy Sean C. Enhanced recovery in gynecologic surgery. Obstetrics and gynecology. 2013;122:319–328. doi: 10.1097/AOG.0b013e31829aa780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lassen Kristoffer, Coolsen Marielle M E, Slim Karem, Carli Francesco, de Aguilar-Nascimento José E, Schäfer Markus, Parks Rowan W, Fearon Kenneth C H, Lobo Dileep N, Demartines Nicolas, Braga Marco, Ljungqvist Olle, Dejong Cornelis H C. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World journal of surgery. 2013;37:240–258. doi: 10.1007/s00268-012-1771-1. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Wang D, Zheng L, Mou T, Liu H, Li G. Is early oral feeding after gastric cancer surgery feasible? A systematic review and meta-analysis of randomized controlled trials. PLOS/ONE. 2014;14(9):e112062. doi: 10.1371/journal.pone.0112062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahmoodzadeh Habibollah, Shoar Saeed, Sirati Freydoon, Khorgami Zhamak. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surgery today. 2015;45:203–208. doi: 10.1007/s00595-014-0937-x. [DOI] [PubMed] [Google Scholar]
- 28.Melloul Emmanuel, Hübner Martin, Scott Michael, Snowden Chris, Prentis James, Dejong Cornelis H C, Garden O James, Farges Olivier, Kokudo Norihiro, Vauthey Jean-Nicolas, Clavien Pierre-Alain, Demartines Nicolas. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World journal of surgery. 2016;40:2425–2440. doi: 10.1007/s00268-016-3700-1. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, Carli F, Demartines N, Griffin S M, Lassen K. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. The British journal of surgery. 2014;101:1209–1229. doi: 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
- 30.Nikfarjam Mehrdad, Weinberg Laurence, Low Nicholas, Fink Michael A, Muralidharan Vijayaragavan, Houli Nezor, Starkey Graham, Jones Robert, Christophi Christopher. A fast track recovery program significantly reduces hospital length of stay following uncomplicated pancreaticoduodenectomy. JOP : Journal of the pancreas. 2013;14:63–70. doi: 10.6092/1590-8577/1223. [DOI] [PubMed] [Google Scholar]
- 31.Nussbaum Daniel P, Penne Kara, Stinnett Sandra S, Speicher Paul J, Cocieru Andrei, Blazer Dan G, Zani Sabino, Clary Bryan M, Tyler Douglas S, White Rebekah R. A standardized care plan is associated with shorter hospital length of stay in patients undergoing pancreaticoduodenectomy. The Journal of surgical research. 2015;193:237–245. doi: 10.1016/j.jss.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 32.Pędziwiatr Michał, Kisialeuski Mikhail, Wierdak Mateusz, Stanek Maciej, Natkaniec Michał, Matłok Maciej, Major Piotr, Małczak Piotr, Budzyński Andrzej. Early implementation of Enhanced Recovery After Surgery (ERAS®) protocol - Compliance improves outcomes: A prospective cohort study. International journal of surgery (London, England) 2015;21:75–81. doi: 10.1016/j.ijsu.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 33.Rohatiner T, Wend J, Rhodes S, Murrell Z, Berel D, Fleshner P. A prospective single-institution evaluation of current practices of early postoperative feeding after elective intestinal surgery. American Surgeon. 2012;78(10):1147–1150. [PubMed] [Google Scholar]
- 34.Scholz A F M, Oldroyd C, McCarthy K, Quinn T J, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. The British journal of surgery. 2016;103:e21–28. doi: 10.1002/bjs.10062. [DOI] [PubMed] [Google Scholar]
- 35.Sierzega Marek, Choruz Ryszard, Pietruszka Szymon, Kulig Piotr, Kolodziejczyk Piotr, Kulig Jan. Feasibility and outcomes of early oral feeding after total gastrectomy for cancer. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2015;19:473–479. doi: 10.1007/s11605-014-2720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugisawa Norihiko, Tokunaga Masanori, Makuuchi Rie, Miki Yuichiro, Tanizawa Yutaka, Bando Etsuro, Kawamura Taiichi, Terashima Masanori. A phase II study of an enhanced recovery after surgery protocol in gastric cancer surgery. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016;19:961–967. doi: 10.1007/s10120-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 37.Teeuwen Pascal H E, Bleichrodt R P, Strik C, Groenewoud J J M, Brinkert W, van Laarhoven C J H M, van Goor H, Bremers A J A. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14:88–95. doi: 10.1007/s11605-009-1037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallström Asa, Frisman Gunilla Hollman. Facilitating early recovery of bowel motility after colorectal surgery: a systematic review. Journal of clinical nursing. 2014;23:24–44. doi: 10.1111/jocn.12258. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang Cheng-Le, Ye Xing-Zhao, Zhang Xiao-Dong, Chen Bi-Cheng, Yu Zhen. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Diseases of the colon and rectum. 2013;56:667–678. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]