Abstract
Rituximab and hyaluronidase human is a new subcutaneous formulation of rituximab that was recently approved by the US Food and Drug Administration for the treatment of adults with follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia. With data to support noninferior pharmacokinetics, similar outcomes, and comparable adverse events, rituximab and hyaluronidase human may offer a suitable alternative to intravenous rituximab that could improve convenience for patients and better utilize health-care resources.
Rituximab is a humanized monoclonal antibody targeting the CD20 transmembrane protein found on B-cells. Although the mechanism of action is not completely understood, this agent exhibits antitumor effects via direct drug-mediated signaling, complement-dependent cytotoxicity, and antibody-dependent cellular cytotoxicity. These pathways make rituximab useful in the treatment of a number of B-cell malignancies, as well as a variety of autoimmune conditions. After initial approval in 1997, intravenous (IV) rituximab has become a mainstay of therapy in malignancies such as acute lymphoblastic leukemia, chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (Genentech, 2016; Weiner, 2010).
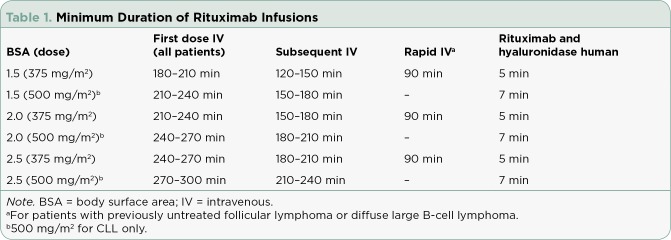
Although rituximab is generally well tolerated, it is associated with rare, serious adverse reactions, including infectious complications, mucocutaneous reactions, arrhythmias, and renal and gastrointestinal toxicities. Infusion reactions occur more commonly and can be severe or even fatal (Kimby, 2005). Due to this risk, the first dose of IV rituximab must be administered slowly and titrated in increments of 50 mg/hr every 30 minutes, generally taking at least 3.5 to 4 hours. Patients who tolerate the first dose without a reaction can receive their infusion at an increased rate starting at 100 mg/hr and titrating by 100 mg/hr every 30 minutes. In 2012, administration instructions for more rapid infusion were added to the prescribing information (specifically for patients with previously untreated follicular lymphoma [FL] or diffuse large B-cell lymphoma [DLBCL] who tolerate the first infusion), further decreasing the time required for rituximab administration (Genentech, 2016). Despite the ability to administer rituximab more rapidly, the duration required for infusion can be logistically challenging for patients, nurses, and providers, especially in patients receiving multiagent chemotherapy regimens (Table 1).
Table 1.
Minimum Duration of Rituximab Infusions
To address the problems associated with prolonged infusion times, a subcutaneous (SC) formulation of rituximab (rituximab and hyaluronidase human; Rituxan Hycela) was developed and approved by the US Food and Drug Administration (FDA) in June 2017 for the treatment of adult patients with FL, CLL, and DLBCL. This formulation is given subcutaneously over 5 to 7 minutes and requires only 15 minutes of monitoring following administration (Genentech, 2017). This new product may offer advantages over IV rituximab; however, many factors should be considered when determining the most appropriate formulation to use. Most importantly, noninferior pharmacokinetics, safety, and efficacy must be established, while additional factors such as cost, patient preference, and data for alternative anti-CD20 agents should also play a role.
PHARMACOKINETICS, SAFETY, AND EFFICACY
Follicular Lymphoma
The SABRINA trial was a randomized phase III study that compared IV rituximab to SC rituximab and hyaluronidase human in combination with standard chemotherapy in 410 patients with previously untreated FL. Patients were randomized to receive all 8 cycles of rituximab at 375 mg/m² IV or 1 cycle of rituximab at 375 mg/m² IV followed by 7 cycles of SC rituximab and hyaluronidase human at 1,400 mg. Both arms received rituximab in combination with either 6 to 8 cycles of cyclophosphamide (Cytoxan), doxorubicin (hydroxydaunorubicin), vincristine (Oncovin), and prednisone (CHOP) or 8 cycles of cyclophosphamide, vincristine, and prednisone (CVP). All responding patients continued with maintenance therapy every 8 weeks for 2 years.
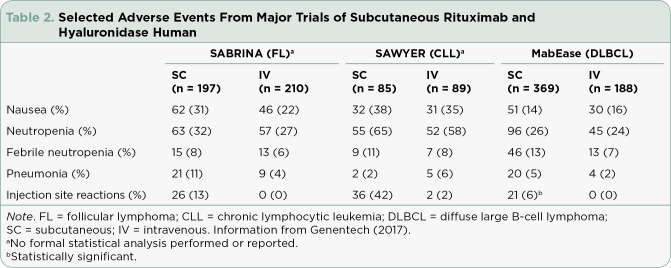
Geometric mean serum trough concentrations of rituximab were 83.13 µg/mL with the IV formulation compared to 134.58 µg/mL with SC administration, resulting in a geometric mean ratio of 1.62 and confirming noninferiority of the SC product compared to the IV formulation. The primary endpoint of overall response rate was similar between groups at 84.9% with IV rituximab compared with 84.4% with SC rituximab and hyaluronidase human. There were few clinically significant differences in rates of adverse events apart from neutropenia (32% vs. 27%) and administration-related reactions (48% vs. 35%), which were higher in the SC rituximab and hyaluronidase human group (Table 2). Administration-related reactions observed included both local and systemic symptoms, with chills (7%) and pruritus (6%) being the most common with IV rituximab. A majority of the reactions seen with SC rituximab were grade 1 or 2 local injection site reactions (Davies et al., 2017).
Table 2.
Selected Adverse Events From Major Trials of Subcutaneous Rituximab and Hyaluronidase Human
Chronic Lymphocytic Leukemia
The SAWYER trial evaluated the pharmacokinetics of SC rituximab and hyaluronidase human in 176 patients with previously untreated CLL in a phase Ib noninferiority study. Patients were randomized to receive rituximab at 375 mg/m² IV with cycle one followed by 500 mg/m2 in cycles two through six or rituximab at 375 mg/m² with cycle one followed by SC rituximab and hyaluronidase human at 1,600 mg in cycles two through six. Both arms received rituximab in combination with fludarabine and cyclophosphamide. The geometric mean trough concentration was higher with rituximab and hyaluronidase human (97.5 µg/mL) compared to IV rituximab (61.5 µg/mL), with an adjusted geometric mean ratio of 1.53 supporting noninferiority of trough concentrations with rituximab and hyaluronidase human.
Although the efficacy endpoints in this study were only exploratory, rates of B-cell depletion were similar between the two groups. Adverse events were similar between groups, except more patients experienced febrile neutropenia (11% vs. 8%) and local injection site reactions (42% vs. 2%) with the SC formulation (Assouline et al., 2016).
Diffuse Large B-Cell Lymphoma
The MabEase study compared the efficacy of IV rituximab (8 cycles) with that of SC rituximab and hyaluronidase human (1 cycle IV followed by 7 cycles of SC rituximab and hyaluronidase human at 1,400 mg) in combination with CHOP in 576 patients with previously untreated DLBCL. Results of the study showed no statistically significant difference in complete response rates (50.6% with SC rituximab vs. 42.4% with IV rituximab; p < .076). With a median follow-upof 35 months, median overall, event-free, and progression-free survival endpoints had not been reached. Adverse events were similar between groups, with a clinically significant difference in injection site reactions (5.7% vs. 0%) with SC rituximab and hyaluronidase human (Genentech, 2017; Lugtenburg et al., 2017).
DOSING AND ADMINISTRATION
The FDA-approved dosing for FL and DLBCL is a flat dose of 1,400 mg of rituximab and 23,400 units of hyaluronidase human (11.7 mL) administered subcutaneously over 5 minutes. For CLL, the dose is 1,600 mg of rituximab and 26,800 units of hyaluronidase human (13.4 mL) given subcutaneously over 7 minutes. Only patients who have received and tolerated 1 full dose of IV rituximab are eligible to receive rituximab and hyaluronidase human subcutaneously, and all patients should be monitored for signs and symptoms of an infusion reaction for 15 minutes following each dose.
Although some data suggest that SC rituximab can be given safely without premedication, the manufacturer recommends that patients should still be premedicated with acetaminophen and an antihistamine, while a glucocorticoid can also be considered (Burrows, Akinbobuyi, Rule, & Crosbie, 2017). In the rare case of a severe reaction, the injection should be stopped and symptomatic treatment should be started, whereas mild or moderate localized reactions typically resolve without treatment. Subcutaneous doses should be administered in the abdomen; there is no data supporting alternative sites of injection. The primary difference between IV and SC formulations is the addition of human hyaluronidase, which breaks down hyaluronan in the extracellular matrix of the SC tissue and leads to increased permeability (Genentech, 2017). This increased permeability allows for administration of larger fluid volumes (up to 15 mL) than what was traditionally feasible via the SC route (1–2 mL) and is an important aspect to consider for those administering the injection (Frost, 2007).
CONSIDERATIONS FOR THE ADVANCED PRACTITIONER
With studies supporting pharmacokinetic noninferiority and similar outcomes between the IV and SC formulations of rituximab, several other factors should be considered when choosing which product is most appropriate for your patient. Factors to evaluate include cost, treatment setting, patient preference, and alternative agents.
Cost
Although drug costs will vary based on a variety of factors that include dose and frequency, treatment setting, patient’s insurance coverage, and others, cost remains an important factor in deciding on the most appropriate treatment. The average wholesale price (AWP) for a 500-mg vial of rituximab is $5,211.78, with a 100-mg vial costing $1,042.36. For a patient with a body surface area (BSA) of 2.0 receiving a dose of 375 mg/m², the cost of the drug would be $8,388.86. Comparatively, the AWP is $7,296.49 for the 1,400-mg SC injection and $8,338.85 for the 1,600-mg syringe (Truven Health Analytics, 2017). Compared to the fixed dosing of SC rituximab, the IV formulation may be more or less expensive depending on the patient’s BSA. Further complicating the ability to compare the drug costs of the IV and SC formulations is the potential for biosimilar IV rituximab products in the near future, with at least six biosimilar products currently in development (Subramanian, Cavenagh, Desai, & Jacobs, 2017). Costs will vary significantly between institutions, so periodic evaluation of organization-specific cost differences may be beneficial.
Treatment Setting
In addition to drug costs, there may be further cost differences related to differences in administration time. A 2013 study done in the UK showed a reduction in both administration chair time and in active health-care provider time (De Cock et al., 2016). Similarly, a systematic survey of 17 Italian hospitals showed significant cost and resource savings with SC rituximab and hyaluronidase human compared to IV rituximab, based mainly on a total time difference of 3.3 hours. This study not only accounted for administration and nursing time, but also reported a shorter pharmacy preparation time as the SC formulation is supplied in a fixed-dosage form (Ponzetti, Canciani, Farina, Era, & Walzer, 2016).
Patient Preference
In a study assessing patient satisfaction in patients with previously untreated DLBCL or FL, over 80% of patients preferred SC rituximab over IV rituximab. The most common reasons cited included less time spent in clinic (69%), feeling more comfortable during administration (37%), and less emotional distress (29%; Rummel et al., 2015). Satisfaction surveys were also administered as part of the MabEase study, which showed higher scores on "impact on activities of daily living," "convenience," and "satisfaction" when comparing SC rituximab with IV rituximab (Lugtenburg et al., 2017). Although the literature supports improved patient satisfaction with the SC formulation, the decision to switch from IV to SC rituximab should be made only after thorough discussion with the patient, as some patients may experience "needle phobia" or otherwise prefer IV administration.
Alternative Agents
Lastly, recently published data suggest that obinutuzumab (Gazyva) increases progression-free survival compared to rituximab when used in combination chemotherapy regimens in adults with FL. This increased efficacy was accompanied by an increased frequency of high-grade adverse events and higher frequency of infusion-related reactions (Marcus et al., 2017). Although rituximab has been the mainstay of therapy in the treatment of many B-cell malignancies, it is important to be aware of data that exist now and potentially in the future for other anti-CD20 therapies such as obinutuzumab and ofatumumab (Arzerra).
CONCLUSION
In summary, rituximab and hyaluronidase human offers an alternative to IV rituximab that increases convenience for patients and health-care providers, and results in increased patient satisfaction. Studies that led to FDA approval have established the noninferior pharmacokinetics, safety, and efficacy of the SC product. Intravenous rituximab will continue to be used upfront in all patients, as they must tolerate a full dose of the IV product before being eligible to switch to the SC formulation. The decision on which formulation to use following the first dose will depend on a variety of patient- and institution-specific factors, including cost, convenience, and patient preference.
Footnotes
The author has no conflicts of interest to disclose.
References
- 1.Assouline Sarit, Buccheri Valeria, Delmer Alain, Gaidano Gianluca, Trneny Marek, Berthillon Natalia, Brewster Michael, Catalani Olivier, Li Sai, McIntyre Christine, Sayyed Pakeeza, Badoux Xavier. Pharmacokinetics, safety, and efficacy of subcutaneous versus intravenous rituximab plus chemotherapy as treatment for chronic lymphocytic leukaemia (SAWYER): a phase 1b, open-label, randomised controlled non-inferiority trial. The Lancet. Haematology. 2016;3:e128–138. doi: 10.1016/S2352-3026(16)00004-1. [DOI] [PubMed] [Google Scholar]
- 2.Burrows Samuel H, Akinbobuyi Oluwatosin, Rule Simon, Crosbie Nicola. Subcutaneous rituximab can be safely administered without pre-medication. British journal of haematology. 2018;181:836–837. doi: 10.1111/bjh.14703. [DOI] [PubMed] [Google Scholar]
- 3.Davies A, Merli F, Mihaljević B, Mercadal S, Siritanaratkul N, Solal-Céligny P, Macdonald D. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): A randomised, open-label, phase 3 trial. Lancet Haematology. 2017;4(6):e272–e282. doi: 10.1016/S2352-3026(17)30078-9. [DOI] [PubMed] [Google Scholar]
- 4.De Cock E, Kritikou P, Sandoval M, Tao S, Wiesner C, Carella A M, Waterboer T. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: A time and motion study in eight countries. PLoS ONE. 2016;11(6):e0157957. doi: 10.1371/journal.pone.0157957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frost Gregory I. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert opinion on drug delivery. 2007;4:427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 6.Genentech, Inc. Rituxan Hycela (rituximab and hyaluronidase human) package insert. 2017 Retrieved from https://www.gene.com/download/pdf/rituxan_hycela_prescribing.pdf.
- 7.Genentech, Inc. Rituxan (rituximab) package insert. 2016 Retrieved from https://www.gene.com/download/pdf/rituxan_prescribing.pdf.
- 8.Kimby Eva. Tolerability and safety of rituximab (MabThera). Cancer treatment reviews. 2005;31:456–473. doi: 10.1016/j.ctrv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Lugtenburg Pieternella, Avivi Irit, Berenschot Henriette, Ilhan Osman, Marolleau Jean Pierre, Nagler Arnon, Rueda Antonio, Tani Monica, Turgut Mehmet, Osborne Stuart, Smith Rodney, Pfreundschuh Michael. Efficacy and safety of subcutaneous and intravenous rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in first-line diffuse large B-cell lymphoma: the randomized MabEase study. Haematologica. 2017;102:1913–1922. doi: 10.3324/haematol.2017.173583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcus Robert, Davies Andrew, Ando Kiyoshi, Klapper Wolfram, Opat Stephen, Owen Carolyn, Phillips Elizabeth, Sangha Randeep, Schlag Rudolf, Seymour John F, Townsend William, Trněný Marek, Wenger Michael, Fingerle-Rowson Günter, Rufibach Kaspar, Moore Tom, Herold Michael, Hiddemann Wolfgang. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. The New England journal of medicine. 2017;377:1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 11.Ponzetti Clemente, Canciani Monica, Farina Massimo, Era Sara, Walzer Stefan. Potential resource and cost saving analysis of subcutaneous versus intravenous administration for rituximab in non-Hodgkin's lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. ClinicoEconomics and outcomes research : CEOR. 2016;8:227–233. doi: 10.2147/CEOR.S97319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rummel M, Kim T, Plenteda C, Capochiani E, Mendoza M, Smith R, Grigg A. Prefmab: Final analysis of patient satisfaction with subcutaneous versus intravenous rituximab in previously untreated CD20 diffuse large b-cell lymphoma or follicular lymphoma. Value in Health. 2015;18(7):A469. [Google Scholar]
- 13.Subramanian Janakiraman, Cavenagh Jamie, Desai Bhardwaj, Jacobs Ira. Rituximab in the treatment of follicular lymphoma: the future of biosimilars in the evolving therapeutic landscape. Cancer management and research. 2017;9:131–140. doi: 10.2147/CMAR.S120589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truven Health Analytics. RED BOOK. 2017 Retrieved from http://www.redbook.com.
- 15.Weiner George J. Rituximab: mechanism of action. Seminars in hematology. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]