Abstract
Hypercalcemia of malignancy (HCM) is a common concern in patients being treated for cancer, affecting over a quarter of this population. There are multiple causes of HCM, including humoral HCM, osteolytic HCM, ectopic hyperparathyroidism, and vitamin D–secreting lymphomas. Common signs and symptoms of HCM can range from mild gastrointestinal disturbances and fatigue to seizures, coma, or even cardiac arrest depending on the severity of the laboratory abnormality. Treatment has evolved in recent years and varies based on the underlying cause of the HCM. Management options include aggressive hydration, bisphosphonates, denosumab, calcitonin, and corticosteroids. It is imperative that advanced practitioners understand the pathophysiology behind the HCM so that proper treatment can be chosen. Early and appropriate treatment is key to successful outcomes. It is also important for continuous monitoring to occur alongside treatment for HCM to prevent potential adverse effects. Finally, the ultimate resolution of HCM comes only from the treatment of the underlying malignancy; therefore, all previously undiagnosed patients should be referred to an oncologist early after HCM is recognized.
Hypercalcemia of malignancy (HCM) has long been recognized as a common paraneoplastic syndrome associated with poor prognosis in cancer patients. It is estimated that HCM affects roughly 30% of patients with cancer, with many studies reporting an even higher incidence in those with advanced stages of cancer (Endres, 2012; Mirrakhimov, 2015). Common malignancies associated with HCM in the United States include breast, lung, multiple myeloma, squamous cell carcinoma of the head and neck, renal, ovarian, and certain lymphomas (Gastanaga et al., 2016). Recent research has indicated that overall HCM prevalence seems to be declining in recent years, likely due to the increased use of prophylactic intravenous (IV) bisphosphonates in high-risk malignancies, particularly in cases of bone metastasis and multiple myeloma (Gastanaga et al., 2016; Terpos et al., 2013). However, HCM is still a frequent oncologic emergency that requires appropriate and prompt intervention to improve overall patient outcomes. The purpose of this paper is to review and summarize current literature and guidelines related to the diagnosis and management of HCM.
PATHOPHYSIOLOGY
Although the primary goal of this article is not to discuss the pathophysiology of HCM at length, at least a brief review is necessary to understand how HCM is diagnosed and managed, as treatment can vary at times depending on the underlying cause. Hypercalcemia of malignancy can be divided generally into four types.
The first and most common cause of HCM is humoral HCM. Approximately 80% of HCM cases are thought to be related to this type. Humoral HCM is caused by malignant tumors secreting parathyroid hormone–related protein (PTHrP), which acts similarly to parathyroid hormone (PTH) in the body. Effects of increased PTHrP secretion result in increased osteoclast activity and bone resorption with subsequent release of calcium into the bloodstream. This occurs secondary to the mechanism in which PTHrP acts on osteoblasts, which enhances production of the receptor activator of nuclear factor-κB (RANK) and its associated ligand (RANK-L). Humoral HCM is typically associated with renal, ovarian, breast, and squamous cell cancers as well as human T-cell lymphotropic virus (HTLV)–associated lymphoma (Dellay & Groth, 2016; Mirrakhimov, 2015; Wijaya, Oehadian, & Sumantri, 2014).
The second most common cause of HCM is known as osteolytic HCM. It accounts for 20% of HCM cases and is a result of excessive calcium release from bone secondary to malignant cells in the bone marrow. Local inflammatory response from these malignant cells stimulates osteoclast activity. This type of HCM is frequently seen in malignancies with bone metastasis as well as breast cancer and multiple myeloma (Dellay & Groth, 2016; Mirrakhimov, 2015; Wijaya et al., 2014).
The last two types of HCM are both relatively rare and account for less than 1% of HCM cases. Vitamin D–secreting lymphomas can cause hypercalcemia by increasing intestinal absorption of calcium and decreasing renal calcium excretion. Ectopic hyperparathyroidism causes increased secretion of PTH and acts nearly the same as PTHrP in the pathway discussed above to cause increased serum calcium. Small cell lung cancer and adenocarcinomas are most commonly associated with ectopic hyperparathyroidism, although a handful of documented cases have shown increased PTH secretion due to a parathyroid carcinoma (Cheng, Kuzhively, & Baim, 2017; Dellay & Groth, 2016; Mirrakhimov, 2015; Wijaya et al., 2014). Finally, it is important to note that primary hyperparathyroidism is possible in cancer patients and needs to be distinguished from HCM (Mirrakhimov, 2015).
CLINICAL MANIFESTATIONS
Signs and symptoms of hypercalcemia can vary significantly and depend on the level of hypercalcemia as well as how rapidly the calcium level is rising. Hypercalcemia of malignancy typically occurs more acutely compared to other causes of hypercalcemia, often making the clinical manifestations appear more severe in these cases (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014).
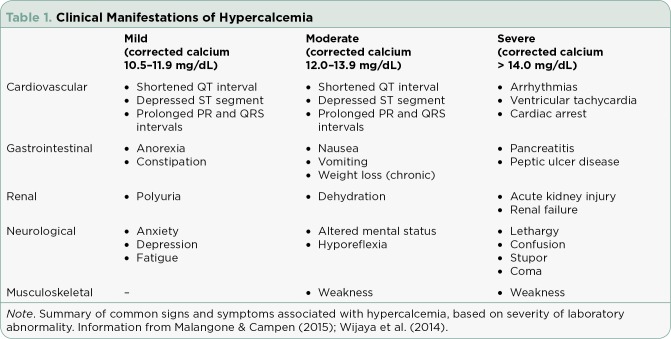
Hypercalcemia is often divided into categories of mild, moderate, severe, and life-threatening depending on the degree of serum calcium elevation. The generally accepted upper limit of normal (ULN) for corrected serum calcium is 10.5 mg/dL. [Note: Corrected serum calcium = serum calcium + 0.8 × (4 – serum albumin)]. It is vital to calculate the corrected serum calcium based on a patient’s serum albumin level if it is below normal; otherwise, many cases of hypercalcemia may be missed. Mild hypercalcemia ranges from the ULN to a corrected serum calcium of 11.9 mg/dL; moderate is defined as a corrected serum calcium from 12.0 mg/dL to 13.9 mg/dL; severe is defined as a corrected serum calcium greater than 14.0 mg/dL. Life-threatening hypercalcemia is often defined as a corrected serum calcium that falls in the severe range accompanied by critical clinical manifestations such as coma or cardiac arrest (see Table 1; Gastanaga et al., 2016; Malangone & Campen, 2015).
Table 1.
Clinical Manifestations of Hypercalcemia
Cardiovascular clinical manifestations include shortened ST segments and QT intervals, depressed ST segments, widened T waves, prolonged PR and QRS intervals, arrhythmias, ventricular tachycardias, and cardiac arrest (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014).
Hypercalcemia of malignancy can also affect the gastrointestinal system. Common signs and symptoms of mild hypercalcemia are anorexia and constipation. Higher degrees of hypercalcemia may also cause nausea, vomiting, weight loss (if chronic), and even pancreatitis or peptic ulcer disease (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014).
Renal manifestations of HCM include nephrogenic diabetes insipidus, which causes polyuria, renal vasoconstriction, and distal renal tubular acidosis, often resulting in acute kidney injury and significant dehydration (which is also related to gastrointestinal signs and symptoms). In chronic hypercalcemia, nephrolithiasis and chronic renal failure can also occur (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014).
Clinical manifestations related to the neurologic and musculoskeletal systems are often much more pronounced at moderate to severe levels of hypercalcemia and include anxiety, depression, cognitive dysfunction, lethargy, weakness, fatigue, hyporeflexia, confusion, stupor, and in the most severe cases, coma. Hypercalcemia of malignancy specifically has been associated with posterior reversible leukoencephalopathy syndrome, which presents with headaches, seizures, and subcortical edema on imaging (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014).
Although the systems listed above are the most commonly affected, other reported signs and symptoms of hypercalcemia include pruritus, generalized abdominal pain, and bone pain (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Wijaya et al., 2014). It is especially important to recognize this constellation of symptoms in patients not previously diagnosed with a malignancy, as the presentation of hypercalcemia may be an important diagnostic clue to finding an underlying cancer in some patients (Mirrakhimov, 2015).
DIAGNOSTIC APPROACH
Once hypercalcemia is confirmed using clinical presentation and corrected serum calcium level, the next step is to determine which pathophysiologic process discussed above is responsible for causing it. In many cases, a clear history of cancer diagnosis will accompany the patient, so a thorough history and physical exam is the obvious first task. However, even with confirmed malignancy, there are varying HCM mechanisms. Therefore, further investigation should be done. This is an important step, as it can have an impact on which treatment will best manage the hypercalcemia (Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Rosner & Dalkin, 2012;).
First, the serum PTH level should be measured. This assists in determining whether or not primary hyperparathyroidism is the sole cause of hypercalcemia (which, as noted above, can still be the case in some cancer patients), or if HCM is likely present. If the serum PTH level is elevated or high normal, it is likely that primary hyperparathyroidism is present and the patient should likely be referred to endocrinology for appropriate management (Malangone & Campen, 2015; Mirrakhimov, 2015). In some cases, chronic hypercalcemia with corrected serum calcium levels less than 12.0 mg/dL is also suggestive of primary hyperparathyroidism and should be considered in the differential (Dellay & Groth, 2016).
If the serum PTH level is decreased or low normal, HCM is the likely diagnosis and checking a serum PTHrP level should be considered for further evaluation (Malangone & Campen, 2015; Mirrakhimov, 2015). In many institutions, this is a send-out laboratory test that may take up to a week to obtain results. Therefore, assessment of PTHrP level should not delay immediate treatment for hypercalcemia, but it can be an important value for selecting continued management options in HCM (Dellay & Groth, 2016).
If the PTHrP level returns elevated, then humoral HCM is likely the cause. If the PTHrP level is normal or low, the next step is to consider evaluating for bony metastatic disease (if this is not already confirmed) as well as checking the vitamin D level (1,25-dihydroxyvitamin D; Dellay & Groth, 2016; Malangone & Campen, 2015; Mirrakhimov, 2015; Rosner & Dalkin, 2012). Elevated vitamin D levels should point advanced practitioners toward considering lymphoma as the underlying cause (Malangone & Campen, 2015).
Finally, if laboratory evaluation reveals normal values of PTH, PTHrP, and vitamin D, other causes of hypercalcemia must be considered. These include use of certain medications such as thiazide diuretics, corticosteroids, lithium, and vitamin A or D oversupplementation, as well as renal failure (Malangone & Campen, 2015; Mirrakhimov, 2015).
MANAGEMENT OPTIONS
Aggressive Hydration
Intravenous fluids are widely accepted as a first-line treatment for hypercalcemia, including HCM. Patients with HCM typically present with dehydration secondary to multiple underlying factors, including anorexia, nausea, vomiting, and nephrogenic diabetes insipidus as discussed previously in clinical manifestations of HCM. Dehydration in turn causes a further decrease in kidney function and a subsequent decrease in calcium excretion (Wijaya et al., 2014).
Aggressive rehydration, generally with 0.9% sodium chloride solution (also known as normal saline), should be initiated rapidly once hypercalcemia is confirmed with laboratory values (see Table 2; Dellay & Groth, 2016; Malangone & Campen, 2015; Wijaya et al., 2014). Practitioners often begin with 1 to 2 liter boluses followed by maintenance IV fluids at a rate of 200 to 500 mL/hr, titrating to a urine output of 100 to 150 mL/hr (Dellay & Groth, 2016; Malangone & Campen, 2015; Wijaya et al., 2014). The goal here is to increase the glomerular filtration rate and increase calcium excretion as well as to dilute serum calcium. Intravenous fluids are an accepted treatment in patients with preexisting renal dysfunction and congestive heart failure; however, careful monitoring of fluid status is mandatory (Sternlicht & Glezerman, 2015).
Table 2.
Common Management Options in the Treatment of Hypercalcemia of Malignancy
Historically, loop diuretics such as furosemide (Lasix) have been used in conjunction with aggressive IV hydration in the treatment of HCM. However, loop diuretics have been shown to carry a high risk of causing volume depletion and further electrolyte imbalances (Sternlicht & Glezerman, 2015). Evidence currently supports restricting the use of loop diuretics to those patients who are experiencing volume overload secondary to extensive IV fluid administration, and even then, close monitoring of ongoing fluid status is required. Routine use of loop diuretics is no longer recommended (Mirrakhimov, 2015; Rosner & Dalkin, 2012; Sternlicht & Glezerman, 2015).
Aggressive hydration usually elicits a clinical response quickly in HCM. Within a few hours, serum calcium levels decrease by approximately 2 mg/dL (Sternlicht & Glezerman, 2015); however, the effect is often transient and further management or treatment of the underlying malignancy is required (Rosner & Dalkin, 2012).
Bisphosphonates
After or often in conjunction with aggressive hydration, IV bisphosphonate treatment should be started to treat HCM. Bisphosphonates are very well studied in this population of patients and are considered to be quite safe and effective (Sternlicht & Glezerman, 2015; Wijaya et al., 2014).
Bisphosphonates act by inhibiting bone resorption. They bind to the bone and reduce or prevent osteoclast activity, which as discussed above is a major factor in the development of HCM (Dellay & Groth, 2016). Oral absorption of bisphosphonates is extremely low (1%–2%); therefore, IV administration is generally the route seen in the clinical setting (Sternlicht & Glezerman, 2015). First-generation bisphosphonates include etidronate (Didronel) and clodronate (Bonefos), neither of which are commonly used in practice anymore. Second-generation bisphosphonates, or those that contain nitrogen, are much more potent and are frequently used today (Mirrakhimov, 2015; Wijaya et al., 2014). Examples of this class include zoledronate (zoledronic acid or Zometa), pamidronate (Aredia), ibandronate (Boniva), and alendronate (Fosamax). Zoledronic acid and pamidronate are the two bisphosphonates that are currently approved by the US Food and Drug Administration (FDA) for the treatment of HCM in the United States (Sternlicht & Glezerman, 2015; Wijaya et al., 2014). Bisphosphonates can also be used for osteoporosis and to prevent skeletal-related events in metastatic bone disease; however, the dosing and timing is often different in these cases (Mirrakhimov, 2015).
Bisphosphonates should be started early in the treatment of HCM, and certainly within the first 48 hours. It generally takes 2 to 4 days to see a clinical response, with the lowest calcium level generally being 7 to 10 days after bisphosphonate administration (Sternlicht & Glezerman, 2015; Wijaya et al., 2014). Effects last roughly a few weeks, averaging 1 to 3 weeks, with some studies reporting up to 4 weeks in duration. Zoledronic acid has been shown to be superior to pamidronate in both clinical response and amount of time until hypercalcemia relapse. Zoledronic acid has shown a nearly 83% response rate in patients at 7 days after administration compared to approximately 64% with pamidronate. In the same study, zoledronic acid averaged 30 days until relapse vs. 17 days with pamidronate (Dellay & Groth, 2016). Due to this finding, zoledronic acid is often the first choice for bisphosphonate treatment (Sternlicht & Glezerman, 2015; Wijaya et al., 2014). It is important to note that due to their mechanism of action, bisphosphonates are usually most effective in HCM due to metastatic bone disease. In humoral HCM, higher levels of PTHrP are associated with a lower response rate, which needs to be taken into consideration when using bisphosphonates (Rosner & Dalkin, 2012).
There are several side effects that accompany bisphosphonate use. The most common are infusion-related fever and flu-like symptoms, including mild bone aches and pains (Mirrakhimov, 2015; Rosner & Dalkin, 2012; Sternlicht & Glezerman, 2015). There is also a risk of nephrotoxic acute tubular necrosis, particularly in patients who have advanced cancer, have been previously treated with bisphosphonates, and with concurrent use of nonsteroidal anti-inflammatory drugs (Mirrakhimov, 2015; Rosner & Dalkin, 2012). Due to this risk, experts recommend careful use of bisphosphonates if patients have preexisting kidney injuries or a decrease in renal function (Dellay & Groth, 2016).
Zoledronic acid is generally contraindicated in patients with a creatinine clearance (CrCl) of less than 30 mL/min. Dose reductions, or even holding a dose altogether, of both zoledronic acid and pamidronate should occur in patients with lower-than-normal CrCl (Rosner & Dalkin, 2012). Ibandronate, although not FDA-approved for the treatment of HCM, is believed to be safer for use with patients who have reduced renal function; however, more research is needed in this area (Mirrakhimov, 2015).
One other rare but serious side effect of bisphosphonate use is osteonecrosis of the jaw (ONJ). The risk of ONJ is believed to be less than 1 in 10,000 and is most common in patients diagnosed with multiple myeloma or metastasis to the bone (Mirrakhimov, 2015; Rosner & Dalkin, 2012). Other risk factors leading to this complication include recent dental extractions, poor dental health, a history of smoking, and long-term, regular use of bisphosphonates (Rosner & Dalkin, 2012). A comprehensive oral exam should be completed for each patient prior to starting bisphosphonate treatment (or as soon as possible after starting treatment). Regular dental care is necessary during and after bisphosphonate treatment as well, and dental procedures should be avoided within one month of bisphosphonate use to decrease the risk of complications (Kapoor, Sikka, Arora, & Chaudhary, 2013).
Bisphosphonate cost varies significantly; one dose of zoledronic acid averages $713, while one dose of pamidronate averages $104. Practitioners’ choice of bisphosphonate should consider the degree of HCM, potential side effect risks, and cost, especially that associated with long-term use for patients (Dellay & Groth, 2016).
Denosumab
Denosumab (Xgeva) was approved by the FDA in 2010 for the prevention of skeletal-related events in patients with solid tumor malignancies as well as osteoporosis (Dietzek, Connelly, Cotugno, Bartel, & McDonnell, 2015; Rosner & Dalkin, 2012). In 2018, the FDA expanded approval to include the prevention of skeletal-related events among patients with multiple myeloma who have developed bone metastases (Varga, Laubach, Anderson, & Richardson, 2018). Denosumab is a fully human monoclonal antibody that binds to RANK-L. This in turn prevents RANK-L from binding to its normal receptor, which as discussed earlier causes a significant decrease in osteoclast activity and subsequent bone resorption (Dellay & Groth, 2016; Dietzek et al., 2015). Although not FDA-approved for the indication of HCM, multiple case reports support denosumab as an option, particularly in the treatment of HCM refractory to bisphosphonates (Ashihara et al., 2016; Dietzek et al., 2015).
Few studies exist on denosumab in the treatment of HCM; however, Hu and colleagues (2014) reported a 64% response rate by 10 days after administration in patients who received denosumab after not having a clinical response to bisphosphonates for 30 days. This study also reported a median duration of response of 104 days, which is significantly greater than what has been reported with bisphosphonate treatment (Hu et al., 2014). Patients who received denosumab for the treatment of HCM refractory to bisphosphonates also demonstrated a decrease in clinical manifestations of HCM, specifically cognitive signs and symptoms such as altered mental status (Hu et al., 2014).
Furthermore, a handful of case studies have depicted safe and successful use of denosumab in patients with HCM and coexisting renal failure (Dellay & Groth, 2016). Denosumab is not eliminated by the kidneys, so dose adjustments are not required in patients with altered renal function (Rosner & Dalkin, 2012); however, more research is needed in this area before routine use in this population can be recommended (Dellay & Groth, 2016).
Denosumab is generally well tolerated, with the most common side effects being arthralgia and mild dyspnea (Hu et al., 2014; Mirrakhimov, 2015; Rosner & Dalkin, 2012). There is also a small risk of ONJ, which is relatively equivalent to the risk associated with zoledronic acid use (Mirrakhimov, 2015). Due to denosumab being a monoclonal antibody, increased infection risk following administration is often a concern; however, current evidence does not support any significant risk of infection associated with its use at this time (Mirrakhimov, 2015).
Denosumab is not commonly used in the treatment of HCM; less than 1% of patients hospitalized for this condition received denosumab as a part of their treatment regimen (Wright et al., 2015). However, with a growing number of studies supporting its safe and effective use, it should be strongly considered as a management option. This is particularly true in patients who are refractory to bisphosphonate use or who have concurrent renal concerns (Dellay & Groth, 2016; Dietzek et al., 2015; Hu et al., 2014; Rosner & Dalkin, 2012). Other factors to consider include ease of administration (subcutaneous vs. IV administration of bisphosphonates), and its fairly high cost as a monoclonal antibody (Ashihara et al., 2016; Dietzek et al., 2015; Hu et al., 2014).
Other Treatment Choices
A few other management options exist in the treatment of HCM, many of which are indicated in specific clinical scenarios. Hemodialysis in the setting of HCM has decreased significantly since bisphosphonates have entered the market; however, it is still appropriate at times (Wijaya et al., 2014). With patients who present with HCM as well as acute or chronic renal failure or a history of heart failure, where aggressive hydration is truly not indicated or is dangerous to the patient, hemodialysis with a very low or no calcium dialysate is feasible and acceptable (Mirrakhimov, 2015; Rosner & Dalkin, 2012; Wijaya et al., 2014).
Another choice in the management of HCM is the administration of calcitonin (Miacalcin, Fortical). Calcitonin acts to inhibit all osteoclast activity as well as to prevent renal absorption of calcium (Dawson, Todd, & Walton, 2014; Dellay & Groth, 2016). This medication exhibits a very rapid, albeit transient, hypocalcemic effect. A decrease in serum calcium level is generally seen about 2 hours following administration; however, calcitonin must be administered every 6 to 8 hours to maintain this effect (Dellay & Groth, 2016). It carries with it great risk for hypocalcemia, rebound hypercalcemia, and tachyphylaxis that usually develops after 2 or 3 days of treatment (Rosner & Dalkin, 2012). Because of this, calcitonin is generally only used in the acute phase of hypercalcemia, and it is often used as a bridging treatment until effects of bisphosphonates administered are seen (Dellay & Groth, 2016). One case study does report successful chronic use of calcitonin in the treatment of HCM (Dawson et al., 2014); however, this is not routinely seen in practice. Side effects most commonly include nausea, vomiting, and pain or irritation at the injection site (Rosner & Dalkin, 2012).
Corticosteroids are considered a management option in the setting of vitamin D–secreting tumors or lymphomas (Dellay & Groth, 2016). They generally work by decreasing gastric absorption of calcium (Mirrakhimov, 2015). It takes approximately 4 days to see a response as evidenced by a decrease in serum calcium level; in cases where no clinical response is achieved by the tenth day of treatment, corticosteroids should be discontinued and another option should be utilized (Dellay & Groth, 2016; Malangone & Campen, 2015). Treatment is often started with IV hydrocortisone for 3 to 5 days, and if there is a clinical response, patients can be transitioned to oral prednisone for an additional 5 to 7 days of treatment (Rosner & Dalkin, 2012). Significant side effects occur with corticosteroid use, including hyperglycemia, hypertension, steroid-induced psychosis, peptic ulcer disease, and an increased risk for tumor lysis syndrome, which contraindicate long-term use (Malangone & Campen, 2015).
In the past, gallium nitrate (Ganite) was also used to treat HCM. The exact mechanism is unknown, but it appeared to inhibit osteoclast activity and bone resorption (Rosner & Dalkin, 2012). This medication was known to carry a risk of many significant side effects (Rosner & Dalkin, 2012; Sternlicht & Glezerman, 2015). Gallium nitrate was withdrawn from the market in the United States in 1995 and discontinued by the manufacturer in 2012, although while in use it did show effectiveness in treating HCM (Mirrakhimov, 2015; Sternlicht & Glezerman, 2015).
There are also new and developing treatment options for HCM. Cinacalcet (Sensipar), a calcimimetic, works by decreasing PTH production. This has been used successfully to treat HCM secondary to parathyroid carcinoma (Mirrakhimov, 2015; Sternlicht & Glezerman, 2015). Minimal side effects include nausea, vomiting, and headaches. Further research is needed to determine if cinacalcet is effective in treating HCM due to other causes (Doyle & Malcolm, 2014; Sternlicht & Glezerman, 2015). Finally, anti-PTHrP antibodies are currently being tested in animal models. This approach offers future hope for additional treatment options in HCM that is refractory to or not appropriate for bisphosphonate use (Mohammad & Guise, 2016; Sternlicht & Glezerman, 2015).
Lastly, it needs to be understood that all the treatment options discussed here are truly only meant for stabilizing the patient until an oncologist can appropriately treat the underlying malignancy, as this is the only true cure for HCM.
PATIENT MONITORING AND OUTCOMES
Careful monitoring of clinical response is vital for all patients during and after treatment for HCM. Many of the management options discussed here predispose the patient to a risk of hypocalcemia, which carries with it its own dangerous clinical manifestations (Body, Niepel, & Tonini, 2017). This risk is highest in patients who are not monitored closely or who are exposed to long-term bisphosphonate use (Narechania, Thiruchelvam, Lokhande, Kistangari, & Daw, 2015; Wijaya et al., 2014). Continued monitoring of the serum calcium level is necessary, and in the event that hypocalcemia occurs, it will likely need to be treated with vitamin D supplementation and calcium repletion (oral or IV, depending on the severity of hypocalcemia), as well as other appropriate supportive measures (Body et al., 2017).
Other steps can also be taken to improve patient outcomes and reduce the risk for redeveloping hypercalcemia. Advanced practitioners should ensure that any calcium supplementation is discontinued, including that in the form of oral replacement therapy or parenteral feeds (Wijaya et al., 2014). It is also important to discontinue vitamin D supplementation, calcitriol, lithium, thiazide diuretics, and any other medications that may independently contribute to hypercalcemia (Sternlicht & Glezerman, 2015; Wijaya et al., 2014). Finally, weight-bearing exercise as tolerated should be encouraged unless contraindicated (Wijaya et al., 2014).
IMPLICATIONS FOR ADVANCED PRACTITIONERS
Hypercalcemia is a serious neoplastic syndrome that is highly prevalent in cancer patients. Prognosis is generally poor, with as many as 50% of those diagnosed with any degree of HCM not surviving for greater than 30 days (Wright et al., 2015). Prompt recognition and treatment of this condition is key. Practitioners need to be aware of how management options and suggested treatments have evolved over the past 5 to 10 years.
A recent study by Wright and colleagues (2015) revealed that many hospitalized patients with HCM did not receive appropriate treatment. Nearly 33% of patients did not receive bisphosphonate treatment, which is widely considered the first-line therapy in the absence of obvious contraindications. Patients frequently received steroid therapy (27% of patients), despite the fact that this is only truly indicated in the setting of vitamin D–secreting lymphomas, which account for only 1% of HCM cases. Additionally, it was common for contraindicated medications such as thiazide diuretics and lithium to be continued while patients were being treated for HCM. Also of note is that treatment was less likely to follow guidelines when provided by hospitalists or family practice providers (Wright et al., 2015). Advanced practitioners are often in front-line positions to quickly recognize evolving diagnoses in patients, as well as to modify treatment plans accordingly. In clinical scenarios where time is of the essence, such as in HCM, advanced practitioners need to be adequately prepared to effectively and efficiently manage their patients. It is imperative that advanced practitioners utilize up-to-date and evidence-based treatment to improve patient outcomes, particularly in the treatment of HCM.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Ashihara N, Nakajima K, Nakamura Y, Kobayashi M, Shirahata K, Maeda C, Ito N. Denosumab is effective for controlling serum calcium levels in patients with humoral hypercalcemia of malignancy syndrome: A case report on parathyroid hormone-related protein-producing cholangiocarcinoma. Internal Medicine. 2016;55(23):3453–3457. doi: 10.2169/internalmedicine.55.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Body Jean-Jacques, Niepel Daniela, Tonini Giuseppe. Hypercalcaemia and hypocalcaemia: finding the balance. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2017;25:1639–1649. doi: 10.1007/s00520-016-3543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng C, Kuzhively J, Baim S. Hypercalcemia of malignancy in thymic carcinoma: Evolving mechanisms of hypercalcemia and targeted therapies. Case Reports in Endocrinology, 2017. 2017;Article ID 2608392. doi: 10.1155/2017/2608392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson C, Todd A, Walton A. Treatment of bisphosphonate-resistant hypercalcemia of malignancy with calcitonin. Journal of Palliative Medicine. 2014;17(10):1084. doi: 10.1089/jpm.2014.0207. [DOI] [PubMed] [Google Scholar]
- 5.Dellay B, Groth M. Emergency management of malignancy-associated hypercalcemia. Advanced Emergency Nursing Journal. 2016;38(1):15–25. doi: 10.1097/TME.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 6.Dietzek Amanda, Connelly Kelly, Cotugno Michael, Bartel Sylvia, McDonnell Anne M. Denosumab in hypercalcemia of malignancy: a case series. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2015;21:143–147. doi: 10.1177/1078155213518361. [DOI] [PubMed] [Google Scholar]
- 7.Doyle Mary-Anne, Malcolm Janine C. An unusual case of malignancy-related hypercalcemia. International journal of general medicine. 2013;7:21–27. doi: 10.2147/IJGM.S51302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Endres David B. Investigation of hypercalcemia. Clinical biochemistry. 2012;45:954–963. doi: 10.1016/j.clinbiochem.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Gastanaga Victor M, Schwartzberg Lee S, Jain Rajul K, Pirolli Melissa, Quach David, Quigley Jane M, Mu George, Scott Stryker W, Liede Alexander. Prevalence of hypercalcemia among cancer patients in the United States. Cancer medicine. 2016;5:2091–2100. doi: 10.1002/cam4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Mimi I, Glezerman Ilya G, Leboulleux Sophie, Insogna Karl, Gucalp Rasim, Misiorowski Waldemar, Yu Bennett, Zorsky Paul, Tosi Diego, Bessudo Alberto, Jaccard Arnaud, Tonini Giuseppe, Ying Wendy, Braun Ada, Jain Rajul K. Denosumab for treatment of hypercalcemia of malignancy. The Journal of clinical endocrinology and metabolism. 2014;99:3144–3152. doi: 10.1210/jc.2014-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor S, Sikka G, Arora P, Chaudhary P. Bisphosphonate-related osteonecrosis of the jaw: An insight. Journal of Orofacial Sciences. 2013;5(2):78–82. [Google Scholar]
- 12.Malangone S, Campen C. Hypercalcemia of malignancy. Journal of the Advanced Practitioner in Oncology. 2015;6(6):586–592. [PMC free article] [PubMed] [Google Scholar]
- 13.Mirrakhimov Aibek E. Hypercalcemia of Malignancy: An Update on Pathogenesis and Management. North American journal of medical sciences. 2015;7:483–493. doi: 10.4103/1947-2714.170600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad Khalid S, Guise Theresa A. Hypercalcemia of Malignancy: A New Twist on an Old Problem. Journal of oncology practice. 2016;12:435–436. doi: 10.1200/JOP.2016.012062. [DOI] [PubMed] [Google Scholar]
- 15.Narechania Shraddha, Thiruchelvam Nirosshan, Lokhande Chetan, Kistangari Gaurav, Daw Hamed. Prolonged zoledronic acid-induced hypocalcemia in hypercalcemia of malignancy. The Journal of community and supportive oncology. 2015;13:374–377. doi: 10.12788/jcso.0166. [DOI] [PubMed] [Google Scholar]
- 16.Rosner Mitchell H, Dalkin Alan C. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clinical journal of the American Society of Nephrology : CJASN. 2012;7:1722–1729. doi: 10.2215/CJN.02470312. [DOI] [PubMed] [Google Scholar]
- 17.Sternlicht Hillel, Glezerman Ilya G. Hypercalcemia of malignancy and new treatment options. Therapeutics and clinical risk management. 2015;11:1779–1788. doi: 10.2147/TCRM.S83681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpos Evangelos, Morgan Gareth, Dimopoulos Meletios A, Drake Matthew T, Lentzsch Suzanne, Raje Noopur, Sezer Orhan, García-Sanz Ramón, Shimizu Kazuyuki, Turesson Ingemar, Reiman Tony, Jurczyszyn Artur, Merlini Giampaolo, Spencer Andrew, Leleu Xavier, Cavo Michele, Munshi Nikhil, Rajkumar S Vincent, Durie Brian G M, Roodman G David. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijaya I, Oehadian A, Sumantri R. Hypercalcemia of malignancy: Clinical characteristics and treatment outcome. Majalah Kedokteran Bandung. 2014;46(2):111–117. [Google Scholar]
- 20.Wright Jason D, Tergas Ana I, Ananth Cande V, Burke William M, Hou June Y, Chen Ling, Neugut Alfred I, Richards Catherine A, Hershman Dawn L. Quality and Outcomes of Treatment of Hypercalcemia of Malignancy. Cancer investigation. 2015;33:331–339. doi: 10.3109/07357907.2015.1047506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Cindy, Laubach Jacob P, Anderson Kenneth C, Richardson Paul G. Investigational agents in immunotherapy: a new horizon for the treatment of multiple myeloma. British journal of haematology. 2018;181:433–446. doi: 10.1111/bjh.15116. [DOI] [PubMed] [Google Scholar]