Abstract
To address the need for improved systemic therapy for non–small‐cell lung cancer (NSCLC), we previously demonstrated that mesenchymal NSCLC was sensitive to polo‐like kinase (Plk1) inhibitors, but the mechanisms of resistance in epithelial NSCLC remain unknown. Here, we show that cMet was differentially regulated in isogenic pairs of epithelial and mesenchymal cell lines. Plk1 inhibition inhibits cMet phosphorylation only in mesenchymal cells. Constitutively active cMet abrogates Plk1 inhibitor–induced apoptosis. Likewise, cMet silencing or inhibition enhances Plk1 inhibitor–induced apoptosis. Cells with acquired resistance to Plk1 inhibitors are more epithelial than their parental cells and maintain cMet activation after Plk1 inhibition. In four animal NSCLC models, mesenchymal tumors were more sensitive to Plk1 inhibition alone than were epithelial tumors. The combination of cMet and Plk1 inhibition led to regression of tumors that did not regrow when drug treatment was stopped. Plk1 inhibition did not affect HGF levels but did decrease vimentin phosphorylation, which regulates cMet phosphorylation via β1‐integrin. This research defines a heretofore unknown mechanism of ligand‐independent activation of cMet downstream of Plk1 and an effective combination therapy.
Keywords: cMet, drug combination, NSCLC, Plk1, vimentin
Subject Categories: Cancer, Pharmacology & Drug Discovery, Respiratory System
Introduction
Lung cancer remains the most lethal cancer, contributing to 27% of cancer‐related deaths in the United States (Siegel et al, 2018). Although personalized treatment approaches based on genetic mutations that underlie non–small‐cell lung cancer (NSCLC) have been developed and immunotherapy is very effective in some patients, the 5‐year overall survival rate for all stages of NSCLC is only 18% and a dismal 5% for those with metastatic disease (Siegel et al, 2018). Therefore, numerous new potential targets, including several cell cycle kinases, are under investigation to further improve patient survival. Polo‐like kinase 1 (Plk1) is a serine–threonine protein kinase that is overexpressed in cancer cells and plays a major role in the regulation of G2–M transition and response to DNA damage. Inhibitors of Plk1 are under active clinical development in oncology (NCT03414034 and NCT03303339).
Despite Plk1's reputation as an essential protein for cell survival, Plk1 inhibitors are well tolerated by patients (Nokihara et al, 2016; Pujade‐Lauraine et al, 2016; Schoffski et al, 2012, 2010; Sebastian et al, 2010; Stadler et al, 2014; Van den Bossche et al, 2016) and there are diverse biological responses to Plk1 inhibition or knockdown in cancer cells (Choi et al, 2015; Craig et al, 2014; Driscoll et al, 2014; Gjertsen & Schoffski, 2015; McCarroll et al, 2015; Medema et al, 2011; Rudolph et al, 2009; Spankuch‐Schmitt et al, 2002). These laboratory results are consistent with the results of clinical trials of Plk1 inhibitors in solid tumors that demonstrated striking clinical responses but a low response rate (4–14%), with a stable disease rate of 26–42% in unselected patients (Nokihara et al, 2016; Pujade‐Lauraine et al, 2016; Schoffski et al, 2012, 2010; Sebastian et al, 2010; Stadler et al, 2014; Van den Bossche et al, 2016). Up to 11% of patients had stable disease for more than a year (Pujade‐Lauraine et al, 2016; Sebastian et al, 2010). Predictive biomarkers have not been used to select patients likely to respond to Plk1 inhibitors. Plk1 inhibitor sensitivity was found to be associated with KRAS and TP53 mutations in colon, breast, and lung tumors in some studies (Degenhardt et al, 2010; Luo et al, 2009; Sanhaji et al, 2013) but not in others (Ferrarotto et al, 2016; Sanhaji et al, 2012). We previously compared gene mutation and basal gene and protein expression among 63 NSCLC cell lines and discovered that mesenchymal NSCLC cell lines were more sensitive to Plk1 inhibitors than were epithelial cell lines in vitro and in vivo; however, KRAS, TP53, and MET mutations did not consistently predict sensitivity. However, only one NSCLC cell line in the analysis had an activating mutation in exon 14 of MET making it impossible to determine whether this molecular subgroup was resistant to Plk1 inhibition. Plk1 inhibitors were equally effective at inhibiting Plk1 in mesenchymal/sensitive and epithelial/resistant NSCLC cell lines (Ferrarotto et al, 2016). The mechanisms of resistance to Plk1 inhibitors in epithelial NSCLC remain unknown, and this represents a major gap in knowledge.
To address this gap, in the current study, we performed an integrated analysis of functional proteomics and drug screening in an independent online database of NSCLC cell lines. When this approach confirmed that epithelial‐to‐mesenchymal transition (EMT)‐related proteins correlated significantly with Plk1 inhibitor sensitivity, we used isogenic pairs of epithelial NSCLC cell lines treated with TGF‐β to induce a mesenchymal phenotype to measure the changes in protein expression and activation after Plk1 inhibition. We observed differential regulation of cMet phosphorylation after Plk1 inhibition in epithelial and mesenchymal NSCLC. We confirmed cMet's role in Plk1 inhibition–induced apoptosis by inhibiting, silencing, and activating cMet in NSCLC in vivo and in vitro. Further, Plk1 inhibition decreases vimentin phosphorylation that subsequently regulates cMet phosphorylation via β1‐integrin only in mesenchymal NSCLC.
Results
NSCLC cell lines with high cMet and epithelial protein expression are resistant to Plk1 inhibitors in vitro
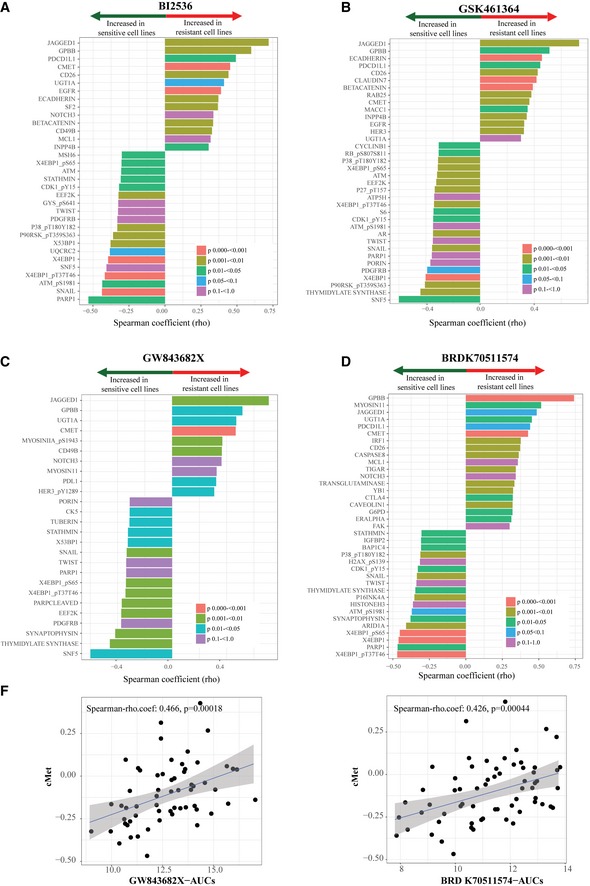
To find pathways that can drive Plk1 inhibitor resistance, we examined data for all NSCLC cell lines from two sources: (i) protein and phosphoprotein expression measured using reverse phase protein array (RPPA) from the MD Anderson Cell Line Project database (Li et al, 2017), and (ii) Plk1 inhibitor sensitivity from the Cancer Therapeutics Response Portal v2 (CTRPv2; https://portals.broadinstitute.org/ctrp/) database (Seashore‐Ludlow et al, 2015). These data were independent of our original study (Ferrarotto et al, 2016), although there was some overlap in the drugs and cell lines tested (Appendix Table S1 and Fig S1). Varying numbers of proteins were associated with sensitivity to four Plk1 inhibitors (selected by Spearman's rho coefficient value > 0.3; associated P‐values are indicated in the figures): 33 proteins were associated with sensitivity to BI2536, 36 with sensitivity to GSK461364, 37 with sensitivity to BRD‐K70511574, and 26 with sensitivity to GW‐843682X (Fig EV1A–D). Thirty‐three proteins were associated with sensitivity to two or more Plk1 inhibitors (Fig 1A). Consistent with our previous findings, we observed that expression of the epithelial proteins E‐cadherin (P < 0.001) and β‐catenin (P < 0.001) was higher and expression of the mesenchymal protein Snail (P < 0.01), as well as ATM and thymidylate synthase, was lower in cell lines resistant to Plk1 inhibitors than in those sensitive to Plk1 inhibitors. We also found that cMet protein expression correlated with drug sensitivity for all Plk1 inhibitors (P < 0.01; Figs 1B and EV1E).
Figure EV1. Basal expression of proteins that correlate with sensitivity to Plk1 inhibitors.
-
A–DSensitivity data for the Plk1 inhibitors BI2536, GSK461364, BRD‐K70511574, and GW‐843682X were obtained from the Cancer Therapeutics Response Portal v2 database. Protein expression data (reverse phase protein array) were obtained from the MD Anderson Cell Line Project database. Spearman's correlations of area under the curve (AUC) values and protein expression in non–small‐cell lung cancer cell lines in vitro are shown for those with a Spearman rho coefficient > 0.3 for BI2536 (A), GSK461364 (B), GW‐843682X (C), and BRD‐K70511574 (D). The color of the bars indicates the P‐value per the legend in the graph.
-
EBasal cMet protein expression in non–small‐cell lung cancer cell lines compared with AUC values of GW‐843682X and BRD‐K70511574. The blue line represents linear regression and 95% confidence interval is indicated in dark gray.
Figure 1. Epithelial‐to‐mesenchymal transition proteins are associated with sensitivity to Plk1 inhibitors in non–small‐cell lung cancer cell lines in vitro in an independent dataset.

-
AProteins with expression levels that correlate with area under the curve (AUC) values for at least two drugs are included in the graph. The color and size of the data point represent the direction and significance (P‐value) of the correlation. Red indicates a positive correlation with drug resistance and green a positive correlation with drug sensitivity (i.e., proteins in red are more highly expressed in resistant cell lines).
-
BAUC values for two Plk1 inhibitors are compared with expression of selected proteins (cMet, E‐cadherin, β‐catenin, and Snail); each data point represents an individual non–small‐cell lung cancer cell line. The gray shaded area represents the 95% confidence interval around the linear regression (blue line). E‐cadherin, β catenin, and cMet protein expression correlated with drug resistance.
The correlation of drug sensitivity with cMet protein expression motivated us to compare Plk1 inhibitor sensitivity [area under the curve (AUC) and effective dose 50 (ED50)] to MET gene copy number in NSCLC cell lines. MET gene copy number was obtained from the MD Anderson Cell Line Project database, CTRPv2, and Kubo et al (2009) in 41, 185, and 29 NSCLC cell lines, respectively. MET gene copy number did not correlate with drug sensitivity for any of the 24 possible comparisons (i.e., two measures of drug sensitivity, four drugs, and three sources of MET copy number) with Spearman's rho coefficient values that ranged from −0.428 to 0.430 and associated P‐values that ranged from 0.078 to 0.872. However, this analysis was limited by the fact that there were drug sensitivity data for only two NSCLC cell lines with MET copy number > 5.
Induction of a mesenchymal phenotype increases Plk1 inhibition–induced apoptosis
To create isogenic cell line pairs for mechanistic studies, we incubated epithelial/resistant NSCLC cells (H1975, HCC366, and HCC4006) with 5 ng/ml TGF‐β for at least 14 days, which led to the expected changes in the expression of vimentin, Snail, Slug, ZEB1, Twist, E‐cadherin, β‐catenin, and claudin 7 (Fig 2A and Appendix Fig S2). Given that gene mutation did not correlate with Plk1 inhibitor sensitivity (Ferrarotto et al, 2016), we chose these cell lines independent of gene mutation status. The induction of a mesenchymal phenotype by TGF‐β led to a significant increase in cleaved poly(ADP‐ribose) polymerase (PARP) protein expression after Plk1 inhibition with volasertib in all isogenic cell lines (Fig 2B and Appendix Fig S3A). Similarly, TGF‐β–treated mesenchymal cells showed increases in volasertib‐induced apoptosis as measured by BrdU‐positive cells (threefold in H1975, 4.1‐fold in HCC4006, and 4.1‐fold in HCC366) compared with their epithelial parental cells (P < 0.05; Fig 2C). Likewise, volasertib‐induced DNA damage was increased in the mesenchymal cell lines compared with the epithelial parental cells (Fig 2B and D, and Appendix Fig S3A), and mesenchymal cell lines were more sensitive to volasertib in vitro (Appendix Fig S3B). The Plk1 inhibitor–induced DNA damage (Driscoll et al, 2014; Wang et al, 2018; Yim & Erikson, 2009) may explain why apoptosis measured by BrdU was more striking than that measured by PARP cleavage. Target inhibition, measured by inhibition of the Plk1 substrate p‐NPM (S4), was similar in the isogenic pairs (Appendix Fig S3A).
Figure 2. TGF‐β–induced mesenchymal phenotype increases sensitivity to Plk1 inhibition.

-
AThree epithelial/resistant non–small‐cell lung cancer cell lines were treated with 5 ng/ml TGF‐β for 14 days to induce a mesenchymal phenotype, which was confirmed with Western blot (left) and qPCR (right) analysis of epithelial‐to‐mesenchymal transition (EMT) markers.
-
BParental and TGF‐β isogenic cell lines were treated with 50 nM volasertib for 72 h. Cells were then harvested, and lysates were immunoblotted for cleaved PARP and γH2AX proteins that were subsequently quantitated and normalized with β‐actin.
-
C, DApoptosis was measured by the Apo‐BrdU assay (C), and DNA damage was measured by the Comet assay (D).
Activation of cMet is differentially regulated in epithelial and mesenchymal NSCLC cell lines following Plk1 inhibition and knockdown
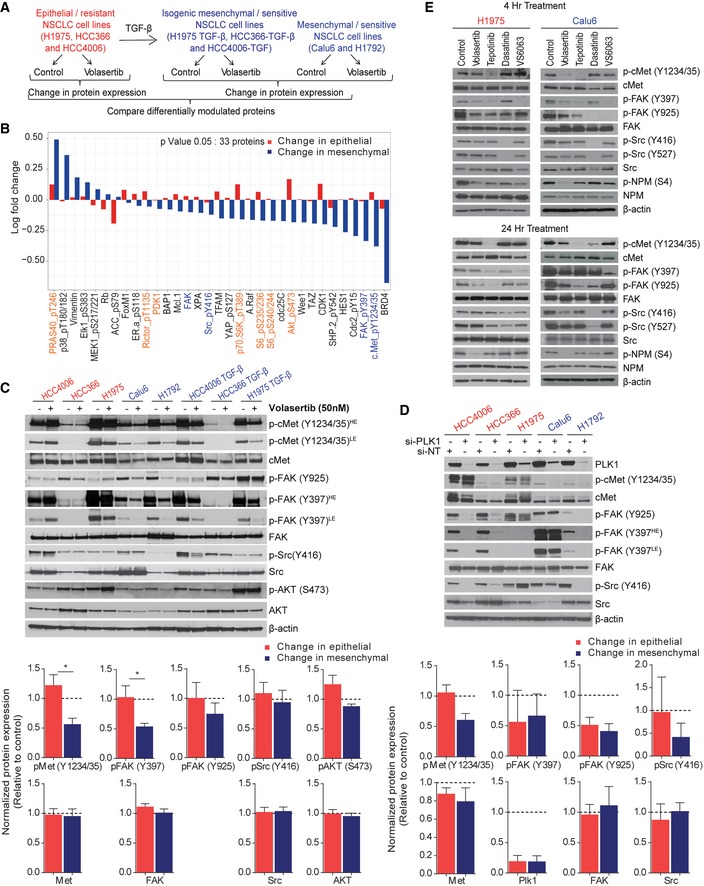
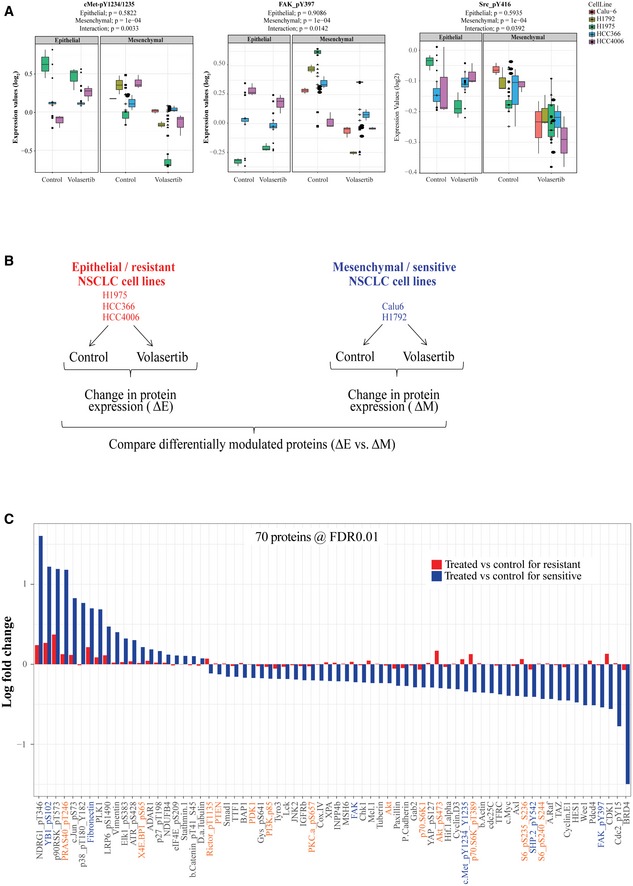
To elucidate the mechanism for EMT‐induced sensitivity to Plk1 inhibition, we analyzed the expression of 301 proteins or phosphoproteins (Kalu et al, 2017) after Plk1 inhibition with volasertib for 24 h using RPPA in the three isogenic pairs and two additional mesenchymal/sensitive cell lines (Calu6 and H1792; Fig 3A). To discover the pathway responsible for driving the resistance to Plk1 inhibition, we looked for differentially expressed proteins between epithelial and mesenchymal cell lines after treatment with volasertib. At a cutoff of P < 0.05, we observed that the mean expression of 33 proteins or phosphoproteins was differentially regulated (Fig 3B). These data revealed differential effects on both the cMet/FAK/Src axis and the PI3K/Akt pathway (Figs 3B and EV2A). We did a similar analysis using only the parental cell lines to eliminate TGF‐β as a confounder, and this led to similar findings (Fig EV2B and C). Western blot analysis confirmed a significant decrease in cMet (Y1234/1235) phosphorylation in sensitive/mesenchymal cells but not in resistant/epithelial cells after treatment with volasertib (Fig 3C). However, changes in Src (Y416), FAK (Y925), and Akt (S473) were not statistically significant (Fig 3C). As with the RPPA results (Fig EV2A), the average decrease in FAK (Y397) phosphorylation was greater in the mesenchymal cells, but the effects of Plk1 inhibition on the epithelial cells were diverse (Fig 3C). Total cMet, FAK, and Src protein levels were not affected, supporting posttranslational changes. Likewise, when we knocked down Plk1 using siRNA, cMet (Y1234/1235) phosphorylation decreased only in sensitive/mesenchymal cells but not in resistant/epithelial cells with variable effects on pSrc and pFAK (Fig 3D).
Figure 3. Activation of cMet is differentially regulated in epithelial and mesenchymal non–small‐cell lung cancer (NSCLC) cell lines following Plk1 inhibition and knockdown.
-
AThree pairs of TGF‐β–treated isogenic NSCLC cell lines (H1975, HCC4006, and HCC366) and two mesenchymal NSCLC cell lines (Calu6 and H1792) were incubated with 50 nM volasertib for 24 h, lysed, and subjected to reverse phase protein array analysis. Experiments were done in triplicate.
-
BThirty‐three proteins were differentially regulated between epithelial and mesenchymal NSCLC cell lines after treatment with volasertib, including those involved in the cMet/FAK/Src signaling axis (blue text) and those involved in the PI3K/Akt signaling axis (orange text).
-
CThe same cell lines treated identically were subjected to immunoblotting for the indicated proteins (upper) with densitometric quantification normalized with β‐actin (lower).
-
DEpithelial (red text) and mesenchymal (blue text) NSCLC cell lines transfected with 10 nM siRNA as indicated for 48 h and subjected to Western blotting with the indicated antibodies (upper) with densitometric quantification normalized with β‐actin (lower). NT, non‐targeting control.
-
EH1975 and Calu6 cells were treated with indicated inhibitors for 4 h or 24 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
Figure EV2. cMet, FAK, and Src phosphorylation regulation in epithelial and mesenchymal non–small‐cell lung cancer cell lines following Plk1 inhibition.
-
ABox plots show protein expression of p‐cMet (Y1234/35), pFAK (Y397), and pSrc (Y416) measured using reverse phase protein array after treatment with volasertib in non–small‐cell lung cancer cell lines. Experiments were performed in triplicate. The median is marked by a horizontal line, the colored boxes are the upper and lower quartiles, and error bars represent standard deviation. FC, fold change.
-
BThree epithelial (H1975, HCC4006, and HCC366) and two mesenchymal (Calu6 and H1792) NSCLC cell lines were incubated with 50 nM volasertib for 24 h, lysed, and subjected to reverse phase protein array analysis. Experiments were done in triplicate.
-
CSeventy proteins were differentially regulated between epithelial and mesenchymal NSCLC cell lines after treatment with volasertib, including those involved in the cMet/FAK/Src signaling axis proteins (blue text) and the PI3K/Akt signaling axis (orange text). FDR, false discovery rate.
Because cMet, FAK, and Src signaling can be bi‐directional (Sen et al, 2011), we used inhibitors of cMet (tepotinib), FAK (VS6063), Src (dasatinib), and Plk1 (volasertib) to distinguish the properties of this signaling axis in NSCLC cell lines (Fig 3E). Consistent with our RPPA findings, these experiments showed that inhibition of Plk1 inhibits phosphorylation of cMet in the mesenchymal cell line but not in the epithelial one. Phosphorylation of the Plk1 substrate NPM1 was inhibited in both cell lines confirming that efficient target inhibition does not underlie the differential sensitivity. cMet inhibition also inhibited the phosphorylation of FAK in both cell lines, but FAK inhibition did not affect cMet activation. Inhibition of Plk1, FAK, or cMet only minimally affected Src activation. Src inhibition decreased phosphorylation of FAK (Y925) but did not significantly affect phosphorylation of cMet or FAK (Y397). Together, these results support a model in which Plk1 is robustly inhibited in all NSCLC cell lines, but only inhibits cMet phosphorylation in mesenchymal NSCLC lines.
The combination of Plk1 and cMet inhibition or knockdown enhances apoptosis in NSCLC cells
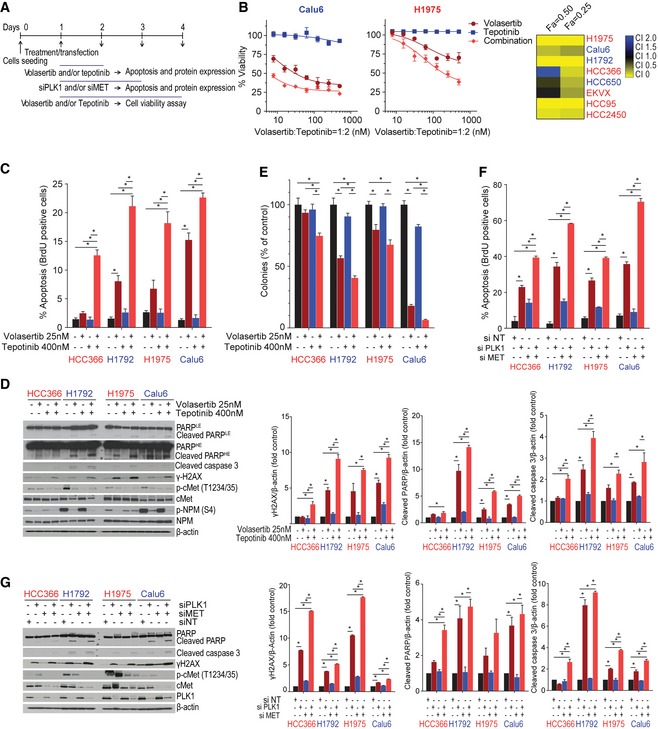
To test the hypothesis that cMet inhibition is important for Plk1 inhibitor–induced apoptosis, we treated eight NSCLC cell lines with a combination of volasertib and tepotinib for 72 h and measured viability (Fig 4A). To inhibit cMet, we chose tepotinib, which has been tested in NSCLC patients (Friese‐Hamim et al, 2017; Reungwetwattana et al, 2017). We used relevant drug concentrations as defined by pharmacokinetic data and target inhibition data. Specifically, 400 nM tepotinib fully inhibits cMet in intact NSCLC cells (Bladt et al, 2013). Results of in vitro kinase assays with 242 kinases showed that only cMet had half‐maximal inhibitory concentration values of less than 600 nM (Bladt et al, 2013). None of the cell lines used in this research harbor MET mutations or MET amplification. A synergistic or additive effect was observed in seven of eight cell lines (Fa = 0.5; Fig 4B and Appendix Table S2). Likewise, the combination led to more apoptosis than did single‐agent treatment in two epithelial and two mesenchymal cell lines, as measured by BrdU, cleaved PARP, and cleaved caspase 3 (Fig 4C and D). We also observed higher DNA damage (γ‐H2AX expression) in all cell lines after treatment with the combination compared with single‐agent treatment or controls (Fig 4D).
Figure 4. Co‐targeting of cMet and Plk1 enhances apoptosis in non–small‐cell lung cancer (NSCLC) in vitro .
-
ASchematic of the experimental plan for Plk1 and cMet inhibition/silencing for viability and apoptosis studies in mesenchymal and epithelial NSCLC cell lines.
-
BCell viability was measured using CellTiter‐Glo in NSCLC cell lines treated with volasertib, tepotinib, or a 1:2 ratio of both for 72 h. Left: representative cell viability graph of cells treated with the indicated drugs. Right: heatmap depicting the calculated combination indices at Fa = 0.25 and Fa = 0.5.
-
CApoptosis was measured using the Apo‐BrdU assay in the indicated cell lines treated with the indicated drugs for 24 h.
-
DImmunoblots from cells treated with indicated drugs for 24 h (left) with densitometric quantification normalized with β‐actin (right).
-
EAll tested cell lines were treated as indicated for 24 h and allowed to grow in drug‐free medium for 15–20 days to form colonies, which were counted using ImageJ.
-
FApoptosis was measured using the Apo‐BrdU assay in the indicated cell lines transfected with 10 nM siRNA as indicated for 48 h. NT, non‐targeting control.
-
GImmunoblots from cells transfected with siRNA as indicated for 48 h (left) with densitometric quantification normalized with β‐actin (right).
We also assessed colony formation in these cells after 24 h of treatment with vehicle control, volasertib, tepotinib, or the combination followed by drug‐free incubation for 12–15 days. The combination treatment significantly decreased the number and size of colonies in all cell lines tested compared with control or single‐agent treatment, although, consistent with our prior results, Plk1 inhibition alone was effective in mesenchymal NSCLC cell lines (Fig 4E and Appendix Fig S4).
To demonstrate the specificity of Plk1 inhibitors, we knocked down PLK1 and MET expression in NSCLC cell lines using siRNA for 48 h (Fig 4A) and observed a significant increase in apoptosis compared with non‐targeting control and single‐gene silencing (Fig 4F). Consistent with our inhibitor studies, silencing of Plk1 alone significantly increased the percentage of apoptotic cells in mesenchymal cell lines, and we observed persistent cMet (Y1234/1235) phosphorylation in epithelial/resistant cell lines and decreased cMet activation in mesenchymal/sensitive cell lines (Fig 4G). All tested cell lines demonstrated significant increases in expression of cleaved PARP, cleaved caspase 3, and γ‐H2AX in combination silencing compared with non‐targeting control or single‐gene silencing (Fig 4G). These results demonstrate that simultaneous inhibition or silencing of cMet potentiates the apoptotic effect of Plk1 inhibition or silencing in NSCLC.
Inhibition of both Plk1 and cMet is more effective than inhibition of either target alone in vivo in NSCLC cell line and patient‐derived xenograft (PDX) models
Encouraged by the in vitro activity, we next investigated the in vivo effect of Plk1 and cMet inhibition for the treatment of lung cancer in PDX and cell line xenograft models of NSCLC (Hao et al, 2015). We selected both epithelial (TC402) and mesenchymal (TC424) PDX and cell line models. We confirmed the xenografts’ EMT status by checking the expression of EMT‐related genes (Fig EV3A).
Figure EV3. Co‐targeting of cMet and Plk1 reduces tumor size in non–small‐cell lung cancer.

-
AmRNA was extracted from two untreated patient‐derived xenograft (PDX) tumors, and the expression of epithelial‐to‐mesenchymal transition genes was normalized with respect to GAPDH expression. Data are means ± standard error of the mean from two independent tumors.
-
BMice bearing epithelial (red text) and mesenchymal (blue text) non–small‐cell lung cancer PDXs were treated with vehicle control, volasertib (30 mg/kg per week intravenously), tepotinib (25 mg/kg per day orally), or the combination for 5 weeks; representative PDX tumors from the end of treatment are pictured.
-
CWaterfall plots show the percent change in individual PDX tumor volumes at the end of treatment (normalized to day zero), as indicated.
-
DmRNA was extracted from two untreated xenograft tumors, and the expression of epithelial‐to‐mesenchymal transition genes was normalized with respect to GAPDH expression. Data are means ± standard error of the mean from two independent tumors.
-
EMice bearing epithelial (red text) and mesenchymal (blue text) NSCLC cell line xenografts were treated with vehicle control, volasertib (30 mg/kg per week intravenously), tepotinib (25 mg/kg per day orally), or the combination for 5 weeks; representative xenograft tumors from the end of treatment are pictured at the top.
-
FWaterfall plots show the percent change in individual xenograft tumor volumes at the end of treatment (normalized to day zero), as indicated.
-
GKaplan–Meier survival curves for mice with epithelial H1975 and mesenchymal Calu6 xenografts, with death due to excessive tumor burden as the endpoint. Log‐rank (Mantel‐Cox) test was used for Kaplan‐Meier survival curves.
-
HMice bearing non–small‐cell lung cancer cell line xenografts (left) were treated for 4 weeks and mice bearing patient‐derived xenografts (right) were treated for 5 weeks with vehicle control, volasertib (30 mg/kg per week intravenously), tepotinib (25 mg/kg per day orally), or the combination as indicated. Mouse weight was recorded twice weekly to generate the graphs. Data are mean ± standard error of the mean from 10 mice for each group.
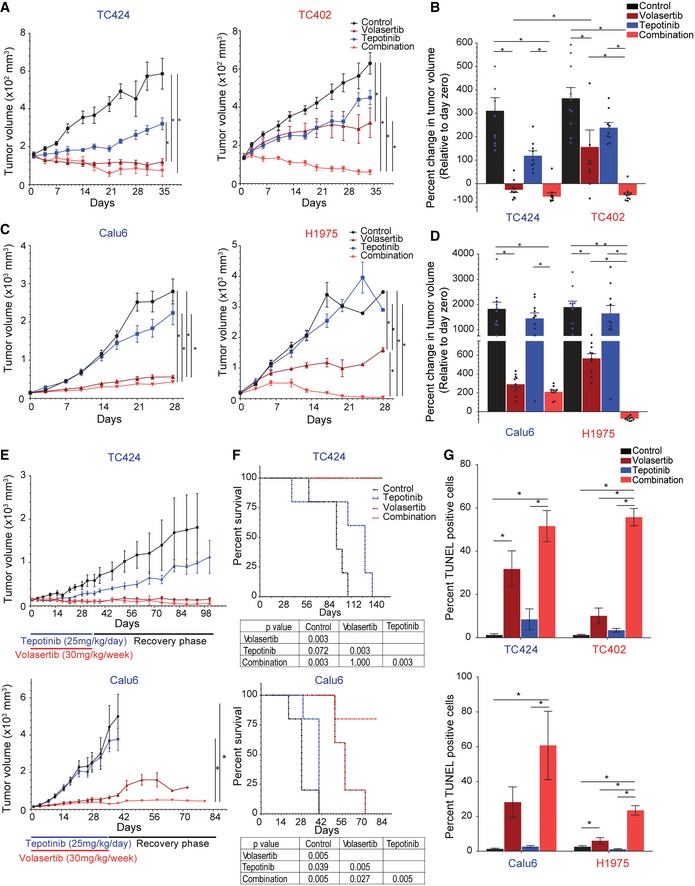
When tumors reached 150 mm3, mice were randomized into one of four treatment groups: vehicle control, volasertib, tepotinib, or the combination of volasertib and tepotinib. Volasertib alone resulted in a significant reduction in tumor growth compared with vehicle control or tepotinib alone in both models. As expected, volasertib reduced tumor size more significantly in the mesenchymal than in the epithelial models (Figs 5A and B, and EV3B). Specifically, volasertib alone led to tumor regression (i.e., tumor smaller than at the start of the experiment) in eight of 10 mesenchymal PDX mice, with an overall −26.5% regression in tumor volume relative to day zero (immediately prior to starting treatment). In contrast, volasertib alone led to tumor regression in only one of 10 epithelial PDX mice and overall tumor size increased by 156% compared with day zero (Figs 5B and EV3C). Likewise, in cell line xenografts (Fig EV3D), Calu6 (mesenchymal) cells showed a better response than did H1975 (epithelial) cells when treated with volasertib alone, although tumors did not regress in either model (Figs 5C and D, and EV3E).
Figure 5. Co‐targeting of cMet and Plk1 induces apoptosis and reduces tumor size in non–small‐cell lung cancer.
-
A–DMice bearing epithelial (red text) and mesenchymal (blue text) non–small‐cell lung cancer tumors were treated with vehicle control, volasertib (30 mg/kg per week intravenously), tepotinib (25 mg/kg per day orally), or the combination for 5 weeks to generate tumor growth curves of patient‐derived xenografts (PDX; A) and cell line xenografts (C). The percent change in tumor volume at the end of treatment (normalized to day zero) was calculated with each data point representing an individual mouse, and the bar is the mean value ± standard error of 10 mice for each group (B, D).
-
ETumor growth curve of mesenchymal PDX TC424 (top) and cell line xenograft Calu6 (bottom) upon treatment for 5 weeks and after cessation of treatment (recovery phase). Data are means ± standard error of the mean from five mice for each group.
-
FKaplan–Meier survival curves for mesenchymal PDX TC424 (top) and cell line xenograft Calu6 (bottom) with death due to excessive tumor burden as the endpoint.
-
GQuantification of TUNEL‐positive cells in paraffin‐embedded xenograft tissue sections. Data are means ± standard error of the mean from six mice for each group.
The combination of Plk1 and cMet inhibitors led to tumor regression in both of the PDX models that was statistically significant starting on day 7 (P < 0.05). The combination therapy led to tumor regression in nine of 10 epithelial and nine of 10 mesenchymal PDX mice (Figs 5B and EV3C). Likewise, both cell line models showed a significant reduction in tumor growth when the mice were treated with the combination compared with control and with tepotinib alone (P < 0.05; Fig 5D). All 10 of the mice bearing the epithelial xenograft showed tumor regression (Figs 5D and EV3F). In the mesenchymal xenograft, the combination was slightly more effective than volasertib alone, but this difference did not reach statistical significance. In both cell line models, mice in the combination arm showed a significant improvement in survival compared with control; volasertib alone improved the survival of mice bearing mesenchymal NSCLC (Fig EV3G). The combination treatment was well tolerated in all mice with no change in body weight over time (Fig EV3H).
To investigate the ability of treated tumors to recover, we treated mice bearing mesenchymal NSCLC for 5 weeks, then stopped drug treatment and examined the mice for tumor growth. The tumors in the mice treated with vehicle control or tepotinib continued to grow steadily. In contrast, tumors remained the same size in both the volasertib‐only and combination treatment groups, with the exception of modest tumor growth in the volasertib‐only Calu6‐bearing mice (Fig 5E). The volasertib‐only and combination treatment groups had significantly longer survival compared with other groups in both models (P < 0.01; Fig 5F).
To asses for apoptosis, we next performed TUNEL staining in paraffin‐embedded tissues (Fig 5G). Similar to the in vitro finding, volasertib alone resulted in a larger increase in TUNEL‐positive cells in the mesenchymal xenograft models (TC424 and Calu6) than in the epithelial xenograft models (TC202 and H1975). Co‐treatment with Plk1 and cMet inhibitors significantly increased the percentage of TUNEL‐positive cells in all mouse models. Taken together, these results support cMet as a driver of Plk1 inhibitor resistance in epithelial NSCLC in vivo, and the findings suggest that co‐inhibition of Plk1 and cMet increases apoptosis, leading to tumor regression in NSCLC.
Constitutive activation of cMet abrogates Plk1 inhibition–induced apoptosis in mesenchymal NSCLC cell lines
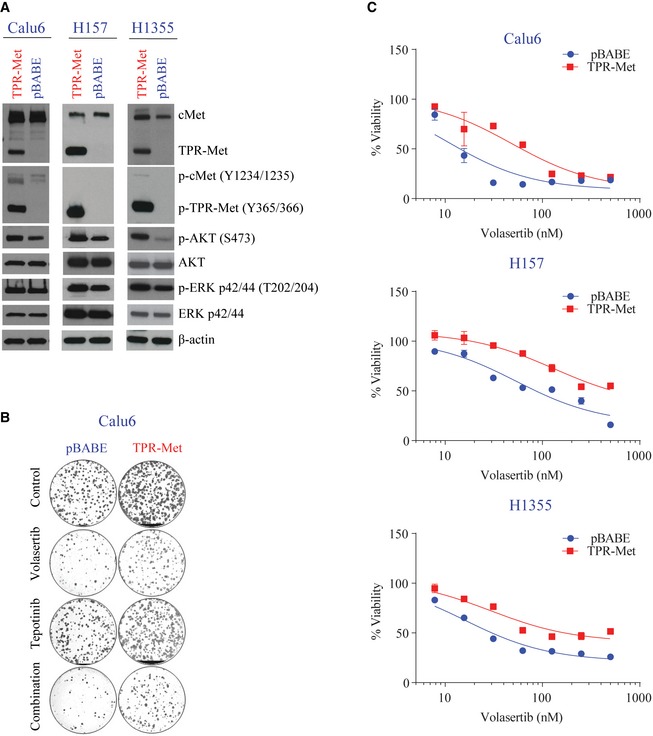
To test cMet as a driver of resistance, we used constitutively active TPR‐Met, which is a fusion protein that lacks the extracellular, transmembrane, and juxtamembrane domains of the cMet receptor and includes the TPR dimerization motif, which allows constitutive and ligand‐independent activation of the kinase. Expression of the TPR‐Met chimeric protein was biologically active in three mesenchymal cell lines (Calu6, H157, and H1355), which in turn increased downstream Akt (S473) and Erk (p42/44 T202/204) phosphorylation (Fig EV4A).
Figure EV4. Expression of the TPR‐Met chimeric protein is biologically active and decreases volasertib sensitivity.
-
ACalu6, H157, and H1355 parental and TPR‐Met–expressing cell lines or vector control (pBABE)–expressing cells were harvested, lysed, and subjected to immunoblotting for the indicated proteins. β‐Actin was used as a loading control.
-
BShown are representative pictures of Calu6 parental and TPR‐Met–expressing cell lines treated with 25 nM volasertib, 400 nM tepotinib, both, or vehicle control for 24 h and allowed to grow in drug‐free medium for 15–20 days to form colonies, which were then stained with crystal violet and photographed.
-
CCell viability of parental and TPR‐Met–expressing Calu6, H157, and H1355 cell lines treated with the indicated concentrations of volasertib for 72 h was measured using CellTiter‐Glo. Experiments were performed in triplicate, and error bars represent standard deviation.
Source data are available online for this figure.
Compared with the control vector–transfected cells, TPR‐Met–expressing cells showed less apoptosis, as measured by Apo‐BrdU, cleaved PARP, and cleaved caspase 3, after treatment with volasertib (Fig 6A and B). Likewise, expression of TPR‐Met increased resistance to Plk1 inhibition compared with control vector, as measured by colony formation (Figs 6C and EV4B) assays and CellTiter‐Glo (Fig EV4C).
Figure 6. Constitutively active cMet expression reduces sensitivity to Plk1 inhibition.

-
ACalu6, H157, and H1355 TPR‐Met–expressing or vector control (pBABE)–expressing cells were treated as indicated for 24 h, and apoptosis was measured using the Apo‐BrdU assay.
-
BParental and TPR‐Met–expressing cell lines were treated with the indicated drugs for 24 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins. β‐Actin was used as a loading control.
-
CParental and TPR‐Met–expressing cell lines were treated as indicated for 24 h and allowed to grow in drug‐free medium for 15–20 days to form colonies, which were counted using ImageJ.
-
DMesenchymal NSCLC cell lines with the noted MET alterations were incubated with 50 nM volasertib for 4 h and subjected to immunoblotting with the indicated antibodies.
To determine whether we could overcome the effects of cMet activation by using a cMet inhibitor, we combined tepotinib and volasertib. Tepotinib inhibited cMet universally but did not completely abrogate cMet signaling in the TPR‐Met–expressing cell lines. The addition of tepotinib significantly increased apoptosis in control vector–transfected cells, but apoptosis was only moderately increased in TPR‐Met–transfected cells, consistent with a lack of full cMet inhibition (Fig 6A and B). The combination of volasertib and tepotinib significantly reduced the clonogenic potential compared with single‐agent treatment in both control and TPR‐Met–transfected cell lines (Fig 6C). Taken together, these results support cMet as a driver of Plk1 inhibitor sensitivity in mesenchymal NSCLC.
The ability of constitutively active TPR‐Met to mediate resistance to Plk1 inhibition led us to ask whether Plk1 inhibition would affect cMet activation in NSCLC with constitutively active cMet. To test this hypothesis, we inhibited Plk1 in mesenchymal NSCLC cell lines with MET amplification and a MET exon 14 skipping mutation (Fig 6D). In both the cell line with marked MET amplification (H920, copy number 16.4) and the one with an activating MET mutation (H596), volasertib did not lead to a decreased in pMet. Both H596 and H920 were resistant to the Plk1 inhibition GSK461364 (Ferrarotto et al, 2016).
Acquired resistance to volasertib leads to a mesenchymal‐to‐epithelial transition and decreased Plk1 inhibitor–mediated cMet inhibition
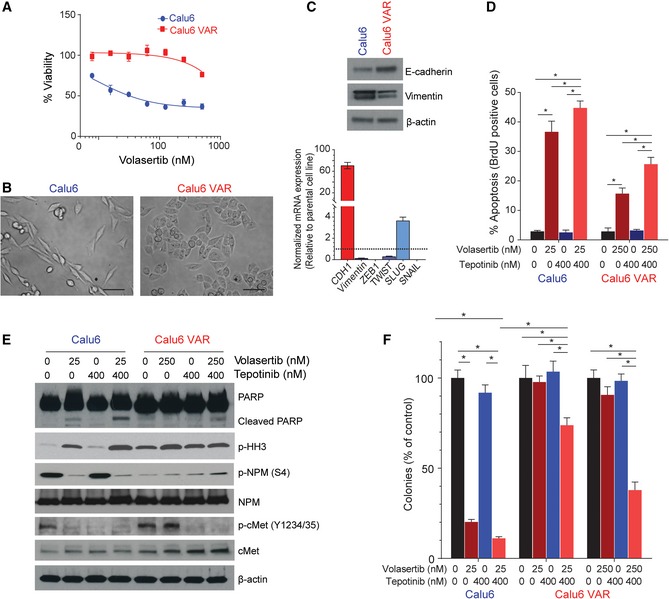
To study the molecular mechanism by which acquired resistance to volasertib emerges, we exposed the Calu6 cell line to increasing concentrations of the drug over time until resistance emerged (Fig 7A). The resulting Calu6‐VAR (volasertib acquired resistance) cell line showed an epithelial morphology (Fig 7B), increased expression of E‐cadherin, and decreased expression of vimentin compared with the parental cell line. Similar to the protein changes, mRNA expression also showed about a 70‐fold increase in CDH1 (P < 0.01) and a decrease in the mesenchymal genes VIM (0.1‐fold), ZEB1 (0.001‐fold), TWIST (0.3‐fold), and SNAIL (0.001‐fold; P < 0.01) compared with the parental cell line (Fig 7C). We did not observe any significant change in basal cMet or p‐cMet (Y1234/1235) protein expression in Calu6‐VAR cells compared with parental cells. We observed persistent p‐cMet (Y1234/1235) phosphorylation after treatment with volasertib in Calu6‐VAR cells, as well as in cell lines with de novo resistance.
Figure 7. The volasertib acquired resistance (VAR) cell line shows mesenchymal‐to‐epithelial transition and persistent cMet phosphorylation.
-
ACell viability measured by CellTiter‐Glo in Calu6 parental and Calu6‐VAR cells treated with different concentrations of volasertib for 72 h.
-
BMicrograph shows acquisition of a mesenchymal‐to‐epithelial transition phenotype in VAR cells. Scale bar: 100 μm.
-
CBasal protein and mRNA expression in parental and VAR cells for the indicated proteins was measured using immunoblotting (upper) and reverse‐transcription PCR (lower), with GAPDH expression used for normalization.
-
DParental and VAR cells were treated as indicated for 48 h, and apoptosis was measured using the Apo‐BrdU assay.
-
EParental and VAR cells were treated with the indicated drug concentrations for 48 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
FParental and VAR cells were treated as indicated for 24 h and allowed to grow in drug‐free medium for 15–20 days to form colonies, which were counted using ImageJ.
The differential effects on cMet activation led us to analyze the effect of co‐targeting Plk1 and cMet with volasertib and tepotinib on apoptosis and cell viability. Plk1 inhibition alone led to less apoptosis in Calu6‐VAR cells than in parental cells (P < 0.01), as measured by Apo‐BrdU and cleaved PARP (Fig 7D and E). Simultaneous inhibition of both cMet and Plk1 significantly increased the percentage of apoptotic BrdU‐positive cells and PARP cleavage compared with single‐agent treatment (P < 0.05) in both parental and Calu6‐VAR cells (Fig 7D and E). We also assessed the colony formation of these cells after 24 h of treatment with vehicle control, volasertib, tepotinib, or both followed by drug‐free incubation for 12–15 days. Volasertib alone was not effective in Calu6‐VAR cells, but combination treatment significantly decreased the number of colonies with both low (25 nM) and high (250 nM) concentrations of volasertib (P < 0.01; Fig 7F and Appendix Fig S5A). Likewise, CellTiter‐Glo analysis showed more sensitivity when Calu6 parental and Calu6‐VAR cells were treated with the combination of volasertib and tepotinib compared with single‐agent treatment (Appendix Fig S5B).
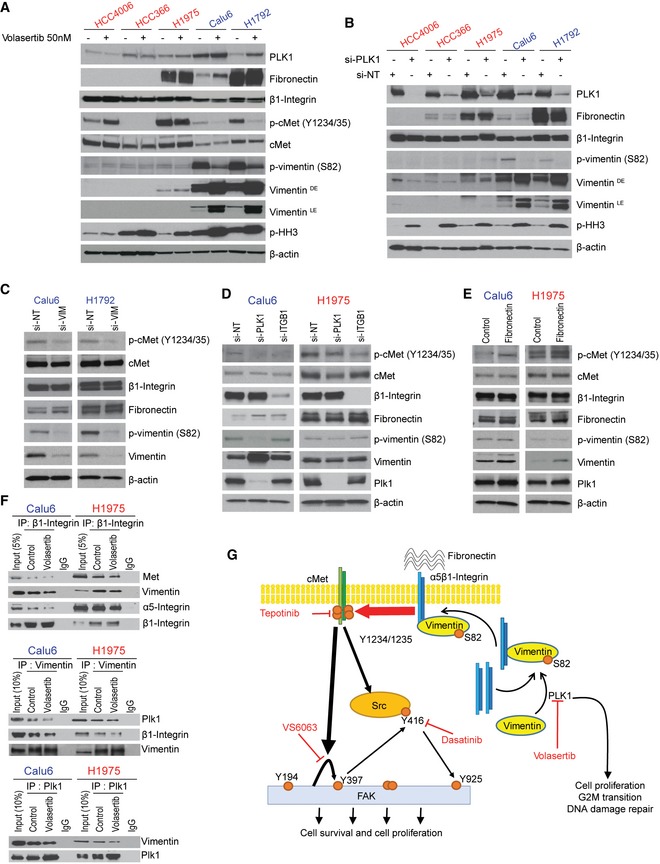
Plk1 inhibition or silencing decreases vimentin phosphorylation and regulates cMet phosphorylation via β1‐integrin trafficking
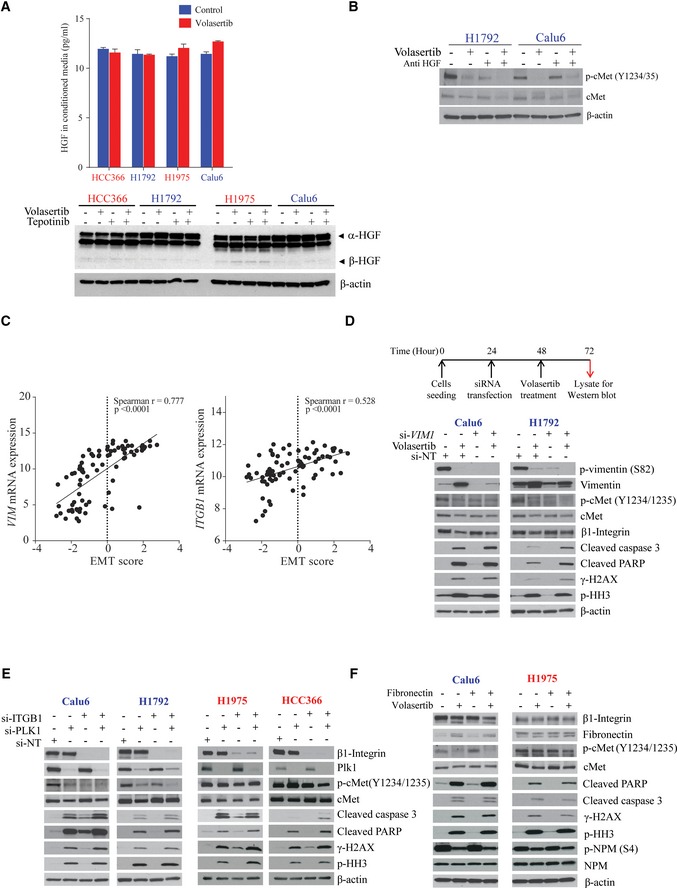
To determine whether cMet inhibition following Plk1 inhibition is ligand‐dependent, we measured hepatocyte growth factor (HGF) in conditioned medium and in cellular lysates after Plk1 inhibition in both mesenchymal and epithelial cell lines. Neither the secreted nor cellular HGF levels were affected by Plk1 inhibition in all tested NSCLC cell lines (Fig EV5A). Likewise, an HGF neutralizing antibody did not affect basal cMet activation or Plk1 inhibition–induced cMet inhibition in the mesenchymal NSCLC cell line Calu6. In a second mesenchymal NSCLC cell line (H1792), the HGF neutralizing antibody did reduce basal cMet activation consistent with cancer cell production of HGF leading to cMet activation that has previously been well established in some NSCLC tumors (Salgia, 2017). In H1792 cells, the combination of the HGF neutralizing antibody and Plk1 inhibition had an additive effect on cMet activation (Fig EV5B).
Figure EV5. Plk1 regulates vimentin phosphorylation, leading to integrin β1–mediated cMet activation.
-
ANon–small‐cell lung cancer cell lines were treated with 50 nM volasertib in serum‐free medium for 24 h. HGF levels were measured in conditioned medium (upper). Data are means ± standard error of the mean from three independent experiments. Non–small‐cell lung cancer cell lines were treated as indicated with 25 nM volasertib or 400 nM tepotinib for 24 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins (lower).
-
BThe indicated mesenchymal cell lines were incubated with 0.5 μg/ml HGF neutralizing antibody or 50 nM volasertib as indicated for 24 h and subjected to immunoblotting.
-
CNSCLC cell line mRNA expression of vimentin (VIM) and integrin β1 (ITGB1) and epithelial‐to‐mesenchymal transition (EMT) scores were obtained from our previous study (22). Spearman's correlations of EMT score and mRNA expression of VIM and ITGB1 in NSCLC cell lines in vitro.
-
DCalu6 and H1792 cell lines were transfected with 10 nM siRNA for 48 h and subsequently treated with 25 nM volasertib for 24 h as indicated. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
ENSCLC cell lines were transfected with ITGB1 and Plk1 siRNA for 48 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
FNSCLC cell lines were treated with volasertib in fibronectin‐coated (5 μg/cm2 for 30 min at room temperature) or fibronectin‐uncoated plates. Cells were then harvested, and lysates were immunoblotted for the indicated proteins. NT, non‐targeting control.
Source data are available online for this figure.
We next investigated the interaction of cMet, Plk1, β1‐integrin (ITGB1), and vimentin (VIM) because this is a ligand‐independent pathway that involves proteins associated with EMT. Plk1 maintains cell surface levels of β1‐integrin via phosphorylation of vimentin on S82 (Rizki et al, 2007). β1‐integrin can form a heterodimer complex with cMet, resulting in ligand‐independent cross‐activation invasive oncologic processes (Jahangiri et al, 2017). We hypothesized that Plk1 regulates vimentin phosphorylation, which in turn regulates the β1‐integrin trafficking to the membrane and maintains cMet phosphorylation in a ligand‐independent manner. Because mesenchymal cells have higher vimentin levels than epithelial cells do, cMet activation using this pathway would be more significant in mesenchymal cells than in epithelial cells. To test this hypothesis, we first checked the expression of vimentin and β1‐integrin in NSCLC cell lines. As expected, we observed a significant positive correlation between expression of mesenchymal markers, according to an EMT score (Byers et al, 2013), and expression of ITGB1 (r = 0.528, P < 0.001) and VIM (r = 0.777, P < 0.001; Fig EV5C).
We then tested the effect of Plk1 inhibition or silencing on vimentin (S82) phosphorylation. Inhibition or silencing of Plk1 by volasertib or siRNA markedly decreased the S82 vimentin phosphorylation in two mesenchymal cell lines. The resulting increase in total vimentin may be a compensatory change. Vimentin levels were very low in epithelial cell lines without consistent effects by Plk1 inhibition (Fig 8A and B); total β1‐integrin levels were not affected.
Figure 8. Plk1 inhibition or silencing decreases vimentin phosphorylation and regulates cMet phosphorylation via β1‐integrin.
-
AEpithelial and mesenchymal non–small‐cell lung cancer (NSCLC) cell lines after treatment with 50 nM volasertib for 24 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
BEpithelial and mesenchymal NSCLC cell lines after Plk1 silencing with siRNA for 48 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
CCalu6 and H1792 mesenchymal NSCLC cell lines after VIM silencing with siRNA for 48 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
DCalu6 (mesenchymal) and H1975 (epithelial) NSCLC cell lines after PLK1 and ITGB1 silencing with siRNA for 48 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
ECalu6 (mesenchymal) and H1975 (epithelial) NSCLC cell lines after treatment with fibronectin for 24 h. Cells were then harvested, and lysates were immunoblotted for the indicated proteins.
-
FCalu6 and H1975 NSCLC cell lines after treatment with 50 nM volasertib for 24 h. Cells were then harvested, and lysates were immunoprecipitated (IP) using β1‐integrin, vimentin, and Plk1 antibodies, then immunoblotted for the indicated proteins.
-
GSchematic summary of the proposed mechanism by which cMet drives primary and acquired resistance to volasertib in NSCLC and possible treatment combinations to overcome this resistance.
Source data are available online for this figure.
To test the role of vimentin, we silenced it in two mesenchymal cell lines, which led to a decrease in cMet phosphorylation at Y1234/1235 (Fig 8C), supporting the role of vimentin in cMet activation. We also observed an increase in cleaved PARP and caspase 3 expression when silencing of vimentin was followed by treatment with volasertib in these cell lines compared with cells treated with volasertib alone (Fig EV5D). Knockdown of vimentin or treatment with volasertib did not affect the expression of total levels of β1‐integrin, cMet, or fibronectin (Figs 8C and EV5D).
To confirm the involvement of β1‐integrin in cMet phosphorylation, we manipulated β1‐integrin levels by siRNA and β1‐integrin activation with fibronectin. Knocking down ITGB1 in both epithelial and mesenchymal cell lines led to a decrease in cMet phosphorylation (Figs 8D and EV5E), demonstrating that this axis is intact in both cell types. Co‐silencing of Plk1 and ITGB1 led to an increase in cleaved PARP and cleaved caspase 3 expression compared with Plk1 silencing alone (Fig EV5E). Consistent with these results, treatment with fibronectin led to an increase in cMet phosphorylation in both epithelial and mesenchymal NSCLC cell lines (Fig 8E). Fibronectin was not able to abrogate Plk1 inhibitor–induced cMet inactivation and likewise had no effect in rescuing the cells from apoptosis by volasertib (Fig EV5F), likely because of a lack of available cell surface β1‐integrin. Manipulation of β1‐integrin did not affect the total levels of cMet, Plk1, or vimentin.
Next, we investigated the interaction between Plk1, vimentin, β1‐integrin, and cMet in NSCLC cell lines after treatment with volasertib. Plk1, vimentin, and β1‐integrin were immunoprecipitated from volasertib‐treated and volasertib‐untreated Calu6 and H1975 cell lysates, and immunoprecipitated protein was resolved by SDS–PAGE and probed for Plk1, vimentin, β1‐integrin, and cMet. Results in Fig 8F show that vimentin co‐immunoprecipitated with Plk1. A reverse immunoprecipitation was performed, and Plk1 was found to co‐immunoprecipitate with vimentin in both NSCLC lines. Similarly, β1‐integrin co‐immunoprecipitated with vimentin and vimentin co‐immunoprecipitated with β1‐integrin. We also observed that cMet co‐immunoprecipitated with β1‐integrin. Altogether, these findings confirm that cMet phosphorylation is regulated by Plk1‐mediated vimentin phosphorylation via β1‐integrin in these cells (Fig 8G).
Discussion
The current study yielded five main findings that follow from our prior discovery that mesenchymal NSCLC is more sensitive to Plk1 inhibitors than epithelial NSCLC (Ferrarotto et al, 2016). First, Plk1 inhibitor sensitivity correlates with cMet and EMT protein expression in a large, independent dataset, validating our prior study. Second, cMet phosphorylation is differentially regulated after Plk1 inhibition in epithelial and mesenchymal NSCLC. Third, pharmacologic and genetic manipulation of cMet affects Plk1 inhibitor–induced apoptosis, establishing this pathway as a bona fide mechanism of resistance. This conclusion was further supported by the finding that a cell line with acquired resistance to Plk1 inhibition showed mesenchymal‐to‐epithelial transition and persistent cMet phosphorylation after Plk1 inhibition, similar to cells with de novo resistance. Fourth, this resistance pathway functions in vivo; significant tumor regression was demonstrated in multiple NSCLC models treated with clinically relevant drugs. Finally, the sensitivity of mesenchymal NSCLC cells to Plk1 inhibition is linked to cMet phosphorylation via the vimentin and β1‐integrin pathway (a ligand‐independent and understudied pathway), leading to cMet activation in NSCLC.
Our work has important clinical implications for the treatment of NSCLC. Previously, we demonstrated that 63 NSCLC cell lines have diverse sensitivities to Plk1 inhibition (Ferrarotto et al, 2016), which is consistent with the results of clinical trials of Plk1 inhibitors for solid tumors, in which response rates were low (4–14%) in unselected patients with stable disease rates of 26–42% (Schoffski et al, 2012, 2010; Sebastian et al, 2010; Stadler et al, 2014). Although approximately 20% of NSCLC tumors are mesenchymal (Akbani et al, 2014; Chen et al, 2014) and predicted to be sensitive to Plk1 inhibition, the reversibility of EMT and intratumoral heterogeneity may diminish the efficacy of Plk1 inhibitors, contributing to low response rates. Recently, Lee et al (2018) measured the same 76‐gene EMT score that we had used to classify NSCLC cell lines (Byers et al, 2013) in 35 regions from 10 NSCLC tumors. Considerable differences in the EMT scores were observed between regions in two of the 10 tumors; in the other eight tumors, different regions within each tumor had very similar EMT scores. The finding that the combination of Plk1 and cMet inhibition led to striking tumor regression in vivo in both epithelial and mesenchymal NSCLC suggests that this combination would be broadly effective in NSCLC patients who may have tumors with heterogeneity or dynamic changes in EMT status. In addition, it is rational to target a pathway that may mediate acquired resistance upfront to achieve a more durable response to therapy (Neel & Bivona, 2017). Because both cMet and Plk1 inhibitors are in clinical development, our work could be rapidly translated to clinical testing.
Recently published work using a novel drug (Poloppin) that inhibits phosphopeptide binding by the Plk1 polo‐binding domain supports our findings (Narvaez et al, 2017). Poloppin led to cell death in KRAS mutant cancer cells in vitro and decreased tumor size in vivo. Because cMet was upregulated in a colon cancer cell line (SW48) expressing KRAS G12D, Narvaez, et al investigated the combination of MET knockdown and cMet inhibition with Poloppin. Both cMet inhibition and knockdown did sensitize five KRAS mutant cell lines and one pancreatic organoid to Poloppin in vitro.
Dysregulation of cMet signaling–mediated proliferation, apoptosis, and migration through cMet overexpression, MET amplification, MET mutation, or HGF‐induced activation has been widely demonstrated in oncogenic processes across multiple tumor types, including NSCLC (Smyth et al, 2014; Tsuta et al, 2012; Van Der Steen et al, 2015). cMet inhibitors have been extensively studied in NSCLC (Salgia, 2017). Recently, mutations in exon 14 of MET that lead to an in‐frame deletion of the negative regulatory juxtamembrane domain were identified in 4% of lung adenocarcinomas. These mutations result in cMet activation and clinical sensitivity to cMet inhibition (Paik et al, 2015). cMet inhibitors are actively being studied in cancers bearing these mutations; a phase II clinical trial of tepotinib in NSCLC harboring MET exon 14 skipping alterations (NCT02864992) is underway. MET is also amplified in 5% of newly diagnosed lung adenocarcinomas (Cappuzzo et al, 2009; Kong‐Beltran et al, 2006; Tsuta et al, 2012), and cMet inhibitors are effective in patients with gene copy numbers > 5 (Salgia, 2017). The presence of cMet/GRB2 complexes is associated with response to cMet inhibitors and may serve as an additional biomarker to select patients who are sensitive to cMet inhibitors in the future (Smith et al, 2017). In the current study, the expression of total cMet protein predicted resistance to four Plk1 inhibitors, although cMet protein and gene expression did not correlate with response in our prior study (Ferrarotto et al, 2016). Our data suggest that vimentin‐dependent cMet activation is independent of canonical mechanisms of cMet activation because a HGF neutralizing antibody can still affect cMet activation in mesenchymal NSCLC and because robust cMet activation by an activating mutation or amplification was not affected by Plk1 inhibition.
Several reports established the cooperativity of cMet and the epidermal growth factor receptor (EGFR) in various cancers, including NSCLC. Co‐targeting of these kinases potentially produces synergistic antitumor effects (Chae et al, 2016; Puri & Salgia, 2008; Wu et al, 2016a), although responses in clinical studies of the combination in NSCLC patients have been modest (Ma, 2015). Both cMet activation and EMT function as resistance mechanisms to EGFR tyrosine kinase inhibitors in NSCLC (Jakobsen et al, 2017; Ninomiya et al, 2018; Rotow & Bivona, 2017; Stewart et al, 2015; Yoshida et al, 2016). Clinical studies combining cMet and EGFR inhibitors in MET‐amplified, EGFR‐mutant NSCLC are ongoing (Rotow & Bivona, 2017). We previously tested the efficacy of Plk1 inhibition in NSCLC cell lines with acquired resistance to EGFR inhibitors and found that cell lines that had undergone EMT, but not those with T790M mutations, became more sensitive to Plk1 inhibition (Wang et al, 2016).
During the reversible process of EMT, epithelial cells lose cell–cell contacts, increase motility, and develop resistance to apoptosis induced by chemotherapy, targeted therapy, and radiotherapy. Changes in gene expression during EMT reflect these characteristics and include repression of E‐cadherin expression with the dissolution of cell–cell junctions; changes in genes encoding cytoskeletal proteins, including the activation of vimentin; and alterations in proteins that affect extracellular matrix interactions, including β1‐integrin. Inhibition of EMT in genetically diverse NSCLC cell lines led to growth inhibition (Burns et al, 2013). Recent studies have demonstrated that Plk1 has a cell cycle–independent function in the regulation of EMT by activating CRAF/ERK signaling in prostate cancer (Wu et al, 2016b) or by activating AKT in gastric cancer (Cai et al, 2016). Plk1 may also indirectly contribute to EMT through regulation of its substrate, the transcription factor Forkhead box M1 (Kong et al, 2014; Wang et al, 2014). However, when we manipulated Plk1, NSCLC cells did not undergo morphologic changes consistent with EMT or show changes in expression of EMT‐related proteins, suggesting that Plk1 is not a driver of EMT in NSCLC (Ferrarotto et al, 2016).
The link between EMT and Plk1 inhibition–induced apoptosis is not explained by canonical signaling pathways, but we hypothesized that proteins that were differentially expressed during EMT, such as vimentin, might be important in this process. CDK1 phosphorylates vimentin at S56, which provides a Plk1 binding site. Plk1 further phosphorylates vimentin at S82 (Oguri et al, 2006; Yamaguchi et al, 2005), which regulates vimentin filament segregation, coordinately with rho‐kinase and Aurora‐B (Yamaguchi et al, 2005). Recently, another study also demonstrated that Plk1 regulates smooth muscle contraction by modulating vimentin phosphorylation at S56 (Li et al, 2016). In addition, Plk1 regulates cell surface levels of β1‐integrin and invasion by phosphorylating vimentin in breast cancer cells (Rizki et al, 2007).
Integrins and other cell surface receptors can lead to ligand‐independent activation of cMet (Mitra et al, 2011; Varkaris et al, 2013). β1‐Integrin positively regulates the endocytosis of activated cMet, as well as cMet signaling after endocytosis in some breast and lung cancer cell lines. cMet and β1‐integrin form a complex on the plasma membrane. cMet activation in either a ligand‐dependent or ligand‐independent manner results in internalization and activation of β1‐integrin. Additionally, β1‐integrin in its active conformation positively regulates the endocytosis of activated cMet. Thus, both cMet and β1‐integrin mutually interact with each other for optimal internalization in a clathrin‐dependent manner. After internalization, the cMet/β1‐integrin complex gradually accumulates on autophagy‐related endomembranes, where β1‐integrin promotes downstream cMet signaling via p52Shc adaptor protein that in turn activates the ERK1/2 signaling pathway. This β1‐integrin–dependent cMet‐sustained signaling regulates cell survival and growth, tumorigenesis, invasion, and lung colonization in vivo (Barrow‐McGee et al, 2016).
Recently, Jahangiri et al demonstrated cMet displaces the α‐heterodimer partner of β1‐integrin, resulting in cMet/β1‐integrin complex formation. This switching of the β1‐integrin binding partner from α5‐integrin to cMet significantly increases fibronectin affinity. Because β1‐integrin lacks enzymatic activity, its signaling depends on the kinase adaptor protein ILK, which binds the cytoplasmic domain of β1‐integrin. In the cMet/β1‐integrin complex, fibronectin promotes ILK‐mediated cMet phosphorylation even in the absence of HGF. Formation of this complex is regulated by tumor microenvironmental factors associated with metastasis and therapeutic resistance (Jahangiri et al, 2017). This activation could lead to Plk1 inhibitor resistance in mesenchymal NSCLC by bypassing Plk1 inhibition–mediated cMet inhibition. This residual cMet/β1‐integrin complex activity, which is dependent upon ligands in the tumor microenvironment, may explain why the combination of PLK1 and cMet inhibition is more effective than single drugs in some mesenchymal NSCLC models and why we observed differences in single‐agent PLK1 inhibitor efficacy in vitro compared with in vivo. Consistent with that study, we also observed that cMet and β1‐integrin interact in NSCLC and that manipulation of β1‐integrin affected cMet phosphorylation. Furthermore, we demonstrated that Plk1 regulates vimentin phosphorylation, which in turn regulates cMet phosphorylation. We are the first to connect these signaling pathways from Plk1 to cMet and the first to study this pathway in NSCLC. Future studies will include fibronectin affinity studies and subcellular localization studies of β1‐integrin, cMet, vimentin, and Plk1 to assess the specific effects of these pathways on β1‐integrin trafficking.
In conclusion, we demonstrated that Plk1 inhibition leads to apoptosis in mesenchymal NSCLC owing to direct effects on Plk1, as well as parallel vimentin and β1‐integrin–mediated, ligand‐independent inhibition of cMet. Moreover, the lack of cMet inhibition in epithelial NSCLC defines a previously unknown pathway of resistance to Plk1 inhibition. Although EMT proteins could serve as candidate biomarkers to select patients for Plk1 inhibition, intratumoral heterogeneity of EMT may limit this approach. The addition of cMet inhibitors is a promising therapeutic strategy to overcome de novo and acquired resistance to Plk1 inhibitors in patients with NSCLC.
Materials and Methods
Reagents and cell lines
All drugs were purchased from Selleck Chemicals (Houston, TX) and prepared as 10 mmol/l stock solutions in dimethyl sulfoxide. Predesigned sets of four independent siRNA sequences of the target genes Plk1 and MET (siGENOME SMARTpool; Dharmacon, Lafayette, CO) were used. Human TGF‐β1 was purchased from Cell Signaling Technology (Danvers, MA). Human fibronectin was purchased from Sigma‐Aldrich (St. Louis, MO). Constitutive active TPR‐Met plasmid and control pBABE plasmid were obtained from Addgene (Cambridge, MA). Antibodies and dilutions used in the study are listed in Appendix Table S3. The HGF neutralizing monoclonal antibody (24612.111) was acquired from Invitrogen (Catalog # MA1‐24767, Thermo Fisher Scientific, Waltham, MA).
Human NSCLC cell lines were obtained, maintained, and genotyped by STR profiling as previously described (Ferrarotto et al, 2016; Peng et al, 2016) and routinely tested for the presence of mycoplasma species using the Mycoplasma Detection Kit (Lonza, Basel, Switzerland).
Development of VAR cell line
For generation of the VAR cell line, the Calu6 cell line was incubated with stepwise increasing concentrations of volasertib. The established Calu6 VAR cell line was maintained in 250 nM volasertib.
Transfections and transduction
Retroviruses were produced by co‐transfecting HEK293 cells with 1 μg of the viral packaging vector pUMVC3 and envelope vector pCMV‐VSV‐G (8:1 ratio) and 1 μg of retroviral plasmid pBABE–TPR‐Met or the control vector using Lipofectamine 3000. The HEK293 cell medium was changed 24 h after transfection, and the cells were incubated at 37°C for 48 h to allow for virus production. After 48 h, HEK293 medium containing viral particles was filtered and transferred onto NSCLC cell culture plates and incubated at 37°C for 48 h. After transduction, fresh RPMI 1640 medium with 10% fetal bovine serum was added to the cell culture plates, and the NSCLC cells were allowed to recover for 24 h. NSCLC cells were selected using 3 μg/ml puromycin.
For knockdown experiments, NSCLC cells were transfected with predesigned sets of four independent siRNAs against Plk1 and cMet using Lipofectamine RNAiMAX Transfection Reagent (Thermo Fisher Scientific, Waltham, MA), as we previously described (Zhang et al, 2017).
Cell viability assays
Cell viability assays were conducted with the CellTiter‐Glo Luminescent Assay (Promega, Fitchburg, WI) after 72 h of exposure to serial fold drug dilutions, as described previously (Ferrarotto et al, 2016; Wang et al, 2016). Drug synergy between volasertib and tepotinib was determined by the combination index, which was generated according to the Chou–Talalay median effect method (Chou, 2010) using the CalcuSyn software (Biosoft, Cambridge, UK).
Colony formation assays
For clonogenic assays, cells were treated for 24 h with dimethyl sulfoxide, volasertib, tepotinib, or the combination of volasertib and tepotinib and then incubated in drug‐free medium for 14–21 days. At the end of the assay, cells were washed, fixed, and stained with crystal violet as described previously (Kalu et al, 2018). The total number of colonies per well was estimated using the ImageJ software program (National Institutes of Health, Bethesda, MD). All clonogenic assays were performed in triplicate, and each test was completed twice on different days.
Reverse phase protein array
Reverse phase protein array was used to compare the expression of 301 proteins (Kalu et al, 2017) using techniques we recently described (Mazumdar et al, 2014), in three parental epithelial NSCLC cell lines, three TGF‐β–induced isogenic mesenchymal NSCLC cell lines, and two mesenchymal NSCLC cell lines after incubation with 50 nM volasertib or vehicle for 24 h. The experiments were performed in triplicate on three different days.
Western blot analysis
Equal amounts of NSCLC cell lysates were separated using 4–20% SDS–PAGE, transferred, and immunoblotted with the indicated primary antibodies, and detected using a horseradish peroxidase–conjugated secondary antibody and an enhanced chemiluminescence reagent, as described previously (Wang et al, 2016). Densitometry quantification of the bands was performed with the ImageJ software program (National Institutes of Health).
Immunoprecipitation assay
Immunoprecipitation was done with Magnetic Dynabeads (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's protocol. Briefly, control and treated cell lysates were incubated with the indicated antibody–Dynabeads conjugate followed by purification of antigen–antibody–Dynabeads conjugate and separation on SDS–PAGE for immunoblotting.
Apoptosis analyses
Apoptosis was measured by TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) staining (Apo‐BrdU Kit; BD Biosciences, San Jose, CA) as described previously (Kalu et al, 2017; Wang et al, 2016). Data were acquired using a flow cytometer (Gallios; Beckman Coulter, Brea, CA) and analyzed using Kaluza software (Beckman Coulter, Brea, CA).
Comet assay
The comet assay was done to detect DNA fragmentation, according to the manufacturer's instructions (Trevigen, Gaithersburg, MD) and as we previously described (Peng et al, 2016; Sen et al, 2012). After fixation, lysis and electrophoresis slides were stained with Vista Green for fluorescence imaging. For evaluation of the comet patterns, about 100 nuclei from each slide were analyzed by Comet Score Pro (TriTek Corp., Sumerduck, VA), and tail moment was calculated by multiplying tail DNA percentage by the length of the tail (Wang et al, 2016).
Quantitative PCR
Total cellular RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and complementary DNAs (cDNAs) were synthesized using iScript Reverse Transcription Supermix (Bio‐Rad Laboratories, Hercules, CA). Quantitative real‐time PCR assays were done with iTaq Universal SYBR Green Supermix (Bio‐Rad Laboratories) and the CFX96 Real‐time System (Bio‐Rad Laboratories) as described previously (Kalu et al, 2017). Primers are listed in Appendix Table S4.
HGF estimation
Secretory HGF was estimated using the HGF ELISA Kit (Abcam, Cambridge, MA) according to the manufacturer's protocol. Briefly, NSCLC cell lines were treated with 50 nM volasertib in serum‐free medium for 24 h, and HGF levels were measured in conditioned medium.
Subcutaneous xenograft animal models
In conducting research using animals, the investigators adhered to the laws of the United States and regulations of the US Department of Agriculture. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Texas MD Anderson Cancer Center and conducted in accordance with MD Anderson's Office of Research Administration and IACUC guidelines. Two million NSCLC cells per mouse were injected subcutaneously, or logarithmically growing PDXs (Hao et al, 2015) were implanted subcutaneously into the flanks of 6‐ to 8‐week‐old female nude mice (Harlan Laboratories, Indianapolis, IN). Tumors were measured by caliper twice weekly by two, non‐blinded, independent investigators. Tumor volumes were calculated as follows: (length × width2)/2. When tumor volumes reached 150 mm3, the mice were randomized and treated with 30 mg/kg volasertib intravenously weekly, 25 mg/kg tepotinib daily (5 days on and 2 days off) by oral gavage, both agents, or vehicle control for 5 weeks. Mice were then euthanized and tumors excised.
A linear model was fit to the data using generalized least squares with two variables (treatment and day) and an autocorrelation structure of order 1 in residuals, given that we had a single grouping variable of mouse identification. The Tukey test was used for pairwise comparisons between treatment groups, and therefore, P‐values corrected for multiple comparisons were produced, as described previously (Zhang et al, 2017). Log‐rank tests were conducted to assess the association of the treatment type with survival. Benjamini–Hochberg correction was applied to P‐values from pairwise comparisons between treatment groups to adjust for multiple comparisons. In addition, Kaplan–Meier curves were generated.
TUNEL tissue staining
Harvested tumor tissues were fixed in 10% formalin, embedded in paraffin, and sectioned at 5 μM. Briefly, after deparaffinization and rehydration, tissue sections were subsequently processed for DNA labeling by TdT dNTP followed by Strep‐HRP/DAB detection using the TUNEL Apoptosis Detection Kit (Trevigen, Gaithersburg, MD). Images were taken at 40× magnification, and image analysis was performed to calculate the percentage of TUNEL‐positive cells using ImageJ software (National Institutes of Health).
Statistical analysis
All statistical analyses were performed using R packages (https://www.r-project.org/), a publically available and widely used statistical computing tool. RPPA protein expression and Plk1 inhibitor (BI2536, GSK461364, BRD‐K70511574, and GW‐843682X) sensitivity data were downloaded from the MD Anderson Cell Line Project (http://tcpaportal.org/mclp/#/) and CTRPv2 (Broad Institute; https://portals.broadinstitute.org/ctrp/) databases and curated for NSCLC cell lines on March 5, 2017. RPPA data for 71 cell lines were available for BI2536, 69 cell lines for GSK461364, 68 cell lines for BRD‐K70511574, and 64 cell lines for GW‐843682X. To identify correlation between drug sensitivity and protein expression, we applied Spearman's correlation using R. For RPPA proteins to be compared between cell lines after treatment with a Plk1 inhibitor, it is necessary to treat cell line as a random effect because the cell line variation is dominant in principal component analysis. Further contrasts were conducted to compare changes in epithelial cells after treatment with the Plk1 inhibitor, changes in mesenchymal cells after treatment with the Plk1 inhibitor, and changes between the two, which is the interaction term. The analysis was carried out by the limma package in R. To correct for multiple hypotheses testing, we adjusted the resulting P‐values using the Benjamini–Hochberg method.
There are a variety of group comparisons with respect to different study designs, including two‐factor and three‐factor factorial designs. All in vitro experiments were repeated at least twice (biological replicates) with three or more technical replicates. Analysis of variance was applied, and various contrasts were set up to test the significance of difference between groups of interest and of the interaction of two factors. Bonferroni or Benjamini–Hochberg correction was applied to adjust for multiple testing. For tumor growth curve analysis, linear modeling using generalized least squares with two variables (treatment and day) and an autocorrelation structure of order 1 in residuals were applied. The Tukey test was used for pairwise comparisons among treatment groups, and P‐values corrected for multiple comparisons were produced. For survival analysis, log‐rank tests were conducted to assess the association of the treatment type with survival. Benjamini–Hochberg correction was applied to P‐values from pairwise comparisons between treatment groups to adjust for multiple comparisons. In addition, Kaplan–Meier curves were generated. A summary of statistical methods for each specific Figure is provided in Appendix Table S5.
Author contributions
RS designed and performed most of the experiments and wrote the first draft of the manuscript. SP, PV, and VS assisted with experimental design and conduct of the animal experiments. BF provided and characterized several PDX models. LS, XR, and JW performed the bioinformatics and statistical analyses. FMJ oversaw the study design and wrote the final draft of the paper.
Conflict of interest
Faye M. Johnson has received research funding from PIQUR Therapeutics and Trovagene. Other authors have no conflicts of interest to declare.
The paper explained.
Problem
Most NSCLC remains incurable despite survival benefits from targeted and immunotherapy. Thus, there is still an urgent need for effective systemic therapy. While Plk1 inhibitors are well tolerated by patients and some striking clinical responses were observed, response rates were generally low. Likewise, Plk1 inhibitors lead to diverse biological effects in cancer cells. Understanding the basis for Plk1 inhibitor–induced apoptosis is essential to maximizing their antitumor efficacy. To address this need, our laboratory previously discovered that mesenchymal NSCLC cell lines are more sensitive to Plk1 inhibitors than epithelial cell lines in vitro and in vivo. However, mechanisms of resistance to Plk1 inhibitors have not been elucidated and this unknown is a major gap in knowledge.
Results
We used isogenic pairs of epithelial and mesenchymal NSCLC cell lines to measure changes in the expression of 301 proteins after Plk1 inhibition. We observed differential regulation of the cMet/FAK/Src axis, which is intact in both mesenchymal and epithelial cells. However, Plk1 inhibition inhibits cMet phosphorylation only in mesenchymal NSCLC cells, leading to subsequent inhibition of FAK and Src. Constitutively active cMet abrogates Plk1 inhibitor–induced apoptosis. Likewise, cMet silencing or inhibition enhances Plk1 inhibitor–induced apoptosis. Additionally, cells with acquired resistance to Plk1 inhibitors are more epithelial than their parental cells and maintain cMet activation after Plk1 inhibition. In both patient‐derived and cell line xenografts, mesenchymal NSCLC was more sensitive to Plk1 inhibition alone than was epithelial NSCLC. The combination of cMet and Plk1 inhibition led to regression of tumors in three models and marked tumor size reduction in the fourth model. When drug treatment was stopped, tumors treated with the combination did not regrow. Plk1 regulates cMet via the vimentin protein that is only expressed in mesenchymal NSCLC.
Impact
NSCLC cell lines have diverse sensitivities to Plk1 inhibition, which is consistent with the results of clinical trials of Plk1 inhibitors in solid tumors. This study reveals a novel mechanism of non‐canonical cMet activation in resistant/epithelial NSCLC after Plk1 inhibition. The addition of cMet inhibitors is a promising therapeutic strategy to overcome de novo and acquired resistance to Plk1 inhibitors in patients with NSCLC.
Supporting information
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8
Acknowledgements
We thank Erica Goodoff of the Department of Scientific Publications at MD Anderson for editing this manuscript. Flow cytometry, bioinformatics, and animal facilities are supported by the National Cancer Institute Cancer Center Support Grant P30CA016672. PDX generation and annotation were supported by philanthropic contributions to The University of Texas MD Anderson Cancer Center Lung Moon Shot Program, Specialized Program of Research Excellence (SPORE) grant CA070907, and University of Texas PDX Development and Trial Center grant U54CA224065. The research was supported by generous donations and by the Office of the Assistant Secretary of Defense for Health Affairs through the Lung Cancer Research Program, under Award No. W81XWH‐17‐1‐0206 (F.M.J.). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
For more information
EMBO Mol Med (2019) 11: e9960
Dr. Ratnakar Singh is currently a postdoctoral fellow at the University of Illinois. Ms. Pavitra Visvanath is currently a graduate student at the University of Utah. Both were employed at The University of Texas MD Anderson Cancer Center at the time this research was performed
References
- Akbani R, Ng PK, Werner HM, Shahmoradgoli M, Zhang F, Ju Z, Liu W, Yang JY, Yoshihara K, Li J, et al (2014) A pan‐cancer proteomic perspective on The Cancer Genome Atlas. Nat Commun 5: 3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow‐McGee R, Kishi N, Joffre C, Menard L, Hervieu A, Bakhouche BA, Noval AJ, Mai A, Guzman C, Robbez‐Masson L, et al (2016) Beta 1‐integrin‐c‐Met cooperation reveals an inside‐in survival signalling on autophagy‐related endomembranes. Nat Commun 7: 11942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladt F, Faden B, Friese‐Hamim M, Knuehl C, Wilm C, Fittschen C, Gradler U, Meyring M, Dorsch D, Jaehrling F, et al (2013) EMD 1214063 and EMD 1204831 constitute a new class of potent and highly selective c‐Met inhibitors. Clin Cancer Res 19: 2941–2951 [DOI] [PubMed] [Google Scholar]
- Burns TF, Dobromilskaya I, Murphy SC, Gajula RP, Thiyagarajan S, Chatley SN, Aziz K, Cho YJ, Tran PT, Rudin CM (2013) Inhibition of TWIST1 leads to activation of oncogene‐induced senescence in oncogene‐driven non‐small cell lung cancer. Mol Cancer Res 11: 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, et al (2013) An epithelial‐mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res 19: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XP, Chen LD, Song HB, Zhang CX, Yuan ZW, Xiang ZX (2016) PLK1 promotes epithelial‐mesenchymal transition and metastasis of gastric carcinoma cells. Am J Transl Res 8: 4172–4183 [PMC free article] [PubMed] [Google Scholar]
- Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, Del Grammastro M, Sciarrotta MG, Buttitta F, Incarbone M, et al (2009) Increased MET gene copy number negatively affects survival of surgically resected non‐small‐cell lung cancer patients. J Clin Oncol 27: 1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae YK, Gagliato Dde M, Pai SG, Carneiro B, Mohindra N, Giles FJ, Ramakrishnan‐Geethakumari P, Sohn J, Liu S, Chen H, et al (2016) The association between EGFR and cMET expression and phosphorylation and its prognostic implication in patients with breast cancer. PLoS One 11: e0152585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al (2014) Metastasis is regulated via microRNA‐200/ZEB1 axis control of tumour cell PD‐L1 expression and intratumoral immunosuppression. Nat Commun 5: 5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Kim W, Cheon MG, Lee CW, Kim JE (2015) Polo‐like kinase 1 inhibitor BI2536 causes mitotic catastrophe following activation of the spindle assembly checkpoint in non‐small cell lung cancer cells. Cancer Lett 357: 591–601 [DOI] [PubMed] [Google Scholar]
- Chou TC (2010) Drug combination studies and their synergy quantification using the Chou‐Talalay method. Cancer Res 70: 440–446 [DOI] [PubMed] [Google Scholar]
- Craig SN, Wyatt MD, McInnes C (2014) Current assessment of polo‐like kinases as anti‐tumor drug targets. Expert Opin Drug Discov 9: 773–789 [DOI] [PubMed] [Google Scholar]
- Degenhardt Y, Greshock J, Laquerre S, Gilmartin AG, Jing J, Richter M, Zhang X, Bleam M, Halsey W, Hughes A, et al (2010) Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function and chromosome instability. Mol Cancer Ther 9: 2079–2089 [DOI] [PubMed] [Google Scholar]
- Driscoll DL, Chakravarty A, Bowman D, Shinde V, Lasky K, Shi J, Vos T, Stringer B, Amidon B, D'Amore N, et al (2014) Plk1 inhibition causes post‐mitotic DNA damage and senescence in a range of human tumor cell lines. PLoS One 9: e111060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarotto R, Goonatilake R, Yoo SY, Tong P, Giri U, Peng S, Minna J, Girard L, Wang Y, Wang L, et al (2016) Epithelial‐mesenchymal transition predicts polo‐like kinase 1 inhibitor‐mediated apoptosis in non‐small cell lung cancer. Clin Cancer Res 22: 1674–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese‐Hamim M, Bladt F, Locatelli G, Stammberger U, Blaukat A (2017) The selective c‐Met inhibitor tepotinib can overcome epidermal growth factor receptor inhibitor resistance mediated by aberrant c‐Met activation in NSCLC models. Am J Cancer Res 7: 962–972 [PMC free article] [PubMed] [Google Scholar]
- Gjertsen BT, Schoffski P (2015) Discovery and development of the Polo‐like kinase inhibitor volasertib in cancer therapy. Leukemia 29: 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Wang L, Peng S, Cao M, Li H, Hu J, Huang X, Liu W, Zhang H, Wu S, et al (2015) Gene mutations in primary tumors and corresponding patient‐derived xenografts derived from non‐small cell lung cancer. Cancer Lett 357: 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahangiri A, Nguyen A, Chandra A, Sidorov MK, Yagnik G, Rick J, Han SW, Chen W, Flanigan PM, Schneidman‐Duhovny D, et al (2017) Cross‐activating c‐Met/beta1 integrin complex drives metastasis and invasive resistance in cancer. Proc Natl Acad Sci USA 114: E8685–E8694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen KR, Demuth C, Madsen AT, Hussmann D, Vad‐Nielsen J, Nielsen AL, Sorensen BS (2017) MET amplification and epithelial‐to‐mesenchymal transition exist as parallel resistance mechanisms in erlotinib‐resistant, EGFR‐mutated, NSCLC HCC827 cells. Oncogenesis 6: e307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalu NN, Mazumdar T, Peng S, Shen L, Sambandam V, Rao X, Xi Y, Li L, Qi Y, Gleber‐Netto FO, et al (2017) Genomic characterization of human papillomavirus‐positive and ‐negative human squamous cell cancer cell lines. Oncotarget 8: 86369–86383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalu NN, Mazumdar T, Peng S, Tong P, Shen L, Wang J, Banerjee U, Myers JN, Pickering CR, Brunell D, et al (2018) Comprehensive pharmacogenomic profiling of human papillomavirus‐positive and ‐negative squamous cell carcinoma identifies sensitivity to aurora kinase inhibition in KMT2D mutants. Cancer Lett 431: 64–72 [DOI] [PubMed] [Google Scholar]
- Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M, Guo YH, Shi J, Gong XD, Zhu YL, Liu F, et al (2014) Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non‐small cell lung cancer. Oncol Rep 31: 2660–2668 [DOI] [PubMed] [Google Scholar]
- Kong‐Beltran M, Seshagiri S, Zha J, Zhu W, Bhawe K, Mendoza N, Holcomb T, Pujara K, Stinson J, Fu L, et al (2006) Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res 66: 283–289 [DOI] [PubMed] [Google Scholar]
- Kubo T, Yamamoto H, Lockwood WW, Valencia I, Soh J, Peyton M, Jida M, Otani H, Fujii T, Ouchida M, et al (2009) MET gene amplification or EGFR mutation activate MET in lung cancers untreated with EGFR tyrosine kinase inhibitors. Int J Cancer 124: 1778–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Diao L, Wang J, Zhang J, Roarty EB, Varghese S, Chow CW, Fujimoto J, Behrens C, Cascone T, et al (2018) Multiregion gene expression profiling reveals heterogeneity in molecular subtypes and immunotherapy response signatures in lung cancer. Mod Pathol 31: 947–955 [DOI] [PubMed] [Google Scholar]
- Li J, Wang R, Gannon OJ, Rezey AC, Jiang S, Gerlach BD, Liao G, Tang DD (2016) Polo‐like kinase 1 regulates vimentin phosphorylation at ser‐56 and contraction in smooth muscle. J Biol Chem 291: 23693–23703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao W, Akbani R, Liu W, Ju Z, Ling S, Vellano CP, Roebuck P, Yu Q, Eterovic AK, et al (2017) Characterization of human cancer cell lines by reverse‐phase protein arrays. Cancer Cell 31: 225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, Wong KK, Elledge SJ (2009) A genome‐wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 137: 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PC (2015) MET receptor juxtamembrane exon 14 alternative spliced variant: novel cancer genomic predictive biomarker. Cancer Discov 5: 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar T, Byers LA, Ng PK, Mills GB, Peng S, Diao L, Fan YH, Stemke‐Hale K, Heymach JV, Myers JN, et al (2014) A comprehensive evaluation of biomarkers predictive of response to PI3K inhibitors and of resistance mechanisms in head and neck squamous cell carcinoma. Mol Cancer Ther 13: 2738–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll JA, Dwarte T, Baigude H, Dang J, Yang L, Erlich RB, Kimpton K, Teo J, Sagnella SM, Akerfeldt MC, et al (2015) Therapeutic targeting of polo‐like kinase 1 using RNA‐interfering nanoparticles (iNOPs) for the treatment of non‐small cell lung cancer. Oncotarget 6: 12020–12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Lin CC, Yang JC (2011) Polo‐like kinase 1 inhibitors and their potential role in anticancer therapy, with a focus on NSCLC. Clin Cancer Res 17: 6459–6466 [DOI] [PubMed] [Google Scholar]
- Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E (2011) Ligand‐independent activation of c‐Met by fibronectin and alpha(5)beta(1)‐integrin regulates ovarian cancer invasion and metastasis. Oncogene 30: 1566–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez AJ, Ber S, Crooks A, Emery A, Hardwick B, Guarino Almeida E, Huggins DJ, Perera D, Roberts‐Thomson M, Azzarelli R, et al (2017) Modulating protein‐protein interactions of the mitotic polo‐like kinases to target mutant KRAS. Cell Chem Biol 24: 1017–1028 e1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel DS, Bivona TG (2017) Resistance is futile: overcoming resistance to targeted therapies in lung adenocarcinoma. NPJ Precis Oncol 1: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya K, Ohashi K, Makimoto G, Tomida S, Higo H, Kayatani H, Ninomiya T, Kubo T, Ichihara E, Hotta K, et al (2018) MET or NRAS amplification is an acquired resistance mechanism to the third‐generation EGFR inhibitor naquotinib. Sci Rep 8: 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokihara H, Yamada Y, Fujiwara Y, Yamamoto N, Wakui H, Nakamichi S, Kitazono S, Inoue K, Harada A, Taube T, et al (2016) Phase I trial of volasertib, a Polo‐like kinase inhibitor, in Japanese patients with advanced solid tumors. Invest New Drugs 34: 66–74 [DOI] [PubMed] [Google Scholar]
- Oguri T, Inoko A, Shima H, Izawa I, Arimura N, Yamaguchi T, Inagaki N, Kaibuchi K, Kikuchi K, Inagaki M (2006) Vimentin‐Ser82 as a memory phosphorylation site in astrocytes. Genes Cells 11: 531–540 [DOI] [PubMed] [Google Scholar]
- Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, Borsu L, Schultz N, Berger MF, Rudin CM, et al (2015) Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov 5: 842–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Sen B, Mazumdar T, Byers LA, Diao L, Wang J, Tong P, Giri U, Heymach JV, Kadara HN, et al (2016) Dasatinib induces DNA damage and activates DNA repair pathways leading to senescence in non‐small cell lung cancer cell lines with kinase‐inactivating BRAF mutations. Oncotarget 7: 565–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade‐Lauraine E, Selle F, Weber B, Ray‐Coquard IL, Vergote I, Sufliarsky J, Del Campo JM, Lortholary A, Lesoin A, Follana P, et al (2016) Volasertib versus chemotherapy in platinum‐resistant or ‐refractory ovarian cancer: a randomized phase II groupe des investigateurs nationaux pour l'etude des cancers de l'ovaire study. J Clin Oncol 34: 706–713 [DOI] [PubMed] [Google Scholar]
- Puri N, Salgia R (2008) Synergism of EGFR and c‐Met pathways, cross‐talk and inhibition, in non‐small cell lung cancer. J Carcinog 7: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reungwetwattana T, Liang Y, Zhu V, Ou SI (2017) The race to target MET exon 14 skipping alterations in non‐small cell lung cancer: the why, the how, the who, the unknown, and the inevitable. Lung Cancer 103: 27–37 [DOI] [PubMed] [Google Scholar]
- Rizki A, Mott JD, Bissell MJ (2007) Polo‐like kinase 1 is involved in invasion through extracellular matrix. Cancer Res 67: 11106–11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotow J, Bivona TG (2017) Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 17: 637–658 [DOI] [PubMed] [Google Scholar]
- Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, Haslinger C, Garin‐Chesa P, Adolf GR (2009) BI 6727, a Polo‐like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res 15: 3094–3102 [DOI] [PubMed] [Google Scholar]
- Salgia R (2017) MET in lung cancer: biomarker selection based on scientific rationale. Mol Cancer Ther 16: 555–565 [DOI] [PubMed] [Google Scholar]
- Sanhaji M, Kreis NN, Zimmer B, Berg T, Louwen F, Yuan J (2012) p53 is not directly relevant to the response of Polo‐like kinase 1 inhibitors. Cell Cycle 11: 543–553 [DOI] [PubMed] [Google Scholar]
- Sanhaji M, Louwen F, Zimmer B, Kreis NN, Roth S, Yuan J (2013) Polo‐like kinase 1 inhibitors, mitotic stress and the tumor suppressor p53. Cell Cycle 12: 1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffski P, Blay JY, De Greve J, Brain E, Machiels JP, Soria JC, Sleijfer S, Wolter P, Ray‐Coquard I, Fontaine C, et al (2010) Multicentric parallel phase II trial of the polo‐like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network Of Core Institutes (NOCI). Eur J Cancer 46: 2206–2215 [DOI] [PubMed] [Google Scholar]
- Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P, Munzert G (2012) A phase I, dose‐escalation study of the novel Polo‐like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer 48: 179–186 [DOI] [PubMed] [Google Scholar]
- Seashore‐Ludlow B, Rees MG, Cheah JH, Cokol M, Price EV, Coletti ME, Jones V, Bodycombe NE, Soule CK, Gould J, et al (2015) Harnessing connectivity in a large‐scale small‐molecule sensitivity dataset. Cancer Discov 5: 1210–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian M, Reck M, Waller CF, Kortsik C, Frickhofen N, Schuler M, Fritsch H, Gaschler‐Markefski B, Hanft G, Munzert G, et al (2010) The efficacy and safety of BI 2536, a novel Plk‐1 inhibitor, in patients with stage IIIB/IV non‐small cell lung cancer who had relapsed after, or failed, chemotherapy: results from an open‐label, randomized phase II clinical trial. J Thorac Oncol 5: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Sen B, Peng S, Saigal B, Williams MD, Johnson FM (2011) Distinct interactions between c‐Src and c‐Met in mediating resistance to c‐Src inhibition in head and neck cancer. Clin Cancer Res 17: 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Peng S, Tang X, Erickson HS, Galindo H, Mazumdar T, Stewart DJ, Wistuba I, Johnson FM (2012) Kinase‐impaired BRAF mutations in lung cancer confer sensitivity to dasatinib. Sci Transl Med 4: 136ra170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68: 7–30 [DOI] [PubMed] [Google Scholar]
- Smith MA, Licata T, Lakhani A, Garcia MV, Schildhaus HU, Vuaroqueaux V, Halmos B, Borczuk AC, Chen YA, Creelan BC, et al (2017) MET‐GRB2 signaling‐associated complexes correlate with oncogenic MET signaling and sensitivity to MET kinase inhibitors. Clin Cancer Res 23: 7084–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth EC, Sclafani F, Cunningham D (2014) Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth‐factor inhibitors. Onco Targets Ther 7: 1001–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spankuch‐Schmitt B, Bereiter‐Hahn J, Kaufmann M, Strebhardt K (2002) Effect of RNA silencing of polo‐like kinase‐1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst 94: 1863–1877 [DOI] [PubMed] [Google Scholar]
- Stadler WM, Vaughn DJ, Sonpavde G, Vogelzang NJ, Tagawa ST, Petrylak DP, Rosen P, Lin CC, Mahoney J, Modi S, et al (2014) An open‐label, single‐arm, phase 2 trial of the polo‐like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer 120: 976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EL, Tan SZ, Liu G, Tsao MS (2015) Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations‐a review. Transl Lung Cancer Res 4: 67–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuta K, Kozu Y, Mimae T, Yoshida A, Kohno T, Sekine I, Tamura T, Asamura H, Furuta K, Tsuda H (2012) c‐MET/phospho‐MET protein expression and MET gene copy number in non‐small cell lung carcinomas. J Thorac Oncol 7: 331–339 [DOI] [PubMed] [Google Scholar]
- Van den Bossche J, Lardon F, Deschoolmeester V, De Pauw I, Vermorken JB, Specenier P, Pauwels P, Peeters M, Wouters A (2016) Spotlight on volasertib: preclinical and clinical evaluation of a promising Plk1 inhibitor. Med Res Rev 36: 749–786 [DOI] [PubMed] [Google Scholar]
- Van Der Steen N, Pauwels P, Gil‐Bazo I, Castanon E, Raez L, Cappuzzo F, Rolfo C (2015) cMET in NSCLC: can we cut off the head of the hydra? From the pathway to the resistance. Cancers 7: 556–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkaris A, Gaur S, Parikh NU, Song JH, Dayyani F, Jin JK, Logothetis CJ, Gallick GE (2013) Ligand‐independent activation of MET through IGF‐1/IGF‐1R signaling. Int J Cancer 133: 1536–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yao B, Wang Y, Zhang M, Fu S, Gao H, Peng R, Zhang L, Tang J (2014) Increased FoxM1 expression is a target for metformin in the suppression of EMT in prostate cancer. Int J Mol Med 33: 1514–1522 [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Wang L, Nilsson M, Goonatilake R, Tong P, Li L, Giri U, Villalobos P, Mino B, et al (2016) Polo‐like kinase 1 inhibition diminishes acquired resistance to epidermal growth factor receptor inhibition in non‐small cell lung cancer with T790M mutations. Oncotarget 7: 47998–48010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu L, Yao Y, Lu G, Xu L, Zhou J (2018) Polo‐like kinase 1 inhibitor BI 6727 induces DNA damage and exerts strong antitumor activity in small cell lung cancer. Cancer Lett 436: 1–9 [DOI] [PubMed] [Google Scholar]
- Wu DW, Chen TC, Huang HS, Lee H (2016a) TC‐N19, a novel dual inhibitor of EGFR and cMET, efficiently overcomes EGFR‐TKI resistance in non‐small‐cell lung cancer cells. Cell Death Dis 7: e2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ivanov AI, Fisher PB, Fu Z (2016b) Polo‐like kinase 1 induces epithelial‐to‐mesenchymal transition and promotes epithelial cell motility by activating CRAF/ERK signaling. Elife 5: e10734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Goto H, Yokoyama T, Sillje H, Hanisch A, Uldschmid A, Takai Y, Oguri T, Nigg EA, Inagaki M (2005) Phosphorylation by Cdk1 induces Plk1‐mediated vimentin phosphorylation during mitosis. J Cell Biol 171: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H, Erikson RL (2009) Polo‐like kinase 1 depletion induces DNA damage in early S prior to caspase activation. Mol Cell Biol 29: 2609–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Song L, Bai Y, Kinose F, Li J, Ohaegbulam KC, Munoz‐Antonia T, Qu X, Eschrich S, Uramoto H, et al (2016) ZEB1 mediates acquired resistance to the epidermal growth factor receptor‐tyrosine kinase inhibitors in non‐small cell lung cancer. PLoS One 11: e0147344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Singh R, Peng S, Mazumdar T, Sambandam V, Shen L, Tong P, Li L, Kalu NN, Pickering CR, et al (2017) Mutations of the LIM protein AJUBA mediate sensitivity of head and neck squamous cell carcinoma to treatment with cell‐cycle inhibitors. Cancer Lett 392: 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Source Data for Expanded View and Appendix
Review Process File
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 5
Source Data for Figure 6
Source Data for Figure 7
Source Data for Figure 8


