Abstract
Tisagenlecleucel is a first-in-class chimeric antigen receptor (CAR) T-cell therapy approved by the US Food and Drug Administration in 2017 for relapsed/refractory (RR) acute lymphoblastic leukemia (ALL) in patients up to 25 years of age. Tisagenlecleucel is an autologous T-cell therapy that is genetically engineered with a lentiviral vector to seek and eliminate CD19-expressing B cells throughout the patient’s body and retain antitumor immune surveillance following remission. This groundbreaking cellular therapy brings unprecedented single-agent efficacy to patients with RR ALL, citing complete response rates of greater than 80% and 6-month relapse-free survivals exceeding 60% in a patient population with poor prognosis and few treatment options. Patients receiving CAR T-cell therapy are at risk for cytokine release syndrome (CRS), neurotoxicity, and infections, along with other toxicities that may be severe or life-threatening. The cornerstone of the management of moderate to severe CRS is treatment with the interleukin-6 antagonist tocilizumab, with dramatic responses often occurring within 24 hours. The optimal management of neurotoxicity following tisagenlecleucel remains undefined. It is critical that providers caring for patients receiving tisagenlecleucel understand the multistep process to prepare a patient for therapy, how to closely monitor patients for toxicity, and how to manage emergent adverse events following cell infusion.
Acute lymphoblastic leukemia (ALL) is an aggressive hematologic malignancy characterized by blood and bone marrow infiltration by malignant lymphoblasts. In 2018, 5,960 people are estimated to be diagnosed with ALL, and 1,470 deaths are anticipated (American Cancer Society, 2018). Acute lymphoblastic leukemia is the most common pediatric malignancy, yet it has a bimodal distribution, with adult diagnoses increasing in the sixth decade of life (American Cancer Society, 2018).
Patients with ALL are typically grouped by age (pediatric, adolescent and young adult, and older adult) and Philadelphia chromosome (Ph) status. Ph-positive patients are treated with a tyrosine kinase inhibitor, often with a corticosteroid and vincristine, or as part of a multiagent regimen (anthracycline, corticosteroid, vincristine, alkylating agent; Foà et al., 2011; Ravandi et al., 2010). Younger Ph-negative patients typically receive similar combination chemotherapy (often adding pegaspargase [Oncaspar]), although older adults may only tolerate less intensive induction regimens (Gökbuget, 2013). Pediatric outcomes are generally favorable, with 5-year overall survival (OS) greater than 90% (Hunger et al., 2012), while 5-year OS for adults more than 30 years old is only 15% to 35% (Rowe et al., 2005). Unfortunately, up to 20% of pediatric patients will relapse, with 5-year OS as poor as 20% (Nguyen et al., 2008). Outcomes for relapsed adults also remain dismal, with a median OS of only 8 months (Gökbuget et al., 2012).
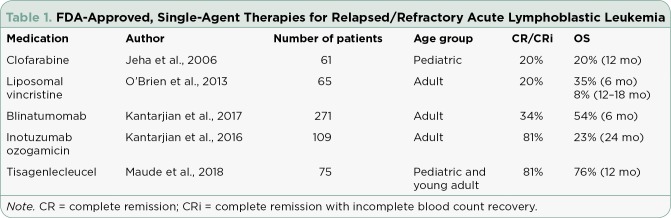
Several agents approved by the US Food and Drug Administration (FDA) for relapsed/refractory (RR) ALL are summarized in Table 1. Single-agent activity in RR ALL has gradually improved, with the most impressive outcomes to date observed with tisagenlecleucel (CTL019; Kymriah; Maude et al., 2018). CTL019 is the chimeric antigen receptor (CAR)-modified T cell developed by the University of Pennsylvania School of Medicine (Penn), the Children’s Hospital of Philadelphia (CHOP), and Novartis Pharmaceuticals. On August 30, 2017, the FDA approved CTL019 for RR B-cell ALL in pediatric and young adult patients (up to 25 years old) in second or later relapse, making it the first-ever CAR T-cell therapy approved for any indication. The approval of CTL019 marks the dawn of a new era in cancer therapeutics, where the immune system is trained to target malignant cells throughout the body and maintain prolonged antitumor surveillance following treatment.
Table 1.
FDA-Approved, Single-Agent Therapies for Relapsed/Refractory Acute Lymphoblastic Leukemia
WHAT IS A CAR T CELL?
Chimeric antigen receptor T cells are autologous T cells genetically modified to express a cell surface receptor against a tumor surface antigen. For CTL019, genetic modification occurs when T cells are infected with a lentivirus containing a DNA sequence encoding a murine anti-CD19 single-chain variable fragment (scFv) receptor, the costimulatory molecule 4-1BB, and the signal transduction molecule CD3ζ. CD19 is a B-cell surface antigen expressed in the majority of B-cell malignancies, including B-cell ALL, chronic lymphocytic leukemia, and B-cell non-Hodgkin lymphoma (NHL), but with minimal expression in multiple myeloma and no expression on hematopoietic stem cells (Wang, Wei, & Liu, 2012). Following infusion, CTL019 cells traffic toward malignant cells, bind CD19 on the cancer cell surface and become activated, resulting in antigen-mediated tumor cell death, proinflammatory cytokine release, and CAR T-cell proliferation. Following tumor cell elimination, CTL019 persists in the blood and bone marrow, conducting tumor surveillance to prevent future relapse (Porter et al., 2015).
Manufacturing of CTL019 requires peripheral blood mononuclear cell harvest via leukapheresis. Cells are cryopreserved and shipped to a centralized manufacturing facility where T cells are isolated, infected with the lentiviral vector, expanded several thousand fold, refrozen into a suspension in a single-infusion bag, and shipped in liquid nitrogen back to the treatment facility (Novartis, 2018). The average turnaround time is 22 days (Novartis, 2018).
In the interim, patients receive lymphodepleting chemotherapy (for ALL, fludarabine at 30 mg/m² daily for 4 days and cyclophosphamide at 500 mg/m² daily for 2 days is advised) to allow for CTL019 expansion in the bone marrow, in addition to providing temporary disease control and tumor burden reduction until CTL019 infusion and expansion. Pediatric and young adult patients with RR ALL receive 0.2 to 5 × 10⁶ CTL019 cells/kg (if ≤ 50 kg) or 0.1 to 2.5 × 10⁸ CTL019 cells (if > 50 kg) over 1 to 3 minutes (10–20 mL/min) 2 to 14 days following the completion of lymphodepletion with fludarabine and cyclophosphamide (Novartis, 2018).
CLINICAL STUDIES
B-Cell Acute Lymphoblastic Leukemia
The first trial reported from the Penn/CHOP group in RR ALL included 25 patients (5–22 years) and 5 older patients (26–60 years) who received physician’s choice of lymphodepleting chemotherapy followed by 0.76 to 20.6 × 10⁶ CTL019 cells/kg. Patients were heavily pretreated, receiving 1 to 4 prior regimens, with 18 out of 30 receiving prior allogeneic stem cell transplant (alloSCT), and 3 receiving prior CD19-directed therapy with blinatumomab. Complete remission (CR) was observed in 90% of patients, including 67% of patients receiving prior blinatumomab and 2 patients with central nervous system (CNS) leukemia prior to CTL019 infusion. Graft-vs.-host disease (GVHD) was not observed in patients receiving prior alloSCT, despite CTL019 being autologous in origin (Maude et al., 2014). Six-month event-free survival and OS were 67% and 78%, respectively. Relapses following CTL019 were due to loss of CTL019 persistence or loss of ALL CD19 expression (Maude et al., 2014). A later report of a total of 53 patients treated on this trial with a median follow-up of 10.6 months demonstrated that more than half of relapses were CD19-negative, highlighting the persistence of CTL019 but the possibility of ALL antigenic escape (Grupp et al., 2015).
Following this single-center success, a multicenter US pediatric RR ALL study (ENSIGN) was conducted with centrally manufactured CTL019. Twenty-nine patients were treated, with a CR/complete remission with incomplete blood count recovery (CRi) rate of 69% (Maude et al., 2016). Six-month relapse-free survival (RFS) and OS were 66% and 76%, respectively (Maude et al., 2016). This was the first multicenter study to demonstrate the feasibility of centralized CAR-T manufacturing, with a median time from enrollment to infusion of 37 days, in addition to demonstrating similar CTL019 efficacy across multiple centers with heterogeneous patient populations.
The FDA approval of CTL019 for RR ALL was based on the phase II, multicenter ELIANA trial, the first global CAR-T trial using centralized manufacturing. Seventy-five pediatric and young adult patients (median age 11 years, range 3–23 years) with heavily pretreated RR ALL (median 3 prior therapies, 61% with prior alloSCT) and extensive disease burden (median marrow blast percentage 74%) were enrolled. Most patients received lymphodepleting chemotherapy followed by a single dose of CTL019 (0.2 to 5.4 × 10⁶ cells/kg), with a median time from study enrollment to CTL019 infusion of only 45 days. With a median follow-up of 13 months, CR/CRi was achieved by 81% of patients, all of whom had minimal residual disease–negative responses by flow cytometry. Relapse-free survival and OS at 12 months were 59% and 76%, respectively, and the median duration of response has not been reached despite only 8 patients receiving alloSCT following CTL019. The majority of patients who relapsed following CTL019 did so with CD19-negative disease (Maude et al., 2018).
B-Cell Non-Hodgkin Lymphoma
Following the early success of CTL019 in RR ALL, researchers targeted B-cell NHL, a more common malignancy with consistent CD19 expression. In the first clinical report published in late 2017, 28 adults received physician’s choice of lymphodepleting chemotherapy followed by 1 to 5 × 10⁸ CTL019 cells. The overall response rate (ORR) was 64%; CR rates were 43% in patients with diffuse large B-cell lymphoma (DLBCL) and 71% in patients with follicular lymphoma, with more than 85% of responses maintained at a median follow-up of 28 months (Schuster et al., 2017).
Preliminary results from a phase II, multicenter, multinational trial of CTL019 in RR DLBCL (JULIET) were presented in late 2017. Ninety-nine patients received a median of 3.1 × 10⁸ CTL019 cells, with an ORR of 53% (39.5% CR) and a 6-month RFS of 73.5%, demonstrating the feasibility of centralized manufacturing of CTL019 for DLBCL (Schuster et al., 2017). The FDA approved CTL019 for the treatment of adults with RR DLBCL after two or more lines of prior systemic therapy on May 1, 2018 (Novartis, 2018).
ADVERSE EVENTS
Cytokine Release Syndrome
The most significant and life-threating adverse event (AE) of CAR T-cell therapy is cytokine release syndrome (CRS), with a median onset of 3 days following CTL019 infusion. In the ELIANA trial, 77% of patients experienced any grade of CRS, with 21% and 25% experiencing grade 3 and 4 CRS, respectively (Maude et al., 2018). Virtually all cases of CRS present with a fever, and other early symptoms often mimic severe flu-like illness. Progressive CRS may result in signs and symptoms of multiorgan involvement, including nausea, vomiting, transaminitis, hyperbilirubinemia, and azotemia (Lee et al., 2014).
For ALL patients treated with CTL019, high tumor burden prior to infusion increases the risk of severe CRS, where symptoms can rapidly progress to high-grade fevers, hypoxia, and hypotension. Roughly half of the patients treated in ELIANA required admission to the intensive care unit and approximately one-quarter required vasopressor support for CRS management (Maude et al., 2018). Numerous inflammatory cytokines are released during CRS, with interleukin (IL)-6 identified as the primary driver (Maude et al., 2014; Teachey et al., 2016). Cytokine concentrations can be measured; however, results are not available in real time at most centers. Surrogates for cytokines, such as ferritin and C-reactive protein, are often elevated during CRS and may be trended to monitor CRS therapy response.
More than one grading scale for CRS is currently in use (Lee et al., 2014; Porter et al., 2015); as such, grading may differ across studies of various CAR T-cell therapy products. CTL019 studies used the Penn Grading System for CRS, which is summarized in Table 2. Severe or life-threatening CRS is managed with the IL-6 receptor antagonist tocilizumab (Actemra), now FDA-approved for CRS (Genentech, 2017). In ELIANA, 48% received at least 1 dose of tocilizumab, with repeat dosing considered for patients with inadequate response (Maude et al., 2018). Responses are often rapid and dramatic, with fever resolution in just a few hours and blood pressure improvement allowing weaning of vasopressors in hours to 1 to 2 days. Cytokine release syndrome management recommendations from the tisagenlecleucel package insert are summarized in Table 3; however, individual centers may use different protocols with slight variations.
Table 2.
Penn Grading System for Cytokine Release Syndrome
Table 3.
Tisagenlecleucel Cytokine Release Syndrome Management Recommendations
Tocilizumab does not appear to impact the efficacy or expansion of CTL019 (Maude et al., 2014). Patients with severe CRS and inadequate response to tocilizumab may require broader anti-inflammatory therapy with corticosteroids to sustain life. There is at least a theoretical risk of abrogating CAR-T efficacy due to the lymphotoxic effects of steroids, but it is unclear what dose or duration of steroid therapy negatively impacts efficacy. The combination of infection and CRS can be particularly fatal, such that patients with active infection should not receive CTL019. Patients with CRS should be managed with broad-spectrum antimicrobials until infection is ruled out (Frey et al., 2014, 2016).
Neurotoxicity
The second most severe CAR T-cell therapy–associated AE is neurotoxicity. Neurologic events (NEs) with CTL019 typically occur following the peak of CRS symptoms, but can also occur without prior CRS (Maude et al., 2014). Most cases occur within 8 weeks of infusion (Novartis, 2018). In the ELIANA trial, 13% of patients experienced grade 3 NEs, with no grade 4 events or cerebral edema observed (Maude et al., 2018). The most common NEs with CTL019 were headache (37%), encephalopathy (34%), and delirium (21%; Novartis, 2018). Although the etiology of NEs remains unclear, studies with other CAR T-cell therapies suggest that loss of vascular integrity and infiltration of leukocytes and inflammatory cytokines into the central nervous system occurs (Gust et al., 2017). However, despite virtually all patients demonstrating CTL019 penetration into the cerebrospinal fluid, severe neurotoxicity remains uncommon (Grupp et al., 2015) and the presence of CNS leukemia does not increase the risk of neurotoxicity (Rheingold et al., 2015). Optimal treatment of NEs following CTL019 is unclear. For mild or moderate cases, only supportive care is advised. Corticosteroids may be beneficial for treating grade 3 to 4 NEs (Maude et al., 2014). Tocilizumab does not seem to treat or prevent NEs (Maude et al., 2014).
Infection
Infection of all grades was observed in 59% of pediatric RR ALL patients treated with CTL019, with 35% developing grade ≤ 3 infections (Novartis, 2018). Infection risk is influenced by the cell product itself, in addition to the underlying disease, immunosuppression and neutropenia related to lymphodepleting chemotherapy, cumulative effects of prior therapies, and hypogammaglobulinemia resulting from B-cell aplasia. Hypogammaglobulinemia was reported in 43% of RR ALL patients and may persist for the duration of CTL019 persistence. Deficient patients may need intravenous gamma globulin supplementation (Novartis, 2018). Herpes simplex virus and Pneumocystis pneumonia prophylaxis are advised for patients receiving CAR T-cell therapy. Some centers use filgrastim when absolute neutrophil count is less than 500/mm³ following CAR T-cell therapy (Brudno & Kochenderfer, 2016); however, colony-stimulating factors (specifically granulocyte-macrophage colony-stimulating factor [GM-CSF]) may theoretically worsen CRS. Myeloid growth factors, particularly GM-CSF, are not recommended during the first 3 weeks following CTL019 or until CRS has resolved (Novartis, 2018).
Other Toxicities
Coagulopathy may occur following CTL019 and correlates with CRS severity with 71% of patients with grade 4 CRS, requiring treatment with cryoprecipitate (Büchner et al., 2017). Despite severe hypofibrinogenemia, significant changes in international normalized ratio, activated partial thromboplastin time, and fatal bleeding events are uncommon (Büchner et al., 2017). Providers should monitor coagulation parameters and for signs of bleeding and administer cryoprecipitate according to institutional standards. Standard institutional tumor lysis syndrome prophylaxis is advised following lymphodepleting chemotherapy and CTL019 infusion. Hypersensitivity reactions to the CTL019 cell product itself or dextran and dimethylsulfoxide in the cell product may occur. Premedication with acetaminophen and diphenhydramine 30 to 60 minutes prior to CTL019, along with monitoring for hypersensitivity reactions, is required.
IMPLICATIONS FOR THE ADVANCED PRACTITIONER
CTL019 is only available via a risk evaluation and mitigation strategies (REMS) program. Sites wishing to use CTL019 must register as Kymriah Treatment Centers after demonstrating the ability to manage the logistics and toxicities of CAR T-cell therapy. Currently, such centers are limited to academic medical centers with experience in alloSCT. Care is typically provided by the stem cell transplant division or a dedicated cellular therapy service. All staff that prescribe, dispense, or administer CTL019 must complete REMS training on toxicity management prior to patient care.
Advanced practitioners (APs) practicing in centers using CTL019 may be involved in patient/family counseling, toxicity monitoring, and management. Advanced practitioners must explain the risk of CRS, neurotoxicity, and infection following CTL019, including the timing of symptom onset and when patients need to return to the hospital for evaluation. Advanced practitioners must be comfortable identifying when intervention for CRS is indicated and when therapy beyond tocilizumab may be required. Advanced practitioners should consider delaying infusion in patients with active or uncontrolled infection, serious and unresolved AEs from prior therapies, active GVHD, or progressive leukemic burden following lymphodepleting chemotherapy to avoid augmenting CTL019 toxicity.
Advanced practitioners must be aware of institutional mechanisms to ensure that at least 2 doses of tocilizumab are immediately accessible for each patient receiving CTL019. Hepatitis B and C screening is required prior to CTL019 as reactivation can occur following B-cell aplasia. Human immunodeficiency virus (HIV) screening is also required, as some HIV assays may cross-react with the lentiviral vector, yielding false-positive results post infusion (Novartis, 2018).
Patients must stay within 2 hours of the treatment facility for 4 weeks following infusion to enable prompt management of CRS or NEs if necessary. Due to the potential for neurotoxicity, patients should not drive or operate heavy machinery for 8 weeks following CTL019. Live virus vaccines should be avoided for at least 2 weeks prior to the start of lymphodepleting chemotherapy, and until immune recovery following CTL019. Patients should be informed of the risk of manufacturing failure, which is approximately 9%, such that all patients receiving leukapheresis may not ultimately receive CTL019 (Novartis, 2018). Insertional mutagenesis from the lentiviral vector may increase the risk of secondary malignancies such that patients should receive lifelong monitoring (Novartis, 2018). A lengthy prior approval process will be necessary to ensure insurance coverage for CTL019 given its $475,000 price tag; however, the manufacturer has stated that Medicaid will not have to pay for the drug for Medicaid-covered children who do not show response by day 30 (Skinner, 2017).
CONCLUSIONS
CTL019 is a first-in-class CAR-T therapy approved for RR ALL in pediatric and young adult patients up to age 25 and for adults with RR DLBCL. In RR ALL, CTL019 offers impressive CR and OS rates for a poor-risk patient population with limited treatment options. With unprecedented efficacy comes unique and potentially life-threatening toxicities such as CRS, neurologic toxicities, and infection, all of which require APs with specialized training for appropriate management.
Footnotes
The author has no conflicts of interest to disclose.
References
- 1.American Cancer Society. Key statistics for acute lymphocytic leukemia. 2018 Retrieved from https://www.cancer.org/cancer/acute-lymphocytic-leukemia/about/key-statistics.html.
- 2.Büchner J, Grupp S A, Maude S L, Hiramatsu H, Teachey D T, Wood P A, De Moerloose B. Management of coagulopathy associated with CTL019 CAR T-cell therapy [Abstract 1276]. Blood (ASH Annual Meeting Abstracts) 2017;130(suppl 1) Retrieved from http://www.bloodjournal.org/content/130/Suppl_1/1276. [Google Scholar]
- 3.Brudno Jennifer N, Kochenderfer James N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foà Robin, Vitale Antonella, Vignetti Marco, Meloni Giovanna, Guarini Anna, De Propris Maria Stefania, Elia Loredana, Paoloni Francesca, Fazi Paola, Cimino Giuseppe, Nobile Francesco, Ferrara Felicetto, Castagnola Carlo, Sica Simona, Leoni Pietro, Zuffa Eliana, Fozza Claudio, Luppi Mario, Candoni Anna, Iacobucci Ilaria, Soverini Simona, Mandelli Franco, Martinelli Giovanni, Baccarani Michele. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 5.Frey N V, Levine B L, Lacey S F, Grupp S A, Maude S L, Schuster S J, Porter D. Refractory cytokine release syndrome in recipients of chimeric antigen receptor (CAR) T cells [Abstract 2296]. Blood (ASH Annual Meeting Abstracts) 2014;124(21) Retrieved from http://www.bloodjournal.org/content/124/21/2296 . [Google Scholar]
- 6.Frey N V, Shaw P A, Hexner E O, Gill S, Marcucci K, Luger S M, Porter D L. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory acute lymphoblastic leukemia [Abstract 7002]. Journal of Clinical Oncology (Meeting Abstracts) 2016;34(15 suppl) [Google Scholar]
- 7.Genentech, Inc. Tocilizumab (Actemra) package insert. . 2017 Retrieved from https://www.gene.com/download/pdf/actemra_prescribing.pdf .
- 8.Gökbuget Nicola. How I treat older patients with ALL. Blood. 2013;122:1366–1375. doi: 10.1182/blood-2012-07-379016. [DOI] [PubMed] [Google Scholar]
- 9.Gökbuget Nicola, Stanze Daniel, Beck Joachim, Diedrich Helmut, Horst Heinz-August, Hüttmann Andreas, Kobbe Guido, Kreuzer Karl-Anton, Leimer Lothar, Reichle Albrecht, Schaich Markus, Schwartz Stefan, Serve Hubert, Starck Michael, Stelljes Matthias, Stuhlmann Reingard, Viardot Andreas, Wendelin Knut, Freund Mathias, Hoelzer Dieter. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032–2041. doi: 10.1182/blood-2011-12-399287. [DOI] [PubMed] [Google Scholar]
- 10.Grupp S A, Maude S L, Shaw P A, Aplenc R, Barrett D M, Callahan C, June C H. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) [Abstract 681]. Blood (ASH Annual Meeting Abstracts) 2015;126(23) Retrieved from https://ash.confex.com/ash/2015/webprogramscheduler/Paper85408.html . [Google Scholar]
- 11.Gust Juliane, Hay Kevin A, Hanafi Laïla-Aïcha, Li Daniel, Myerson David, Gonzalez-Cuyar Luis F, Yeung Cecilia, Liles W Conrad, Wurfel Mark, Lopez Jose A, Chen Junmei, Chung Dominic, Harju-Baker Susanna, Özpolat Tahsin, Fink Kathleen R, Riddell Stanley R, Maloney David G, Turtle Cameron J. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer discovery. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunger Stephen P, Lu Xiaomin, Devidas Meenakshi, Camitta Bruce M, Gaynon Paul S, Winick Naomi J, Reaman Gregory H, Carroll William L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeha Sima, Gaynon Paul S, Razzouk Bassem I, Franklin Janet, Kadota Richard, Shen Violet, Luchtman-Jones Lori, Rytting Michael, Bomgaars Lisa R, Rheingold Susan, Ritchey Kim, Albano Edythe, Arceci Robert J, Goldman Stewart, Griffin Timothy, Altman Arnold, Gordon Bruce, Steinherz Laurel, Weitman Steven, Steinherz Peter. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1917–1923. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 14.Kantarjian Hagop M, DeAngelo Daniel J, Stelljes Matthias, Martinelli Giovanni, Liedtke Michaela, Stock Wendy, Gökbuget Nicola, O’Brien Susan, Wang Kongming, Wang Tao, Paccagnella M Luisa, Sleight Barbara, Vandendries Erik, Advani Anjali S. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. The New England journal of medicine. 2016;375:740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantarjian Hagop, Stein Anthony, Gökbuget Nicola, Fielding Adele K, Schuh Andre C, Ribera Josep-Maria, Wei Andrew, Dombret Hervé, Foà Robin, Bassan Renato, Arslan Önder, Sanz Miguel A, Bergeron Julie, Demirkan Fatih, Lech-Maranda Ewa, Rambaldi Alessandro, Thomas Xavier, Horst Heinz-August, Brüggemann Monika, Klapper Wolfram, Wood Brent L, Fleishman Alex, Nagorsen Dirk, Holland Christopher, Zimmerman Zachary, Topp Max S. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. The New England journal of medicine. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee Daniel W, Gardner Rebecca, Porter David L, Louis Chrystal U, Ahmed Nabil, Jensen Michael, Grupp Stephan A, Mackall Crystal L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maude Shannon L, Frey Noelle, Shaw Pamela A, Aplenc Richard, Barrett David M, Bunin Nancy J, Chew Anne, Gonzalez Vanessa E, Zheng Zhaohui, Lacey Simon F, Mahnke Yolanda D, Melenhorst Jan J, Rheingold Susan R, Shen Angela, Teachey David T, Levine Bruce L, June Carl H, Porter David L, Grupp Stephan A. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude Shannon L, Laetsch Theodore W, Buechner Jochen, Rives Susana, Boyer Michael, Bittencourt Henrique, Bader Peter, Verneris Michael R, Stefanski Heather E, Myers Gary D, Qayed Muna, De Moerloose Barbara, Hiramatsu Hidefumi, Schlis Krysta, Davis Kara L, Martin Paul L, Nemecek Eneida R, Yanik Gregory A, Peters Christina, Baruchel Andre, Boissel Nicolas, Mechinaud Francoise, Balduzzi Adriana, Krueger Joerg, June Carl H, Levine Bruce L, Wood Patricia, Taran Tetiana, Leung Mimi, Mueller Karen T, Zhang Yiyun, Sen Kapildeb, Lebwohl David, Pulsipher Michael A, Grupp Stephan A. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. The New England journal of medicine. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maude S L, Pulsipher M A, Boyer M W, Grupp S A, Davies S M, Philips C. L, Levine J E. Efficacy and safety of CTL019 in the first US phase II multicenter trial in pediatric relapsed/refractory acute lymphoblastic leukemia: Results of an interim analysis [Abstract 2801]. Blood (ASH Annual Meeting Abstracts) 2016;128(22) Retrieved from http://www.bloodjournal.org/content/128/22/2801/tab-figures-only?sso-checked=true. [Google Scholar]
- 20.Nguyen K, Devidas M, Cheng S-C, La M, Raetz E A, Carroll W L, Winick N J, Hunger S P, Gaynon P S, Loh M L. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novartis. Tisagenlecleucel (Kymriah) package insert. 2018 Retrieved from https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kymriah.pdf .
- 22.O’Brien Susan, Schiller Gary, Lister John, Damon Lloyd, Goldberg Stuart, Aulitzky Walter, Ben-Yehuda Dina, Stock Wendy, Coutre Steven, Douer Dan, Heffner Leonard T, Larson Melissa, Seiter Karen, Smith Scott, Assouline Sarit, Kuriakose Philip, Maness Lori, Nagler Arnon, Rowe Jacob, Schaich Markus, Shpilberg Ofer, Yee Karen, Schmieder Guenter, Silverman Jeffrey A, Thomas Deborah, Deitcher Steven R, Kantarjian Hagop. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:676–683. doi: 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter D L, Hwang W T, Frey N V, Lacey S F, Shaw P A, Loren A W, June C H. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Science Translational Medicine. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravandi Farhad, O’Brien Susan, Thomas Deborah, Faderl Stefan, Jones Dan, Garris Rebecca, Dara Samuel, Jorgensen Jeffrey, Kebriaei Partow, Champlin Richard, Borthakur Gautam, Burger Jan, Ferrajoli Alessandra, Garcia-Manero Guillermo, Wierda William, Cortes Jorge, Kantarjian Hagop. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rheingold S R, Chen L N, Maude S L, Aplenc R, Barker C, Barrett D M, Grupp S A. Efficient trafficking of chimeric antigen receptor (CAR)-modified T cells to the CSF and induction of durable CNS remissions in children with CNS/combined relapsed/refractory ALL [Abstract 3769]. Blood (ASH Annual Meeting Abstracts) 2015;126(23) Retrieved from http://www.bloodjournal.org/content/126/23/3769. [Google Scholar]
- 26.Rowe Jacob M, Buck Georgina, Burnett Alan K, Chopra Raj, Wiernik Peter H, Richards Susan M, Lazarus Hillard M, Franklin Ian M, Litzow Mark R, Ciobanu Niculae, Prentice H Grant, Durrant Jill, Tallman Martin S, Goldstone Anthony H. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–3767. doi: 10.1182/blood-2005-04-1623. [DOI] [PubMed] [Google Scholar]
- 27.Schuster Stephen J, Svoboda Jakub, Chong Elise A, Nasta Sunita D, Mato Anthony R, Anak Özlem, Brogdon Jennifer L, Pruteanu-Malinici Iulian, Bhoj Vijay, Landsburg Daniel, Wasik Mariusz, Levine Bruce L, Lacey Simon F, Melenhorst Jan J, Porter David L, June Carl H. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. The New England journal of medicine. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster Stephen J, Svoboda Jakub, Chong Elise A, Nasta Sunita D, Mato Anthony R, Anak Özlem, Brogdon Jennifer L, Pruteanu-Malinici Iulian, Bhoj Vijay, Landsburg Daniel, Wasik Mariusz, Levine Bruce L, Lacey Simon F, Melenhorst Jan J, Porter David L, June Carl H. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. The New England journal of medicine. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner G. Kymriah, the first gene therapy, arrives with a $475,000 price tag. 2017 Retrieved from https://www.consumerreports.org/drug-prices/kymriah-first-gene-therapy-costs-475000-dollars-childhood-cancer/
- 30.Teachey David T, Lacey Simon F, Shaw Pamela A, Melenhorst J Joseph, Maude Shannon L, Frey Noelle, Pequignot Edward, Gonzalez Vanessa E, Chen Fang, Finklestein Jeffrey, Barrett David M, Weiss Scott L, Fitzgerald Julie C, Berg Robert A, Aplenc Richard, Callahan Colleen, Rheingold Susan R, Zheng Zhaohui, Rose-John Stefan, White Jason C, Nazimuddin Farzana, Wertheim Gerald, Levine Bruce L, June Carl H, Porter David L, Grupp Stephan A. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer discovery. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Wei G, Liu D. CD19: A biomarker for B cell development, lymphoma diagnosis and therapy. Expert Hematology and Oncology. 2012;1(1):36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]