Abstract
In group-living species with parental care, the accurate recognition of one's own young is critical to fitness. Because discriminating offspring within a large colonial group may be challenging, progeny of colonial breeders often display familial or individual identity signals to elicit and receive parental provisions from their own parents. For instance, the common murre (or common guillemot: Uria aalge) is a colonially breeding seabird that does not build a nest and lays and incubates an egg with an individually unique appearance. How the shell's physical and chemical properties generate this individual variability in coloration and maculation has not been studied in detail. Here, we quantified two characteristics of the avian-visible appearance of murre eggshells collected from the wild: background coloration spectra and maculation density. As predicted by the individual identity hypothesis, there was no statistical relationship between avian-perceivable shell background coloration and maculation density within the same eggs. In turn, variation in both sets of traits was statistically related to some of their physico-chemical properties, including shell thickness and concentrations of the eggshell pigments biliverdin and protoporphyrin IX. These results illustrate how individually unique eggshell appearances, suitable for identity signalling, can be generated by a small number of structural mechanisms.
Keywords: coloration, coloniality, discrimination, egg recognition, individual recognition, maculation
1. Introduction
Caring for dependent young often incurs energetic and temporal costs for the parents [1]. Recognition mechanisms that enable the delivery of parental care to genetically related young, therefore, are favoured by natural selection [2]. In many parental species, identifying one's own progeny reliably can be achieved indirectly through the recognition of the site at which young are housed and developing (e.g. hive, nest or den) [3]. However, in colonially breeding species, especially those without fixed dens or nests, or with mobile offspring, the direct recognition of own offspring is necessary for accurate parental investment [4]. In birds, individually unique coloration and maculation of eggs provides a potential mechanism for direct offspring recognition [5].
Avian eggs are often highly variable in appearance, both among and within species [6,7]. This variation includes diversity in size, shape, background coloration and, when present, maculation [8,9]. Several adaptive functions may explain intraspecific variability of avian eggshell appearance [10,11], including direct individual egg recognition by parents in species without nests [12]. A notable textbook (e.g. [13]) exemplar of this is the highly variable egg of the common murre (or common guillemot: Uria aalge, hereafter: murre) (figure 1 inset). Murres are seabirds that breed in dense cliff colonies, and do not build a nest but instead individually recognize and retrieve their own egg when faced with a choice between it and the unattended egg of a neighbour or other conspecific [14] (figure 1).
Figure 1.

Common murres at their breeding colony off Gotland, Sweden. Inset: Representative human-visible shape, colour, and pattern variation between eggs, collected from different individual common murres in Iceland. Photo credits: M. Hauber and B. Stauffer. (Online version in colour.)
Traits that subserve individual recognition have a characteristic set of qualities that discriminate them from display trait sets that function in different recognition context, such as kinship or Fisherian attractiveness [5]. Specifically, individual recognition traits would show a high level of variability, multimodal distribution, lack of correlation between component traits, and strong degree of genetic determination [15]. Murre eggs appear to conform to these trait qualities, in that they show multimodality and lack of correlation between their shell background colours' hue and saturation [16], and high variability between individuals with strong high putative genetic control within individuals (sensu [17]) for egg shape [18], coloration and maculation: [19,20], and size [20].
Here, we address the structural basis of individual recognition signalling in egg coloration and maculation in murres. Chemically, avian eggshell colour variation is under the influence of just two known pigments: the blue-green biliverdin and the rusty-black protoporphyrin IX [21], and their interaction with the white calcite of the eggshell matrix [8]. These two pigments are ubiquitous across the avian radiation [22], and had originated once in non-avian dinosaurs [23], and so our study is relevant to understanding the structural bases in variation of avian and reptilian eggshell coloration and maculation more generally. Additional structural and environmental constituents of eggshells can contribute to variation in avian egg appearance, including a vaterite layer covering the shell background [24], or dirt accumulating during incubation [25]. However, these mechanisms do not explain the structural origin of individually consistent but interindividually variable murre egg phenotypes [20].
Here we quantified the relationship between avian-perceivable visual components of murre eggshells (background coloration and maculation density) with two of the shells' structural elements known to be related to coloration and maculation (pigment concentrations: [8], and thickness: [26]). These analyses tested two predictions related to the eggshell identity signal hypothesis:
Prediction A: shell background colour and maculation density contributing to avian-perceivable egg appearance are statistically uncorrelated within the same eggs, and
Prediction B: variation in the physico-chemical structure of the shell generates avian-visible diversity across murre eggs.
This work, thus, complements our recent report of intraindividual repeatability of eggshell phenotypes laid by known females across different years [20]. Specifically, this new study helps to uncover the structural bases underlying uniquely high phenotypic variability to yield a signal that facilitates individual recognition [5].
2. Methods
2.1. Murre eggshell collection
We collected murre eggs (n = 50) from Gull Island, Witless Bay, Newfoundland & Labrador, Canada. Sympatric gulls, notably the American herring gull Larus (argentatus) smithsonianus and the great black-backed gull L. marinus, depredate murre eggs regularly at this site [27]. We collected only recently depredated eggs (within the last week) from gull territories because depredated eggs show human-visible signs of weathering/fading after a few weeks of exposure (G.J.R., personal observation, 2013). The depredated murre eggs might not represent a random subset of the population of breeding birds at the site because disproportionately more eggs may be from younger/inexperienced murres, relegated to nesting at the margins of the colony [28]. However, our sample of collected eggshells did display the full range of human-visible egg coloration present in the colony as a whole (G.J.R., personal observation, 2013), and, thus, were deemed suitable for representative pigment analyses. After collecting the eggs, we washed off any surface debris and fouling from each egg with seawater and then packaged them in a box for shipment to New York City, NY, USA for laboratory analysis at Hunter College. Upon delivery, the eggshells were stored in a dark container at 4°C until processing.
2.2. Eggshell measurements
For each egg, we broke the shell into two approximately 1 cm2 fragments/egg from different parts of the egg. We washed the fragments with double distilled water, measured each fragment's thickness to an accuracy of 0.01 mm with a point micrometer (Series 112 Mitutoyo), and then weighed each fragment to an accuracy of 1 mg (XS403S Mettler Toledo). We then took digital photos of the fragments that included a size scale.
We collected avian-visible spectral (300–700 nm) reflectance data using an Ocean Optics USB2000 spectrometer, illuminated by a DT mini-lamp (Ocean Optics, Inc., Dunedin, FL, USA). All measurements were taken at a 90° angle (following [29]). Spectral reflectance measurements were taken in triplicate from an average of two fragments from their shell background (N = 50 eggs), excluding areas with maculation. We did not analyse spot coloration as this trait is not statistically repeatable across eggs laid by the same female in different years [20].
Averaged reflectance spectra for the separate fragments of each egg (figure 2) were transformed into avian tetrahedral colour space coordinates [30] using the tcspace function from the pavo package v. 1.3.1 in R 3.5.0 [31]. We then calculated the repeatability (R) of r.achieved (a measure of avian-perceived ‘colourfulness’), hue.theta and hue.phi (colour hue angles), using the rpt function from the rptR v. 0.9.21 package in R. For all repeatabilities, we performed significance tests against a null distribution of N = 2000 permuted samples and estimated the 95% confidence interval of R with N = 2000 bootstrap samples. Because repeatabilities of modelled avian-perceived within-egg fragment colours were all significant (all p < 0.001) and highly repeatable (all R > 0.83; data not shown), we took the mean of the two egg fragments' background reflectance spectra for each egg to obtain a ‘whole egg’ background reflectance spectrum for each egg.
Figure 2.
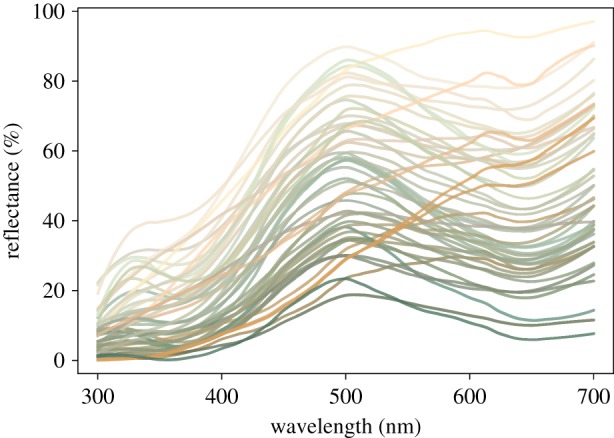
Avian-visible reflectance spectra of eggshell fragments used for pigment extractions in this study. For illustrative purpose we plotted spectra to reflect the human-visible appearance of the eggs as calculated from each shell's average reflectance spectrum. (Online version in colour.)
We used a receptor-noise limited visual model to model avian perceived colour discriminability between all collected murre eggs' background spectral reflectance [32]. Visual models were run using the coldist function from the pavo package in R. Because no published data of murre visual physiology exist, we constructed a general avian visual model using available knowledge of predicted species-specific vision. Murres likely possess a violet sensitive (VS vision) rather than ultra-violet sensitive (UVS vision) visual system, based on predicted amino acid spectral tuning sites using SWS1 opsin gene fragments for closest relatives in the order Charadriiformes [33]. Therefore, we used an average avian VS visual system for subsequent perceptual modelling (avg.v, peak cone sensitivities = 416 nm, 478 nm, 542 nm, 607 nm), but with photoreceptor densities of the red-billed leiothrix (Leiothrix lutea) ([34]; VS-cone = 1, SWS-cone = 2, MWS-cone = 2, LWS-cone = 4), a receptor noise Weber fraction of 0.1 with the long-wave sensitive cone (LWS) as the reference cone, and excluded any effect of light transmission through ocular media (ideal transmission). The visual model also assumed that murre eggs were illuminated by full sunlight (D65) and perceived colour differences were independent of the viewing background appearance (ideal background). Quantum cone catch values for all murre egg colours were transformed into chromatic perceivable differences from one another (chromatic just noticeable differences, hereafter: JNDs). These chromatic JND values were then mapped into perceptually-corrected Cartesian coordinates using the jndxyz function in pavo, with the mean murre egg colour in our sample set as the central origin point in 3D-space and Euclidean distances from the origin equal to JNDs from the mean egg colour for each egg. For each egg background colour, we obtained X, Y and Z coordinates in 3D JND space.
We quantified eggshell maculation density by visualizing the shell fragments in Image J (v. 1.51; [35]) and dividing the total number of unconnected human-visible marks (i.e. spots and/or lines) by the surface area of each shell fragment. Repeatability was high between fragments sourced from the same shell (R = 0.87), and so we used the mean value per egg in the statistical analyses.
2.3. Eggshell pigment concentrations
We washed the fragments with 70% ethanol and then, after drying, manually pulverized them. We used an ethylenediaminetetraacetic acid (EDTA) protocol [21] to extract pigments from the eggshell fragments, resulting in 1 ml of dissolved sample in acetonitrile–acetic acid (4:1 v/v). Samples were run in a Cary 300 UV–Vis spectrophotometer for UV absorbance, with biliverdin and protoporphyrin absorbance observed at 377 nm and 405 nm, respectively [22].
We performed ultra-high performance liquid chromatography (UHPLC) using the same method as described in similar avian eggshell extraction studies from our laboratory (e.g. [24,36]). Briefly, samples were run at a flow rate of 0.4 ml min−1 using as solvents A and B water with 0.01 M formic acid or acetonitrile with 0.1 M formic acid, respectively. The linear gradient was set to 2% A and 98% B at 6.5 min. Biliverdin eluted at approximately 3.5 min and protoporphyrin IX at approximately 5.6 min. We calculated relative proportions of the two pigments using Beer–Lambert's law (A = ɛlc) (following [24]). Additionally, pigment presence or absence was independently confirmed through mass spectrometry (following [36]). Samples were standardized by dividing the amount of pigment (in moles) with the mass of the initial eggshell sample (M/g) (following [24]). Repeatability of pigment concentrations was moderately high between fragments sourced from the same shell (Rbiliverdin = 0.65 and Rprotoporphyrin = 0.61), and we used the mean value per egg in our analyses.
2.4. Statistical tests
Murre eggshell thickness, as well as patterning, varies among different shell regions [20,28]. We did not keep track of which eggshell fragment yielded which spectral and maculation versus thickness and pigment concentration data point, and therefore we averaged each set of data types per egg in the subsequent statistical analyses.
A statistical inspection of the correlations between the X, Y and Z coordinates revealed strong covariations (all pairwise |R| ≥ 0.48, all p ≤ 0.0004) and visual inspection of the eggshell JND space coverage indicated a planar distribution (sensu [37]). Nevertheless, we opted to analyse the data using each of these coordinates separately as their dimensions can be directly interpreted as perceptual JND distances within the avian visual system.
To test the prediction of the individual recognition hypothesis, namely that different components of multiple visible cues are independent of each other within eggs, we used standard least squares models in JMP 12.0 (SAS Institute, Cary, NC).
To analyse the putative relationship between physico-chemical properties of each eggshell and its background coloration JND scores or maculation density, we used a second set of standard least squares models. Predictors in these models were the ln-transformed average concentrations of each of the two pigments and the untransformed value of the average shell thickness per egg because raw pigment concentration data did (Shapiro–Wilk tests, both W > 0.58, p < 0.0001) but thickness did not differ (W = 0.99, p = 0.93) from normal distributions. The response variables were the three JND coordinate scores from averaged background coloration analysis and the average maculation density. We omitted non-significant terms and interactions from the final model statistics reported for the significant terms. For illustrative purpose we plotted the relationships of eggshell properties using coloured data points that reflect the human-visible appearance of the eggs as calculated from each shell's average reflectance spectrum (figures 3–6).
Figure 3.
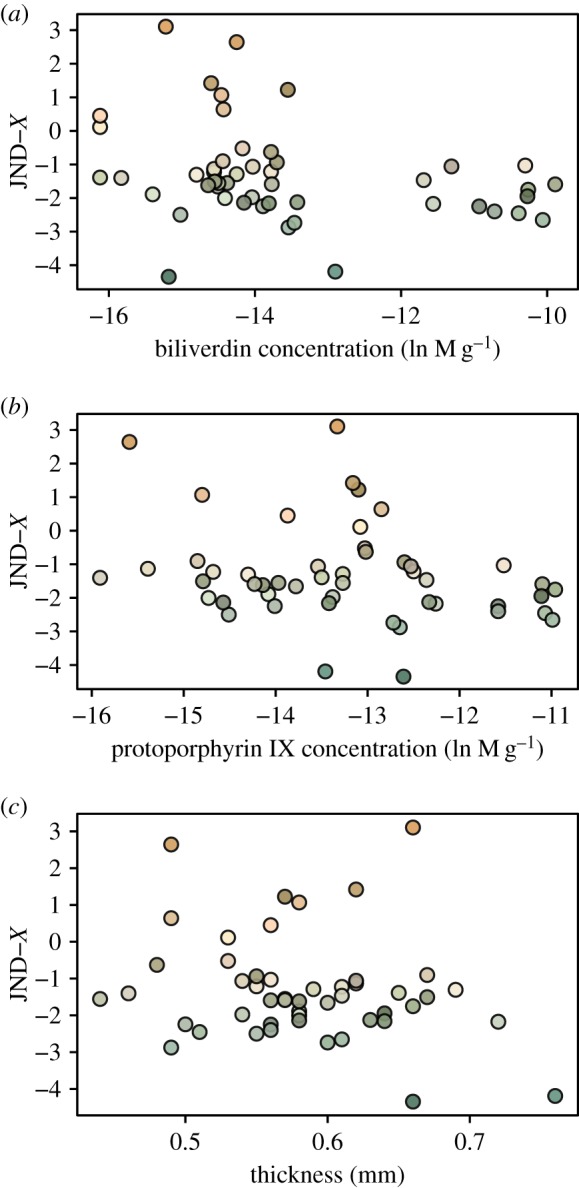
Relationship between murre eggshells' avian perceivable colour space coordinate X (in units of JND) with their physico-chemical traits (a,b: pigment concentrations, c: thickness). (Online version in colour.)
Figure 4.
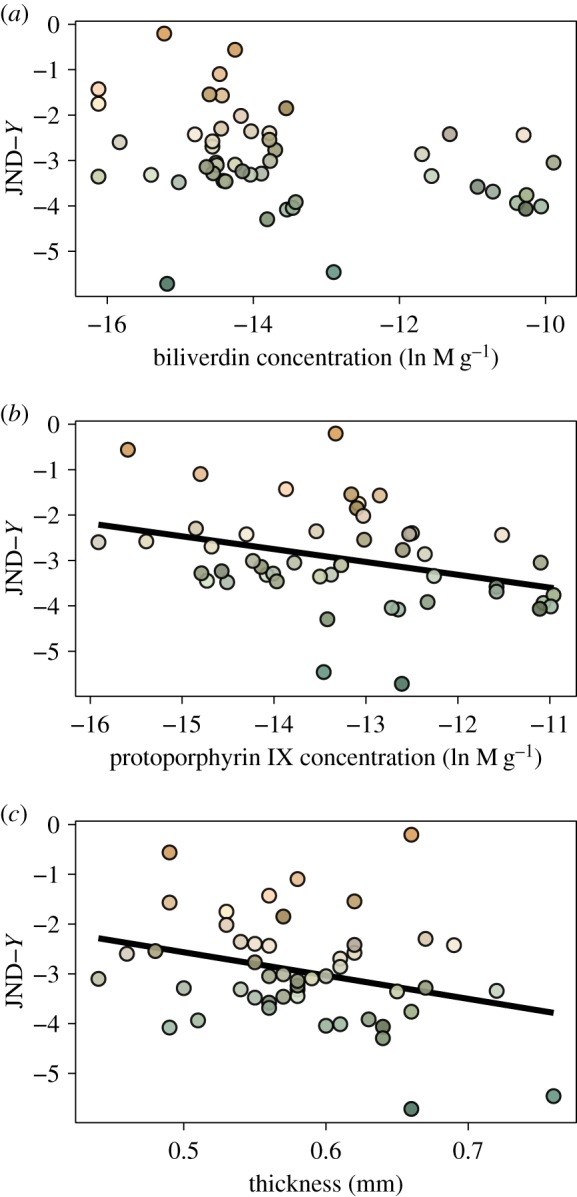
Relationship between murre eggshells' avian perceivable colour space coordinate Y (in units of JND) with their physico-chemical traits (a,b: pigment concentrations, c: thickness). Significant relationships from the Results are shown with linear regression lines. (Online version in colour.)
Figure 5.
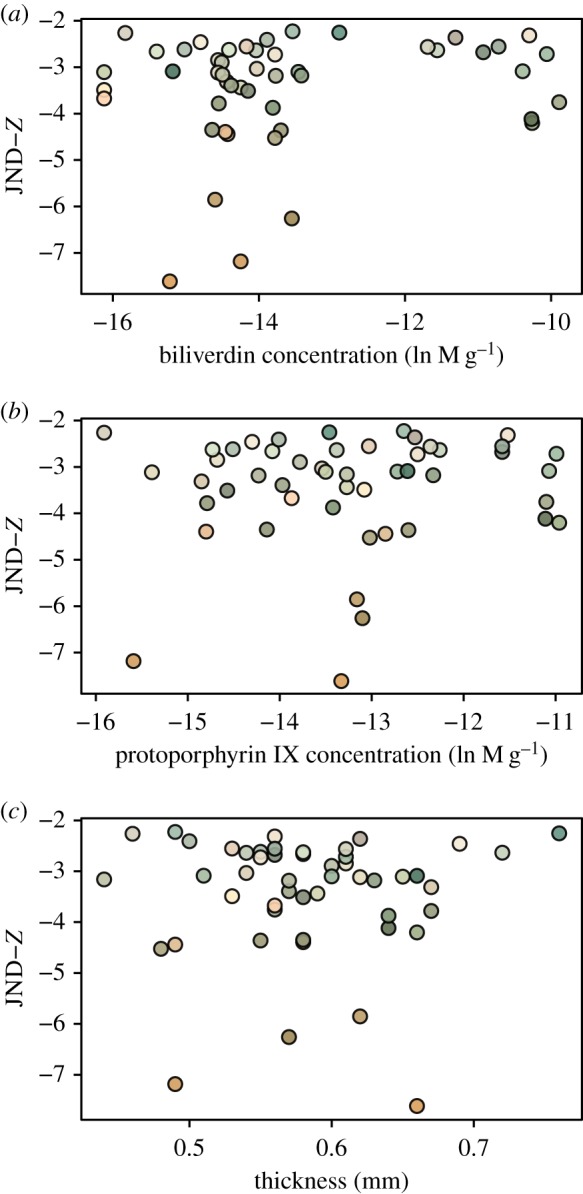
Relationship between murre eggshells' avian perceivable colour space coordinate Z (in units of JND) with their physico-chemical traits (a,b: pigment concentrations, c: thickness). (Online version in colour.)
Figure 6.
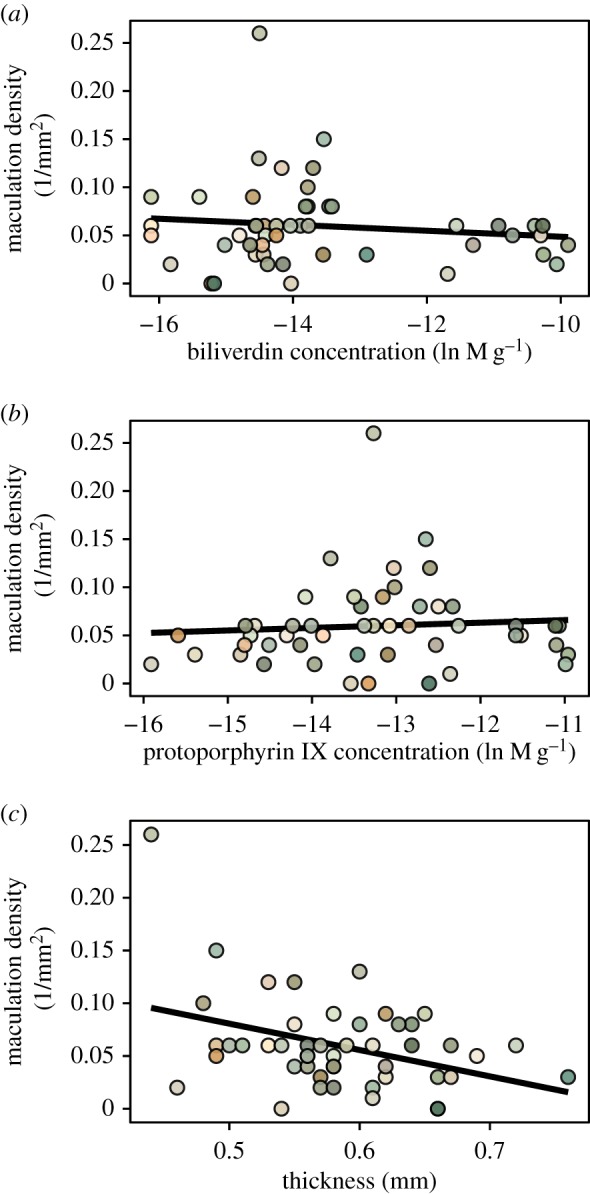
Relationship between murre eggshells' maculation density with their physico-chemical traits (a,b: pigment concentrations, c: thickness). Significant relationships from the Results are shown with linear regression lines. (Online version in colour.)
3. Results
As predicted by (A), the individual recognition signal hypothesis, there was no statistical relationship within the same egg between any one of its X, Y and Z JND coordinates and its maculation density metric (all |R| < 0.05, p > 0.60).
For prediction (B) regarding the shell's physico-chemical properties, there was no statistically significant relationship between JND coordinate X and shell thickness or pigment concentrations (all F < 1.7, all p > 0.20) (figure 3). In contrast, coordinate Y was negatively correlated with thickness (F = 3.8, p = 0.05), not correlated with biliverdin (F = 0.1, p = 0.76), and negatively correlated with protoporphyrin IX (F = 4.6, p = 0.048) concentrations (figure 4). The JND coordinate Z was also not statistically related to thickness, biliverdin or protoporphyrin IX (all F < 1.5, p > 0.23) (figure 5). Finally, maculation density was negatively correlated with thickness (F = 7.5, p = 0.0086), negatively correlated with biliverdin (F = 4.4, p = 0.042), and positively correlated with protoporphyrin IX (F = 4.2, p = 0.047) (figure 6).
4. Discussion
4.1. Identity signalling hypothesis
Contrary to American coots Fulica americana [38] and common moorhens Gallinula chloropus [39], the context of individual egg recognition, and of the resulting conspecific egg discrimination, is unlikely to be conspecific brood parasitism, as murres lay only a single egg and do not build nests. Nonetheless, behavioural experimentation shows that murres can reliably recognize and retrieve their own eggs when facing a choice of unattended conspecific eggs in their dense breeding colonies [4,14] (figure 1). They do so likely through the unique combination of their own egg's visible traits (though critical experiments with manipulated real or model murre eggs remain to be conducted; sensu [40]).
As assumed by the individual identity signalling hypothesis [5], the appearance of eggs of common murres is repeatable both within the same shell (this study) and across eggs laid by the same female in different years [20]. However, as predicted by (A) of the same hypothesis, the multiple components of each murre egg's visible appearance, including its avian-perceivable background shell coloration and maculation density, are not statistically related to each other even within the same eggs ([16], this study).
4.2. The pigmentary basis of egg phenotypes
Here, we also assessed the structural basis underlying the immense variation in visible traits of murre eggs. As predicted by (B), even when analysing just two aspects of the physico-chemical properties of each shell, namely its thickness and pigment concentrations, these covaried statistically with some of our metrics of avian perceivable background colour spectra and maculation density taken from the same egg. Specifically, using the avian perceivable colour space coordinates (X, Y and Z) in units of JND (figures 3–5) allowed us to measure the pigment-based covariation of colour appearance of the shells within our dataset. This variation reached, on average, an extent of up to approximately 2.0 JNDs, which is predicted to be discriminable by the visual system of the murres. The results, therefore, illustrate how individually unique eggshell appearances, suitable for individual identity signalling, can be generated by a small number of structural and physiological mechanisms involved in the formation of avian eggshells and their pigmentation during oviposition [9].
Regarding the statistical relationships we found between eggshell thickness with shell background coloration and maculation density, earlier work on murre eggs, also collected at Gull Island, showed no significant covariation between these traits [28]. However, such differences may have arisen due to divergent methodologies used between the two studies, i.e. using human-assessed (background colour, spot pattern) versus avian perceivable metrics (JND coordinates based on eggshell background reflectance spectra, count-based maculation density), respectively. In turn, prior work on great tit Parus major eggs from England also showed an ecological and structural linkage between thinner shells and pigmentation leading to denser spotting patterns [26]. Here, we found a statistically negative relationship of biliverdin and a positive relationship of protoporphyrin IX with maculation density (figure 6a,b), which is in support of the latter known as the pigmentary basis of avian eggshell maculation patterns in other taxa [41].
Specifically, as seen in the great tit, we also found a negative relationship between eggshell thickness and maculation density (figure 6c). However, given that the thinnest murre eggshells in our sample were already 30% thicker than size-matched average chicken Gallus domesticus shell's thickness [42], it therefore remains a relevant subject for future research whether the covariation in murre eggshell thickness with spotting patterns translates into biologically relevant variation in structural strength. For example, the known overall thicker-than-predicted-by-size shells of murres, and the thicker equatorial than blunt end shell regions, may be an adaptation to incubation and rolling on a rough cliff-edge breeding surface [14] and/or to withstand microbial contamination through contact with the faeces-soiled breeding substrate in the colony [43].
5. Conclusion
Previous work suggested that the pigmentary composition of avian eggshells might be a poor predictor of the visible appearance of shells across and within species ([36,44,45], but see [29]). In contrast, here we imply that the extraordinary amount of interindividual variation in murre egg appearance seems to be generated by consistent differences in some, but certainly not all, aspects of the pigment compositions and concentrations among eggs. However, whether and how a putatively tighter physico-chemical control of eggshell appearance in murres is under more direct genetic control so as to generate adaptively variable, but individually consistent, shell colours and spotting patterns remain to be investigated in the future. In general, across different bird species and lineages, the two eggshell pigments may interact with diverse physical properties of the eggshell differently to produce avian perceivable variation in eggshell colours and maculation patterns [8]. Further micro- and nanostructural analyses of the shell matrix structure in murres and other avian taxa (following [46,47]) should also be informative to evaluate these alternatives.
Acknowledgements
Ketti Barateli, Ian Hays, Daniel Hanley, Phill Cassey, Olof Olsson, Jonas Sundberg and many others provided helpful discussions, assistance and/or comments.
Ethics
Our work on murre eggs was approved by USDA Research Permit no. 125504. Access to the Gull Island, part of the Witless Bay Islands Seabird Ecological Reserve, was provided to G.J.R. by the Parks and Natural Areas Division, Government of Newfoundland and Labrador. Eggs were collected and possessed under Canadian Wildlife Service Scientific Permit SS2803 issued G.J.R. We also obtained confirmation from the University of Illinois, Urbana-Champaign, IACUC, that because the work only included unhatched, depredated avian eggs, it required no additional animal ethics approval.
Data accessibility
All data analysed in this study are included in the figures of the published article. They can also be accessed via the Dryad Digital Repository at: https://doi.org/10.5061/dryad.360b25r [48].
Authors' contributions
M.E.H. and E.S.H. designed the study; A.L.B., A.-L.K. and G.J.R. collected all samples; M.D. and M.H. processed and measured all the samples, A.L. conducted the visual modelling and generated the figures, M.E.H., A.L. and J.D. conducted data analyses, M.E.H. wrote the first draft, and all authors edited additional drafts and approved the submitted version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by Human Frontier Science Program (Young Investigator Award).
References
- 1.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Sherman PW, Reeve HK, Pfennig DW. 1997. Recognition systems. In Behavioural ecology: an evolutionary approach (eds Krebs JR, Davies NB), pp. 69–96. Hoboken, NJ, USA: Blackwell Science. [Google Scholar]
- 3.Hauber ME, Sherman PW. 2001. Self-referent phenotype matching: theoretical possibilities and empirical tests. Trends Neurosci. 24, 609–616. ( 10.1016/S0166-2236(00)01916-0) [DOI] [PubMed] [Google Scholar]
- 4.Birkhead TR. 1978. Behavioural adaptations to high density nesting in the common guillemot Uria aalge . Anim. Behav. 26, 321–331. ( 10.1016/0003-3472(78)90050-7) [DOI] [Google Scholar]
- 5.Tibbets EA, Dale J. 2007. Individual recognition: it is good to be different. Trends Ecol. Evol. 22, 529–537. ( 10.1016/j.tree.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 6.Hauber ME. 2014. The book of eggs. Chicago, IL: University of Chicago Press. [Google Scholar]
- 7.Birkhead TR. 2016. The most perfect thing. London, UK: Bloomsburry Publishing. [Google Scholar]
- 8.Hanley D, Grim T, Cassey P, Hauber ME. 2015. Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol. Lett. 11, 20150087 ( 10.1098/rsbl.2015.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoddard MC, Yong EH, Akkaynak D, Sheard C, Tobias J, Mahadevan L. 2017. Avian egg shape: form, function and evolution. Science 356, 1249–1254. ( 10.1126/science.aaj1945) [DOI] [PubMed] [Google Scholar]
- 10.Kilner RM. 2006. The evolution of egg colour and patterning in birds. Biol. Rev. 81, 383–406. ( 10.1017/S1464793106007044) [DOI] [PubMed] [Google Scholar]
- 11.Hays IR, Hauber ME. 2018. How the egg rolls: a morphological analysis of avian egg shape in the context of displacement dynamics. J. Exp. Biol. 221, jeb.178988 ( 10.1242/jeb.178988) [DOI] [PubMed] [Google Scholar]
- 12.Gaston AJ, De Forest LN, Noble DG. 1993. Egg recognition and egg stealing in murres (Uria spp.). Anim. Behav. 45, 301–306. ( 10.1006/anbe.1993.1034) [DOI] [Google Scholar]
- 13.Faaborg J. 1988. Ornithology: an ecological approach. Upper Saddle River, NJ, USA: Prentice Hall. [Google Scholar]
- 14.Tschantz B. 1959. Zur brutbiologie der Trottellumme (Uria aalge aalge Pont.). Behaviour 14, 1–108. ( 10.1163/156853959X00018) [DOI] [Google Scholar]
- 15.Dale J, Lank DB, Reeve HK. 2001. Signalling individual identity vs. quality: a model and case studies with ruffs, queleas, and house finches. Am. Nat. 158, 75–86. ( 10.1086/320861) [DOI] [PubMed] [Google Scholar]
- 16.Dale JR. 2006. Intraspecific variation in coloration. In Bird coloration function and evolution, vol. 2 (eds Hill GE, McGraw KJ), pp. 36–86. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Bell AM, Hankinson SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkhead TR, Thompson JE, Biggins JD. 2017. Egg shape in the common guillemot Uria aalge and Brunnich's guillemot U. lomvia: not a rolling matter? J. Ornithol. 158, 679–685. ( 10.1007/s10336-017-1437-8) [DOI] [Google Scholar]
- 19.Birkhead TR, Montgomerie R. 2018. Rare red eggs of the common guillemot (Uria aalge): birds, biology and people at Bempton, Yorkshire, in the early 1900s. Arch. Nat. Hist. 45, 69–79. ( 10.3366/anh.2018.0483) [DOI] [Google Scholar]
- 20.Hauber ME, Luro A, McCarthy CK, Barateli K, Cassey P, Hansen ES, Dale J. 2019. Interannual repeatability of shell phenotype in individually unique eggs of common murres Uria aalge . Can. J. Zool. 10.1139/cjz-2018-0172. [DOI] [Google Scholar]
- 21.Gorchein A, Lim CK, Cassey P. 2009. Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. 23, 602–606. ( 10.1002/bmc.1158) [DOI] [PubMed] [Google Scholar]
- 22.Igic B, et al. 2010. Detecting pigments from the colourful eggshells of extinct birds. Chemoecology 20, 43–48. ( 10.1007/s00049-009-0038-2) [DOI] [Google Scholar]
- 23.Wiemann J, Yang T-R, Norell MA. 2018. Dinosaur egg color had a single evolutionary origin. Nature 563, 555–558. ( 10.1038/s41586-018-0646-5) [DOI] [PubMed] [Google Scholar]
- 24.Hauber ME, Dainson M, Baldassarre DT, Hossain M, Holford M, Riehl C. 2018. The perceptual and chemical basis of egg discrimination in communally nesting greater anis Crotophaga major. J. Avian Biol. 49, e01776 ( 10.1111/jav.01776) [DOI] [Google Scholar]
- 25.Mayani-Paras F, Kilner RM, Stoddard MC, Rodriguez C, Drummond H. 2015. Behaviorally induced camouflage: a new mechanism of avian egg protection. Am. Nat. 186, E91–E97. ( 10.1086/682579) [DOI] [PubMed] [Google Scholar]
- 26.Gosler AG, Connor OR, Bonser RHC. 2011. Protoporphyrin and eggshell strength: preliminary findings from a passerine bird. Avian Biol. Res. 4, 214–223. ( 10.3184/175815511X13207833399666) [DOI] [Google Scholar]
- 27.Veitch BG, Robertson GJ, Jones IL, Bond AL. 2016. Great black-backed gull (Larus marinus) predation on seabird populations at two colonies in eastern Canada. Waterbirds 39(Special Publication 1), 235–245. ( 10.1675/063.039.sp121) [DOI] [Google Scholar]
- 28.Pirie-Hay DW, Bond AL. 2014. Thickness of common murre (Uria aalge) eggshells in Atlantic Canada. Can. Field Nat. 128, 72–76. ( 10.22621/cfn.v128i1.1553) [DOI] [Google Scholar]
- 29.Igic B, Cassey P, Grim T, Greenwood DR, Moskat C, Rutila J, Hauber ME. 2012. A shared chemical basis of avian host–parasite egg colour mimicry. Proc. R. Soc. B 279, 1068–1076. ( 10.1098/rspb.2011.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoddard MC, Prum RO. 2008. Evolution of avian plumage color in a tetrahedral color space: a phylogenetic analysis of New World buntings. Am. Nat. 171, 755–776. ( 10.1086/587526) [DOI] [PubMed] [Google Scholar]
- 31.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 32.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ödeen A, Håstad O. 2011. The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol. Biol. 13, 36 ( 10.1186/1471-2148-13-36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maia R, Eliason C, Bitton P-P, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Ecol. Evol. 4, 906–913. [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dainson M, Mark M, Hossain M, Yoo B, Holford M, McNeil S, Riehl C, Hauber ME. 2018. How to make a mimic? Brood parasitic Striped Cuckoo eggs match host shell color but not pigment concentration. J. Chem. Ecol. 44, 940–946. ( 10.1007/s10886-018-0986-5) [DOI] [PubMed] [Google Scholar]
- 37.Spottiswoode CN, Stevens M. 2012. Host–parasite arms races and rapid changes in bird egg appearance. Am. Nat. 179, 633–648. ( 10.1086/665031) [DOI] [PubMed] [Google Scholar]
- 38.Lyon B. 2007. Mechanism of egg recognition in defenses against conspecific brood parasitism: American coots (Fulica americana) know their own eggs. Behav. Ecol. Sociobiol. 61, 455–463. ( 10.1007/s00265-006-0273-2) [DOI] [Google Scholar]
- 39.Petrie M, Pinxten R, Eens M. 2009. Moorhens have an internal representation of their own eggs. Naturwissenschaften 96, 405–407. ( 10.1007/s00114-008-0486-5) [DOI] [PubMed] [Google Scholar]
- 40.Hauber ME, Tong L, Ban M, Croston R, Grim T, Waterhouse GIN, Shawkey MD, Barron AB, Moskat C. 2015. The value of artificial stimuli in behavioral research: making the case for egg rejection studies in avian brood parasitism. Ethology 121, 521–528. ( 10.1111/eth.12359) [DOI] [Google Scholar]
- 41.Orlowski G, Pokorny P, Dobicki W, Lukaszewicz E, Kowalczyk A. 2017. Speckled and plain regions of avian eggshells differ in maternal deposition of calcium and metals: a hitherto overlooked chemical aspect of egg maculation. Auk 34, 721–731. ( 10.1642/AUK-17-7.1) [DOI] [Google Scholar]
- 42.Sun CJ, Chen SR, Xu GY, Liu XM, Yang N. 2012. Global variation and uniformity of eggshell thickness for chicken eggs. Poult. Sci. 91, 2718–2721. ( 10.3382/ps.2012-02220) [DOI] [PubMed] [Google Scholar]
- 43.Birkhead TR, Thompson JE, Jackson D, Biggins JD. 2017. The point of a guillemot's egg. Ibis 159, 255–265. ( 10.1111/ibi.12458) [DOI] [Google Scholar]
- 44.Brulez K, et al. 2016. Eggshell pigment composition co-varies with phylogeny but neither with life history nor with nesting ecology traits of British passerines. Ecol. Evol. 6, 1637–1645. ( 10.1002/ece3.1960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassey P, Portugal SJ, Ewen JG, Boulton RL, Hauber ME, Blackburn TM. 2010. Variability in eggshell colour: a comparative study of museum eggshells. PLoS ONE 5, e12054 ( 10.1371/journal.pone.0012054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Igic B, Braganza K, Hyland MM, Silyn-Roberts H, Cassey P, Grim T, Rutila J, Moskat C, Hauber ME. 2011. Alternative mechanisms of increased eggshell hardness of avian brood parasites relative to host species. J. R. Soc. Interface 8, 1654–1664. ( 10.1098/rsif.2011.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igic B, et al. 2015. A nanostructural basis for gloss of avian eggshells. J. R. Soc. Interface. 12, 20141210 ( 10.1098/rsif.2014.1210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ME Hauber, AL Bond, A-L Kouwenberg, GJ Robertson, ES Hansen, Holford M, Dainson M, Luro A, Dale J.. 2015. Data from: The chemical basis of a signal of individual identity: shell pigment concentrations track the unique appearance of common murre eggs Dryad Digital Repository. ( 10.5061/dryad.360b25r) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- ME Hauber, AL Bond, A-L Kouwenberg, GJ Robertson, ES Hansen, Holford M, Dainson M, Luro A, Dale J.. 2015. Data from: The chemical basis of a signal of individual identity: shell pigment concentrations track the unique appearance of common murre eggs Dryad Digital Repository. ( 10.5061/dryad.360b25r) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data analysed in this study are included in the figures of the published article. They can also be accessed via the Dryad Digital Repository at: https://doi.org/10.5061/dryad.360b25r [48].


