Abstract
The diagnosis of celiac disease (CD) no longer rests on a malabsorptive state or severe mucosal lesions. For the present, diagnosis will always require the gold-standard of a biopsy, interpreted through its progressive phases (Marsh classification). Marsh classification articulated the immunopathological spectrum of gluten-induced mucosal changes in association with the recognition of innate (Marsh I infiltration) and T cell-based adaptive (Marsh II, and the surface re-organisation typifying Marsh III lesions) responses. Through the Marsh classification the diagnostic goalposts were considerably widened thus, over its time-course, permitting countless patients to begin a gluten-free diet but who, on previous criteria, would have been denied such vital treatment. The revisions of this classification failed to provide additional insight in the interpretation of mucosal pathology. Morever, the subclassification of Marsh 3 imposed an enormous amount of extra work on pathologists with no aid in diagnosis, treatment, or prognosis. Therefore, it should now be apparent that if gastroenterologists ignore these sub-classifications in clinical decision-making, then on that basis alone, there is no need whatsoever for pathologists to persist in reporting them. Since new treatments are under critical assessment, we might have to consider use of some other higher level histological techniques sensitive enough to detect the changes sought. A promising alternative would be to hear more voices from imaginative histopathologists or morphologists together with some more insightful approaches, involving molecular-based techniques and stem cell research may be to evaluate mucosal pathology in CD.
Keywords: Celiac disease, classification, duodenal mucosa, histopathology, morphometry
INTRODUCTION
Diagnosis includes collation of data regarding patients’ clinical histories and initiation of appropriate treatment. The field of internal medicine has immensely expanded in the post-war years owing to both improved knowledge and the advances in techniques for sampling tissues from conscious subjects. Biopsies of the bone marrow, kidney, and liver can now be performed (1–3), enabling substantial consolidation of clinicopathological correlations.
The gastric mucosa was first obtained in 1949 by Wood (4), closely followed by intestinal biopsies (5) and severe celiac lesion recognition by Shiner. Histological interpretations of “atrophy” (6) were most likely influenced by true gastric atrophy of pernicious anemia. The resemblance between the two lesions is admittedly striking. Addisonian pernicious anemia is associated with life-long and irreparable loss of intrinsic factor secretion, which is exemplary of a true autoimmune damage to the gastric mucosa; in contrast, mucosal changes of gluten sensitivity are not due to autoimmune destruction as they are reversible. Nonetheless, parenthetically, previous investigations have shown that approximately 50% patients with celiac disease (CD), dermatitis herpetiformis (DH), or tropical sprue were hypochlorhydric or achlorhydric (7) (Table 6.4; P. 179).
Subsequently, Shiner reported additional subjective categories of “partial,” “subtotal,” and “total” villous atrophy (8), although the precise criteria were never defined, and against which uncertainties arose. First of all, there is considerable confusion regarding the variable and sometimes modest changes seen across the duodenum, jejunum, and ileum in CD and cognate enteropathies, such as tropical sprue (9); second, Anderson’s demonstration of villous recovery with gluten restriction (10) strongly proved that atrophy was not a causal mechanism. Nonetheless, based on flawed histological interpretation, the inappropriate diagnostic usage of the term “atrophy” has survived for over 60 years.
We might question the reason for the continued use of this term in the present day diagnostic histopathology, thereby rendering it an entirely subjective enterprise. Imagine the following: without any universally agreed quantitative guidelines, how do we distinguish “subtotal” from partial villous atrophy? Finally, whether all followers of Shiner realize this point, it should be understood that Shiner’s definition of “atrophy” inevitably commits its users to the false concept that each villus is individually reduced to nothing as the mucosa deteriorates. Instead, the remains of villi appear to constitute a various structures (convolutions and mosaic plateau) down the line, with the surface epithelia lying well above the openings of individual crypt tubes (Figure 1).
Figure 1.
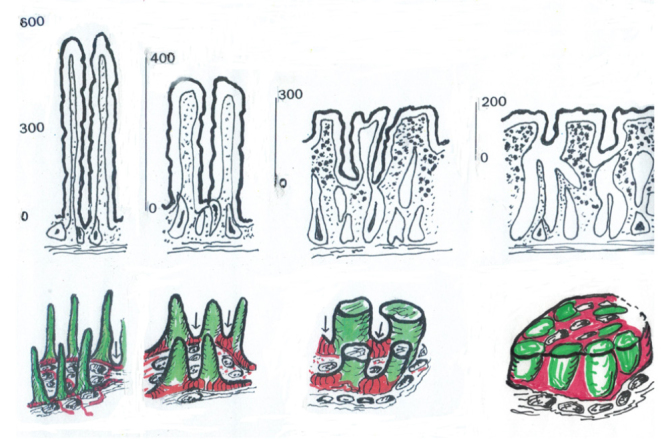
These diagrams represent schematised accounts of the mucosal progression to “flattening”. The upper panel (a) gives some realistic indication of the reductions in mucosal height across the spectrum, from normal villi to the final mosaic platform.
The lower panel (b) provides a coloured description of the gradual upward growth of the inter-villous ridges (red) and their relationship to the villi (green) which are progressively shortened, by ~65–70%. Ultimately, the mosaic comprises a mixture of hypertrophied ridges which amalgamate the adjacent reduced villi: this seems to be the only intelligible way of explaining how mosaic plateaux are formed. Mosaic platforms, although containing villous cells, do not comprise individual villi, and cross sections of these plateaux must not be misinterpreted as such. The differential heights (a) give the proper interpretative clue.
(a) Surfing the surface: Insights from above
Two further insights regarding the surface microstructure, with both interpretative and diagnostic values, have been provided. The first was based on wax modeling of “control” mucosa (11) and revealed that individual crypts open into “basins;” the latter was described as collectively opening into larger, surface wells approximately 200 μm in diameter. This complex microgeometry indicates that crypts open onto the basal mucosal surface neither directly nor vertically; instead, they are often angulated in their upper reaches while connecting to their respective basins. Such long-forgotten observations contraindicate the concept of a villus-crypt unit, because given that a single villus may be surrounded by 20 crypt tubes or more, the question of which villus with respect to which crypts arises (12). In the two-dimensional approach of conventional histopathology, the small intestinal mucosal architecture is characterized by villi and crypts lying side-by-side on the vertical plane, thereby justifying the concept of villus/crypt ratios for practicing pathologists.
The second observation highlighted inter-villous ridges and their capacity for progressive, upward growth in severer lesions (13,14). These ridges form a series of crisscross elevations across the mucosal surface, leading to a mucosal “ground plan” from which villi originate, as revealed by scanning electron microscopy (EM) (15) (Figure 1, 2). Moreover, as flattening occurs, their thickening coupled with upward growth leads to the amalgamation of villi, a process that avoids necrosis of intervening epithelia via the amalgamation of adjacent villi (Figure 1a, b).
Figure 2.
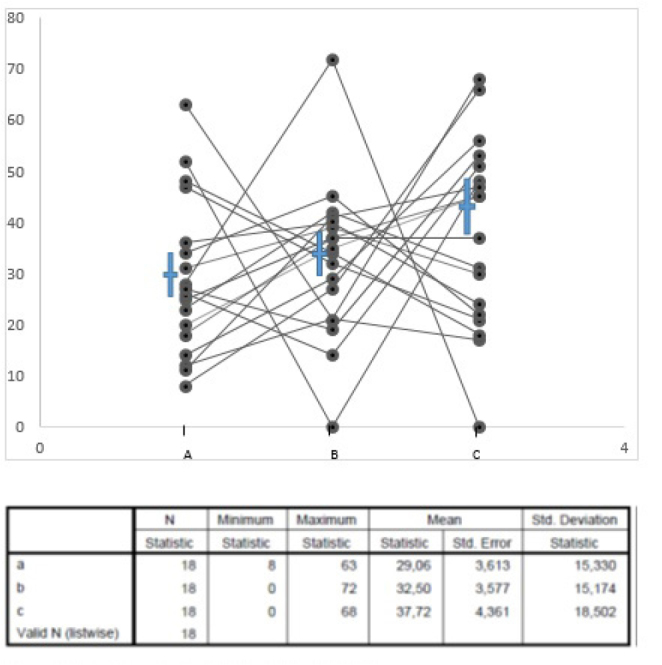
This is a plot, expressed as percentages on vertical axis, of over 3,000 biopsies classified as Marsh III a, b, c. The means (non-parametric) do not differ. However, the spread of individual values for each set of data is very wide, revealing their enormous variation in practice. Such classifications, however, have never been shown to have any real meaning.
(b) Forget mucosal atrophy
During the process of villous amalgamation, linear convolutions and finally distinct islands or a mosaic plateau (16) with variable heights of approximately 80–200 μm are formed (Figure 1). Lying immediately below their surface, epithelial coverings are healthy enterocytes expressing the specific brush border enzymes alkaline phosphatase and esterase (17). This critically emphasizes that convolutions and mosaic plateau comprise a villous territory, although villous projections are obviously not present (or visible) at this stage of mucosal remodeling despite many inaccurate claims to the contrary.
In summary, it should be clearly understood that the interpretation of mucosal histopathology has been based on a flawed supposition of “atrophy.” Moreover, the current widespread but indiscriminate use of the term “atrophy” in mucosal interpretation and reporting no longer reasonably connotes the pathological process. Other informal terms, such as villous attenuation or attrition, shortening, and amalgamation, offer a much superior understanding and are thus obviously preferable. In other words, despite continuing claims or beliefs into that possibility, villi are never individually reduced to nothing. Hence, the apparent demonstration of villi of 1 μm in height by Taavela (18) is almost unbelievable. The epithelial covering alone would require at least a 35-μm (19) height; in contrast, a scanning EM has never revealed such miniscule structures. To allege otherwise is pure fantasy.
Clinical- and research-based consequences
Beyond Shiner’s legacy: Perhaps
From the beginning, celiac mucosae were deemed flat, although it was never argued upon how that flattening came about. The picture was subsequently further complicated by lymphocytic infiltrates of the epithelium in DH (20). Here, the interpretational problem was to discover whether and how this paradoxical, gluten-induced lesion was related to or even connected with the flat celiac mucosa. However, other clues, such as the fact that symptomatic patients thought to have CD might first present with “normal” mucosae that flatten at a later period of follow-up or reinvestigation, were also published (21,22); over time, loading treated patients with increasing amounts of gluten demonstrated a repeatable series of changes comprising villous infiltration by small lymphocytes, gradual crypt enlargement, and then flattening (23,24). Relatives of patients with CD could have (unrecognized) infiltrated mucosae and be largely asymptomatic (25); further, an infiltrated mucosae with crypt hyperplasia (26) was the outcome in a mild, murine GVH model. All such changes were thought to be consistent (at the time) with T-cell-mediated activity (27).
(a) New classification reflecting mucosal immunopathology
Based on the foregoing, it became evident (to one of the authors) that celiac mucosae exist in various forms; this indicated that in reflecting those changes, mucosae must necessarily evolve over time via “normal,” pre-infiltrative, or infiltrative±crypt hypertrophy and finally to a villous phase. Indeed, we know of no exceptions to this rule. This new classification (28,29) was published 30 years after the first biopsies were performed (1960), and the findings were corroborated by time/dose oral challenges (24,25). Moreover, abundant data are now available that attest to large numbers of patients with minimal-change lesions (Marsh 0 and I/II) who whether symptomatic have experienced great improvement and shown mucosal normalization following gluten restriction.
Any reference made by authors to early-change biopsies being described as non-specific, non-celiac, or inconsistent with published guidelines must be rejected. Indeed, patients with minimal-change biopsies not only have a higher mortality (30), but may also suffer severe symptoms and malabsorptive defects despite minimal histological changes (31). This reaffirms the age-old adage that symptoms bear no relationship with the extent of tissue damage (32–34). A list of differential diagnoses associated with mucosal changes has been published, incidentally revealing that flat (Marsh III) lesions are also noticeably non-specific as well; perhaps, the categories listed should help those with continuing doubts or even uncertainties (Table 1).
Table 1.
Conditions associated with mucosal remodelling
| Infections | Autoimmune associations |
| • HIV | • MVID |
| • Tb | • Autoimmune enteropathy |
| • Whipple’s disease | • Collagenous enteropathy |
| • Bacterial overgrowth | • IDDM |
| • Immunodeficiencies | • Cerebellar ataxia |
| • Helicobacter pylori | • Dermatitis herpetiformis |
| • Postinfectious diarrhoea | • Sarcoidosis |
| Antigen-based | Miscellaneous |
| • Tropical Sprue | • Malnutrition |
| • GVHD | • ZES enteropathy |
| • Parasitic | • Lymphoma |
| • Giardiasis | • Intestinal lymphangiectasia |
| • Cryptosporidiosis | • Eosinophilic enteritis |
| • Enterocytozoon bieneusi | • Crohn’s disease |
| • Collagenous enterocolitis | |
| Drug-induced | Food antigens |
| • Vinca | • Gluten |
| • Olmesartan | • Milk |
| • Neomycin | • Egg |
| • Cytotoxic drugs | • Soya |
| • Azothiopyrin | • Fish |
| • Colchicine | |
| • Ipilimumab |
We agree with Corazza and Villanacci (36) that the Marsh II stage (villous infiltration+crypt hypertrophy) had not been proven to be diagnostically beneficial; however, we retain it as reminder of its apparent pivotal role in heralding progression toward the end stages of mucosal remodeling (Marsh III), as panoramically displayed elsewhere (37). Few investigators seem to have comprehended that the Marsh classification is structural and does not address temporal characteristics. This finding is crucial considering that the Marsh II stage may be transient and thereby less likely to be encountered. It is also possible that the original Marsh II stage needed better clarification (38,39) to permit easier distinction between shortened villi with or without crypt hyperplasia. We know from public demonstrations that this stage is poorly recognized, thereby considerably bearing on the quality of histopathological training in mucosal interpretation. Secondly, but more importantly, we do not know whether the Marsh I lesion always progresses at some future time or whether it can also regress like lymphocytic insulitis in (female) NOD mice (40). The possibility that circulating levels of islet-derived 3-alpha predict progression from the Marsh I/II to III stages is intriguing (41).
Taavela (19) reported that the Marsh classification “is often too imprecise (how often, and in what way, might we ask?) and ignores minor but significant changes in mucosa.” Unfortunately, the “minor but significant changes” have not been highlighted. Moreover, Taavela considers that “validated (by whom?) continuous variables” are needed for mucosal studies. Curiously, the Marsh classification is reportedly sufficiently pliant for challenge purposes (42–44). Note that this classification (in principle) could never have evolved by repeating Taavela’s “continuous variables” in the employment of (suspect) crypt/villus ratios (in practice) and that the principle has not restricted the determination of variations in the mucosal structure through time or across patients’ disease history, irrespective of severe childhood (45,46) or adult (25) enteropathies. There is nothing original or magical about continuous variables; everyone measures changing histological features all the time!
(b) Mud in the waters: Austrian style with Oberhuber
The simplistic nature of the Marsh classification with two or three basic categories was unfortunately confused by the later attempts to standardize it (47). This merely substituted Shiner’s atrophy categories with letters (48). Corazza and Villanacci (37) challenged the usefulness of the categorization that increased pathologists’ burden to 7–8 categories. They showed that histopathologists’ performances were poorly executed with this unwanted approach when compared across institutions, a confusion later upheld in further inter-observer comparisons (49–51). Interestingly, the scheme was avidly taken up and applied universally even by specialist units worldwide, although it was never subject to any severe critical appraisal. We have plotted the subclassification of Marsh III lesions (Figure 2), revealing that there is a considerable variation in the assessments across units but an overall equality in each sub-division. Despite the extra work imposed on pathologists, to our knowledge, there is no study that crucially aids the diagnosis, treatment, or prognosis in these subcategories. Therefore, it should now be blindingly apparent that if gastroenterologists ignore these subclassifications in clinical decision-making, there is no need whatsoever for pathologists to exclusively continue reporting the classifications. Many enlightened histologists have already started to opt out.
Many recent studies have now confirmed that this revision lacks value and in fact does not reflect progressive mucosal deterioration. Degenerate or atrophic villi in Marsh III lesions have never been documented using scanning EM (52); there is no increase in CD3+ intraepithelial lymphocytosis (IEL) across these sub-divisions (53–54) or changed titers of tTG-IgA/IgG or DGP antibodies (55) as the mucosa flattens; moreover, a logical regression analysis failed to find diagnostic help from any subcategory (56). Considering costs, pathologists should stop wasting their time in chasing these so-called standardizations, which we daringly call “classification chaos” (39) because they have no clinically useful contributions.
Mucosal histopathology: Further interpretations and misinterpretations
We have reviewed key articles illustrating the complex three-dimensional (3D) morphology of the small intestinal mucosa. In our opinion, a complete understanding of this literature is crucial if the marked changes associated with its diseases are to be appropriately assessed; their diagnostic relevance might therefore be immediately apparent. Thus, certain outcomes need attention.
(a) Handling biopsies and beyond
We whole-heartedly agree with Green (57) that if endoscopy is worth performing, it should be performed together with all the ancillary activities. Besides endoscopic amplification, it is important that at least six biopsies are obtained from several regions of the duodenum under optimal conditions (58), and sufficient biopsies should be obtained for all necessary procedures, as might be anticipated in a prospective study (59,60). Indeed, we would urge units specialized in CD research or experienced histopathologists capable of reading mucosal biopsies that a technician should be employed to undertake perform biopsy examination and preparation, as exemplified by Biagi’s unit (42).
To overcome this problem, more fragile forceps biopsies should be placed in the organ culture medium at body temperature to encourage relaxation and unrolling; during this procedure, surface mucus may float free of the tissue. Each fragment should be carefully examined using dissecting microscopy, and its features should be precisely recorded and then placed on a firm fabric base and fixed or frozen. As stated already, surface microscopy forms a critically important part of mucosal evaluation. We would suggest, contra Green, that there are indeed not impossible to deploy or not worthwhile but utilizable procedures concerning specimen preparation.
(b) What is “Normal”?
In recent years, the trend to report biopsies as normal has considerably grown, although no supporting quantitative data are provided or even considered necessary; such statements are therefore purely subjective and perhaps of reduced value. Admittedly, the data are extraordinarily scarce (Table 2), although it is hoped that the current Third Study of the Bucharest Consensus of histopathologists will at least rectify these lacks. As noted recently (60), the villi size is dependent on many factors (Table 3) rarely considered in studies. The recent history of the patient may have a considerable bearing on how any random biopsy is interpreted, particularly whether categorized as a disease-control or used in comparative measurement studies.
Table 2.
Mucosal morphometric parameters
| Villus Height (μm) | Crypt Depth (μm) | Epithelial Cell Height (μm) | |
|---|---|---|---|
| Shiner (1959) | 430 | - | 34 |
| (320–570) | (29–41) | ||
| Booth (1961) | 600 | 100 | - |
| Crowe & Marsh (1994) | 510 | 110 | 37 |
| (30–43) | |||
| Catassi (2007) | 372 | 135 | - |
| (340–385) | (125–150) | - | |
| Cummins (2011) | 753 | 200 | - |
| (731–775) | |||
| Vazquez (1996) | 403 | 173 | - |
| (380–420) | (165–81) |
Morphometric data extracted from a very limited and somewhat disparate set of figures. Since villi do not exist when mucosal surface structure is <300–350μm, data on so-called “villi” for Marsh III lesions hardly seem to make sense. The figures here are provisional because there is no accurately-based criterion for distinguishing the upper limit of individual crypt tubes – or the lowest limit of a villus. There is also a very wide disparity in the measurements of crypt depth. Clearly, new data are required to fill these deficits: deficits which could well improve morphometric analyses of the intestinal mucosa, especially the poorly-conceived notion of crypt/villus ratios. (Papers not included in main reference list are: Catassi et al. Am J Clin Nutr 2007: 85; 160–6; Vasquez et al. Eur J Gastro Hepatol 1996: 8; 15–21).
Table 3.
Factors influencing villous height
| • Age | • Diurnal variation |
| • Ethnicity | • Parasites |
| • Gender | • Infections |
| • Genetic background | • Drug ingestion |
| • Geo-cultural habitat | • Allergy/Atopy |
| • Handling artefacts- | • Microbiome - |
| • forceps trauma | • Local intestinal |
| • fixation contraction | • Maternal |
| • embedding medium | • Diet |
| • imperfect sectioning | • Motility/ Peristalsis |
| • Food sensitivities |
There is one important distinction to be made based on mucosal microdissections (61), which reveal marked differences between the villous height of disease-control mucosae and the normalcy of the pre-infiltrative stage or Marsh 0 stage (Table 2). Given the difference, the implication would be that pathological factors causing some degree of attrition in the overall height of Marsh 0 villi are already operative. This implication should not be surprising given the EM evidence of enterocyte and microvillus damage (62), elevated 1-FABP levels (63,64), deficits in disaccharidase (65), and anti-TG2 antibodies along epithelial and microvascular basement membranes (66), together with the continued activation of chemokines and their respective genes at this early phase of mucosal reorganization (67,68). Likewise, similar changes precede the clinical presentation of inflammatory bowel disease (69). In both stages, disease-control or Marsh 0 specimens, villi, and crypts should be easily identifiable and separable by microdissection. Further confirmation regarding these critical differences and proofs of inferences that Marsh 0 lesions and their villi are subject to pathological influences are needed.
(c) Morphometry and mucosal pathology
(i) Concerning ratios in biopsy interpretation
Most of the biopsy interpretation is based on ratios between two measured commodities. These procedures work; however, we suggest them only because the end-result is anticipated if not already known.
IEL are said to be probably raised in Marsh III lesions. A computerized morphometric analysis showed a percentage fall in total IEL that was much less than the loss of enterocytes (70). Comparatively, an infiltrated lesion has 8 IELs per enterocyte; yet, there are still 3 IELs per enterocyte with a flattened flat (20); therefore, the mucosa subjectively appears to be infiltrated. Another related factor is that IELs in the flat mucosae are considerably larger than those in control mucosae. However, larger objects create more profile-discs in section, thereby resulting in higher apparent numbers when counted (71). This is an important observation because it affirms the long-lost principle that histological sections do not map directly onto the 3D tissue that they purport to represent. While we tolerate this conundrum, there are now stringent norms with which it is possible to work (38,53,72,73); an upper control range centered around 25 IELs/100 enterocytes, depending on observer error, has been set.
Changes in the numbers of cell types within the lamina propria are often subjectively gauged via a comparison of the fields of view. The problem here is that lamina propria volumes vary across the spectrum between the control and Marsh III lesions (73–75). Thus, the area surveyed for assessing changes in the inflammatory cell content must be adjusted to compensate for altered volumes of distribution (76), as we did by using an external reference area (104 μm2) of the muscularis mucosae. The absolute values for any cell type(s) under investigation can be obtained in this manner and be usefully deployed in comparative work. Such approaches additionally bypass subjectively-based observer terms, such as “mild,” “moderate,” or “severe” increases.
The C/V ratios are frequently used in mucosal evaluations, usually without accompanying data on each pair of measurements. Here again, despite, these ratios seem to work despite simultaneously involving two moving parts because the outcome is generally known, although that alone would not necessarily guarantee good quality results.
For example, we are invariably made to believe that a C/V ratio >2 indicates a normal mucosa. However, given an average crypt depth of 100 μm, a ratio of 2 implies villi of approximately 200 μm in height, which is anatomically impossible. As stated by Cummins, ratios for normalcy should be in a much higher range bracket (77–79) of 3.5–6.2 or 3.6–3.9, bearing in mind his use of high values (200 μm) for mean crypt depths. The question is how normal are the villi being measured? The subjectivity of C/V ratios is further highlighted by the fact that individual crypt tubes often enter their respective basins at an angle to ensure that they cannot be used, whereas the junctional zone between the start of a villus and the upper margins of a crypt tube are ill-defined and hence extremely dependent on subjective assessments. One alternative could determine the villous fraction of Marsh III mucosae either with monoclonal antibodies against enterocyte alkaline phosphatase or esterase or by identifying the lowest cells in the villous epithelium expressing their corresponding gene-activated mRNAs.
(ii) Use of biopsies in evaluating other ancillary treatments
Two recent studies concerning the effects of non-dietary therapies for CD have been published (79,80) and are open to comments. Here, we only examined biopsies as monitors for these novel treatments. Of note, both studies applaud old-fashioned IEL counts and C/V ratios. Regarding the latter, Ludvigson’s committee concluded that only “validated morphometric analysis” [was able to] “produce excellent reproducibility and validity,” with specific reference to Taavela (19) who, with several other departmental colleagues, comprised its membership. The two panels chose not to refer to other studies (except Taavela’s) concerning the application of sound morphometric pursuits variously employed over the years (61,73,75,81) but which importantly would need to satisfy the Weibel’s strict criteria (82).
Both studies seem to confuse evolutionary schemes (i.e., Marsh in particular) from morphometric techniques. However, both studies aimed at answering research questions that might require higher-level morphometric procedures and techniques.
We do not make these criticisms lightly because if new treatments are under critical assessment, we might have to consider the use of some other higher-level histological techniques sensitive enough to detect the changes sought. Furthermore, it needs to be considered whether any approach would start with patients having marked mucosal changes but not under dietary control or those already under dietary restrictions and shown some mucosal improvement. It seems a matter of profound regret that more voices from imaginative histopathologists or morphologists were not heard in those discussions and that some more insightful approaches involving molecular-based techniques did not arise from the ad hoc panels. We believe that stem-cell research may be a promising alternative to evaluate mucosal pathology in patients with CD. The results of few studies have suggested that the intestinal stem-cell compartment is depleted in patients with active CD, which seems corroborate with the concept of deranged regenerative potential of the mucosa (83).
CONCLUSION
The diagnosis of CD no longer rests entirely on an overt malabsorptive state or on the presence of a severe mucosal lesion. As of now, diagnosis will always require a biopsy, which is the gold standard, interpreted through its progressive phases as defined, for example, using the Marsh classification. It should be noted that the latter (without the added encumbrance of Oberhuber’s unnecessary modifications) is a static display of the prominent phases in gluten-induced host response. We have no cohesive ideas about the temporal aspects of that transition. Only further extensive case studies would perhaps help answer the questions whether (i) regression of earlier Marsh stages does occur and (ii) progression toward more marked changes is inevitable, which may not be the case.
For example, the Marsh I (infiltrative) lesion is characteristic of many cases of DH, whereas a similar innate response is seen in giardiasis and tropical sprue (in what was earlier mis-interpreted as a separate nosology termed “tropical enteropathy”).
As a comprehensive scheme, reflecting both the innate and adaptive responses to antigenic stimulation, the Marsh classification covers the necessary landscapes. In addition, it has served in evaluating dynamic tissue reactions over a set time period, for example, part of a diagnostic challenge routine. However, this classification does not detract from other forms of tissue analysis, although the drawbacks to some of the commoner-used processes have been discussed in this article. Regarding ratios, many papers still fail to provide the background measurements from which the secondary data described herein have been derived. Furthermore, there is a critical need to structurally and functionally gain precise definitions of a villus and to use molecular techniques to specify precisely where villi begin and crypts terminate. Until we have the said data, C/V ratios can never be considered seriously in mirroring changed mucosal structure.
This article has briefly drawn attention to the divide between service histology and the sterner requirements for mucosal studies in answering specific research-based questions. Indeed, we feel that if these changes to technique cannot be generally updated, the advances of molecular genetics and other serological discoveries will overtake those not prepared to advance and therefore lag. Moreover, individuals without much interest in microscopy and histological examinations would not be sorry to discontinue commerce with histopathologists. The question remains who will measure up to these newer demands on performance and place histological approaches, including image analysis and even the exploration of more robust molecular techniques, to CD diagnosis and its further enquiries onto a firmer, certain, and more enlightened footing. Of course, that is another type of challenge; however, who will be capable of responding?
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - A.E., M.N.M.; Design - A.E., M.N.M.; Supervision - A.E., M.N.M.; Analysis and/or Interpretation - A.E., M.N.M.; Literature Search - A.E., M.N.M.; Writing Manuscript - A.E., M.N.M.; Critical Review - M.N.M.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Morena M, Gatti R. A history of bone marrow trephine. Immunol Allerg Clin North Am. 2010;30:1–15. doi: 10.1016/j.iac.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Iversen P, Brun C. Aspiration biopsy of the kidney. Am J Med. 1951;11:324–30. doi: 10.1016/0002-9343(51)90169-6. [DOI] [PubMed] [Google Scholar]
- 3.Menghini G. One-second needle biopsy of the liver. Gastroenterology. 1958;35:190–9. [PubMed] [Google Scholar]
- 4.Wood I, Doig R, Motteram R, Hughes A. Gastric biopsy: report on fifty-five biopsies using a new flexible gastric biopsy tube. Lancet. 1949;1:18–21. doi: 10.1016/S0140-6736(49)90344-X. [DOI] [PubMed] [Google Scholar]
- 5.Crosby W, Kugler H. Intraluminal biopsy of the small intestine. The intestinal biopsy capsule. Dig Dis Sci. 1957;2:236–41. doi: 10.1007/BF02231100. [DOI] [PubMed] [Google Scholar]
- 6.Sakula J, Shiner M. Coeliac disease with atrophy of the small-intestine mucosa. Lancet. 1957;2:876–77. doi: 10.1016/S0140-6736(57)90010-7. [DOI] [PubMed] [Google Scholar]
- 7.Marsh MN. Mucosal pathology in gluten sensitivity. In: Marsh MN, editor. Coeliac Disease. Oxford: Blackwell Scientific Publications; 1992. p. 136. [Google Scholar]
- 8.Shiner M, Doniach I. Proc World Congr Gastroenterol. Washington [DC] Baltimore: The Williams & Wilkins Company; 1959. Histopathologic studies in steatorrhea; p. 586. [PubMed] [Google Scholar]
- 9.Baker S. Idiopathic small intestinal disease in the tropics. In: Chandra RK, editor. Critical Reviews in Tropical Medicine. Vol. 1. New York [NY]: Plenum Press; 1982. p. 197. [DOI] [Google Scholar]
- 10.Anderson C. Histological changes in the duodenal mucosa in coeliac disease: reversibility during treatment with a gluten-free diet. Arch Dis Child. 1960;35:419–27. doi: 10.1136/adc.35.183.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocco A, Dohrmann M, Hendrix T. Reconstruction of normal jejunal biopsies: three-dimensional histology. Gastroenterology. 1996;51:24–31. [PubMed] [Google Scholar]
- 12.Ensari A, Marsh MN. Exploring the Villus. Gastroenterology Hepatology from Bed to Bench. 2018;11:181–90. [PMC free article] [PubMed] [Google Scholar]
- 13.Creamer B, Leppard P. Post-mortem examination of a small intestine in the coeliac syndrome. Gut. 1965;6:466–71. doi: 10.1136/gut.6.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loehry C, Creamer B. Three-dimensional structure of the human small intestinal mucosa in health and disease. Gut. 1969;10:6–12. doi: 10.1136/gut.10.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toner P, Carr K, Ferguson A, Mackay C. Scanning and transmission electron microscopic studies of the human small intestinal in health and disease. Gut. 1970;11:471–81. doi: 10.1136/gut.11.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes R, Hourihane O’B, Booth C. The mucosa of the small intestine. Postgrad Med J. 1961;37:717–24. doi: 10.1136/pgmj.37.434.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Padykula H, Stauss E, Ladman A, Gardner F. A morphological and histochemical analysis of the normal human jejunal epithelium in nontropical sprue. Gastroenterology. 1961;40:735–65. [PubMed] [Google Scholar]
- 18.Taavela J, Koskinen O, Huhtala H, et al. Validation of morphometric analyses of small-intestinal biopsy readouts in celiac disease. PloS ONE. 2013;8:e76163. doi: 10.1371/journal.pone.0076163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe P, Marsh MN. Morphometric analysis of small intestinal mucosa: IV - Determining cell volumes. Virchows Archiv A Pathol. 1993;422:459–66. doi: 10.1007/BF01606454. [DOI] [PubMed] [Google Scholar]
- 20.Fry L, Seah P, McMinn R, Hoffbrand V. Lymphocytic infiltration of epithelium in diagnosis of gluten-sensitive enteropathy. Br Med J. 1972;3:371–374. doi: 10.1136/bmj.3.5823.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maki M, Holm K, Koskimies S, et al. Normal small bowel biopsy followed by celiac disease. Arch Dis Child. 1990;65:1137–41. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh MN. Studies of intestinal lymphoid tissue: XIII - Immunopathology of the evolving celiac sprue lesion. Pathol Res Pract. 1989;185:774–7. doi: 10.1016/S0344-0338(89)80237-7. [DOI] [PubMed] [Google Scholar]
- 23.Leigh R, Marsh MN, Crowe P, et al. Studies of intestinal lymphoid tissue: IX- Dose-dependent, gluten-induced lymphoid infiltration of coeliac jejunal epithelium. Scand J Gastroenterol. 1985;20:715–9. doi: 10.3109/00365528509089201. [DOI] [PubMed] [Google Scholar]
- 24.Marsh MN, Loft D, Garner V, Gordon D. Time/dose responses of celiac mucosae to graded oral challenges with Frazer’s fraction III of gliadin. Eur J Gastroenterol Hepatol. 1992;4:667–73. [Google Scholar]
- 25.Marsh MN, Bjarnason I, Shaw J, et al. Studies of intestinal lymphoid tissue: XIV - HLA status, mucosal morphology, permeability and epithelial lymphocyte populations in first degree relatives of patients with coeliac disease. Gut. 1990;31:32–6. doi: 10.1136/gut.31.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mowat A, Ferguson A. Intraepithelial lymphocyte counts and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982;83:417–23. [PubMed] [Google Scholar]
- 27.Ferguson A. Models of immunologically-driven small intestinal damage. In: Marsh MN, editor. The Immunopathology of the Small Intestine. Chichester: Wiley; 1987. p. 239. [Google Scholar]
- 28.Marsh MN. Grains of truth: evolutionary changes in small intestinal mucosa in response to environmental antigenic challenge. Gut. 1990;31:111–4. doi: 10.1136/gut.31.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh MN. Gluten, major histocompatibility complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. doi: 10.1016/0016-5085(92)91819-P. [DOI] [PubMed] [Google Scholar]
- 30.Ludvigsson J, Montgomery S, Ekbom A. Small-intestinal histopathology and mortality in celiac disease. JAMA. 2009;302:1171–8. doi: 10.1001/jama.2009.1320. [DOI] [PubMed] [Google Scholar]
- 31.Zanini B, Caselani F, Magni A, et al. Celiac disease with mild enteropathy is not mild disease. Clin Gastroenterol Hepatol. 2013;11:253–8. doi: 10.1016/j.cgh.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Dickey W, Hughes D, McMillan S. Patients with serum IgA endomysial antobodies and intact duodenal villi: clinical characteristics and management options. Scand J Gastroenterol. 2005;40:1240–3. doi: 10.1080/00365520510023747. [DOI] [PubMed] [Google Scholar]
- 33.Ludvigsson J, Brandt L, Montgomery S. Symptoms and signs in individuals with serology positive for celiac disease but normal mucosa. BMC Gastroenterol. 2009;9:57–67. doi: 10.1186/1471-230X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monzon H, Forne M, Gonzalez C, et al. Mild enteropathy as a cause of iron-deficiency anaemia of previously unknown origin. Dig Liver Dis. 2011;43:448–53. doi: 10.1016/j.dld.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Rostami K, Aldulaimi D, Holmes G, et al. Microscopic enteritis: The Bucharest Consensus. World J Gastroenterol. 2015;21:2593–604. doi: 10.3748/wjg.v21.i9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corazza G, Villanacci V. Coeliac disease: an update for pathologists. J Clin Pathol. 2005;58:573–4. doi: 10.1136/jcp.2004.023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsh MN, Heal C. Evolutionary developments in interpreting the gluten-induced mucosal celiac lesion: an Archimedian heuristic. Nutrients. 2017;9:1–20. doi: 10.3390/nu9030213. [DOI] [Google Scholar]
- 38.Ensari A. Gluten-sensitive enteropathy (celiac disease): Controversies in diagnosis and classification. Arch Pathol Lab Med. 2010;134:826–36. doi: 10.5858/134.6.826. [DOI] [PubMed] [Google Scholar]
- 39.Özakinci H, Kirmizi A, Savas B, et al. Classification chaos in coeliac disease: Does it really matter? Pathol Res Pract. 2016;212:1174–8. doi: 10.1016/j.prp.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Hattori M, Fukuda M, Ichikawa T, et al. A single recessive non-MHC diabetogenic gene determines the development of insulitis and the presence of an MHC-linked diabetogenic gene in NOD mice. J Autoimmunity. 1990;3:1–10. doi: 10.1016/0896-8411(90)90057-Y. [DOI] [PubMed] [Google Scholar]
- 41.Giuffrida P, Biagi F, Schiepatti A, et al. Serum regenerating islet-derived 3-alpha predicts the evolution of potential coeliac diease into overt coeliac disease. Dig Liver Dis. 2018;50:e96. doi: 10.1016/S1590-8658(18)30329-3. [DOI] [Google Scholar]
- 42.Wahab P, Bart J, Crusius A, et al. Gluten challenge in borderline gluten-sensitive enteropathy. Am J Gastroenterol. 2001;96:1464–9. doi: 10.1111/j.1572-0241.2001.03812.x. [DOI] [PubMed] [Google Scholar]
- 43.Biagi F, Rondonotti E, Campanella J, et al. Video capsule endoscopy and histology for small-bowel mucosa evaluation: a comparison performed by blinded observers. Clin Gastroenterol Hepatol. 2006;4:998–1003. doi: 10.1016/j.cgh.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Leffler D, Schuppan D, Pallav K, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62:996–1004. doi: 10.1136/gutjnl-2012-302196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan P, Marsh MN, Mirikian R, et al. Chronic diarrhea and malnutrition - histology of the small intestinal lesion. J Ped Gastroenterol Nutr. 1991;12:195–203. doi: 10.1097/00005176-199102000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan P, Marsh MN. Small intestinal mucosal histology in the syndrome of persistent diarrhoea and malnutrition: a review. Acta Paediatr Scand. 1992;(Suppl 381):72–7. doi: 10.1111/j.1651-2227.1992.tb12375.x. [DOI] [PubMed] [Google Scholar]
- 47.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of celiac disease: time for a standardised report scheme for histopathologists. Eur J Gastroenterol Hepatol. 1999;11:1185. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Rostami K, Ensari A, Ciacci C, et al. Coeliac biopsies: numbers are valid, alphabets not. Gut. 2017;67:2069–70. doi: 10.1136/gutjnl-2017-315517. [DOI] [PubMed] [Google Scholar]
- 49.Arguelles-Grande C, Tennyson C, Lewis K, et al. Variability in small bowel histopathology reporting between different pathology practice settings: impact on the diagnosis of coeliac disease. J Clin Pathol. 2012;65:242–7. doi: 10.1136/jclinpath-2011-200372. [DOI] [PubMed] [Google Scholar]
- 50.Picarelli A, Borghini R, Conato G, et al. Weaknesses of histological analysis in celiac disease diagnosis: new possible scenarios. Scand J Gastroenterol. 2014;49:1318–24. doi: 10.3109/00365521.2014.948052. [DOI] [PubMed] [Google Scholar]
- 51.Corazza G, Villanacci V, Zambelli C, et al. Comparison of the inter-observer reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838–43. doi: 10.1016/j.cgh.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Marsh MN, Johnson M, Rostami K. Mucosal histopathology in celiac disease: a rebuttal of Oberhuber’s sub-division of Marsh III. Gastroenterol Hepatol Bed Bench. 2015;8:99–109. [PMC free article] [PubMed] [Google Scholar]
- 53.De Andres A, Camarero C. Distal duodenum versus bulb: intraepithelial lymphocytes have something to say about coeliac disease diagnosis. Dig Dis Sci. 2015;60:1004–9. doi: 10.1007/s10620-014-3414-x. [DOI] [PubMed] [Google Scholar]
- 54.Rostami K, Marsh MN, Johnson M, et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution and role in coeliac disease mucosal interpretation. Gut. 2017;66:2080–6. doi: 10.1136/gutjnl-2017-314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parizade M, Bujanover Y, Weiss B, et al. Performance of serology assays for diagnosing celiac disease in a clinical setting. Clin Vacc Immunol. 2009;16:1576–82. doi: 10.1128/CVI.00205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charlesworth R, Andronicus N, Scott D, et al. Can the sensitivity of the histopathological diagnosis of celiac disease be increased and can treatment progression be monitored using mathematical modelling of histological section? - A pilot study. J Adv Med Sci. 2017;62:136–42. doi: 10.1016/j.advms.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Green P. Celiac disease: how many biopsies? Gastrointest Endosc. 2008;67:1088–90. doi: 10.1016/j.gie.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 58.Lebwohl B, Kapel R, Neugot A, et al. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biagi F, Vattiato C, Burrone M, et al. Is a detailed grading of villous atrophy necessary for the diagnosis of enteropathy? J Clin Path. 2016;69:1–4. doi: 10.1136/jclinpath-2016-203711. [DOI] [PubMed] [Google Scholar]
- 60.Marsh MN, Rostami K. What is a normal intestinal mucosa? Gastroenterology. 2016;151:784–8. doi: 10.1053/j.gastro.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Cummins A, Alexander B, Chung A, et al. Morphometric evaluation of duodenal biopsies in celiac disease. Am J Gastroenterol. 2011;106:145–50. doi: 10.1038/ajg.2010.313. [DOI] [PubMed] [Google Scholar]
- 62.Sbarbati A, Valletta E, Bertini M, et al. Gluten sensitivity and ‘normal’ histology: is the intestinal mucosa really normal? Digest Liver Dis. 2003;35:768–73. doi: 10.1016/S1590-8658(03)00457-2. [DOI] [PubMed] [Google Scholar]
- 63.Not T, Ziberna F, Vatta S, et al. Cryptic genetic gluten intolerance revealed by intestinal antitransamine antibodies and response to gluten-free diet. Gut. 2011;60:1487–93. doi: 10.1136/gut.2010.232900. [DOI] [PubMed] [Google Scholar]
- 64.Vreugdenhil A, Wolters V, Adriaanse M, et al. Additional value of serum I-FABP levels for evaluating celiac disease activity in children. Scand J Gastroenterol. 2011;46:1435–41. doi: 10.3109/00365521.2011.627447. [DOI] [PubMed] [Google Scholar]
- 65.Mones R, Yankah A, Duelfer D, Bustami R, Mercer G. Disaccaraidase deficiency in pediatric patients with celiac diseasd and intact villi. Scand J Gastroenterol. 2011;46:1429–34. doi: 10.3109/00365521.2011.619276. [DOI] [PubMed] [Google Scholar]
- 66.Kaukinen K, Peraaho M, Collin P. Small-bowel mucosal transglutaminase 2-specific IgA deposits in celiac disease without villous atrophy: a prospective and randomised clinical study. Scand J Gastroenterol. 2005;40:564–72. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- 67.Brottveit M, Beitnes A-C, Tollefson S, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842–50. doi: 10.1038/ajg.2013.91. [DOI] [PubMed] [Google Scholar]
- 68.Diosdado B, van Bakel H, Strengman E, et al. Neutrophil recruitment and barrier impairment in celiac disease: a genomic study. Clin Gastroenterol Hepatol. 2007;5:574–81. doi: 10.1016/j.cgh.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 69.van Schaik F, Oldenburg B, Hart A, et al. Serological markers predict inflammatory bowel disease years before the diagnosis. Gut. 2013;62:683–8. doi: 10.1136/gutjnl-2012-302717. [DOI] [PubMed] [Google Scholar]
- 70.Crowe P, Marsh MN. Morphometric analysis of small intestinal mucosa: VI - Principles in enumerating intra-epithelial lymphocytes. Virchow Arch. 1994;424:301–6. doi: 10.1007/BF00194615. [DOI] [PubMed] [Google Scholar]
- 71.Marsh MN. Studies of intestinal lymphoid tissue: III - Quantitative studies of epitheliallymphocytes insmall intestinal mucosa of control human subjects and of patients with celiac sprue. Gastroenterology. 1980;79:481–92. [PubMed] [Google Scholar]
- 72.Cabanne A, Vasquez H, Argonz J. Clinical utility of couting intraepithelial lymphocytes in celiac disease intestinal mucosa. Acta Gastroenterol Latinoam. 2007;37:20–8. [PubMed] [Google Scholar]
- 73.Guix M, Skinner J, Whitehead R. Measuring intraepithelial lymphocytes, surface area, and volume of lamina propria in the jejunal mucosa of coeliac disease. Gut. 1979;20:275–8. doi: 10.1136/gut.20.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marsh MN, Hinde J. Morphometric analysis of small intestinal mucosa: III - Determination of lamina propria volumes, plasma cell and neutrophil populations within control and celiac disease mucosae. Virchows Archiv (Pathol Anat) 1986;409:11–22. doi: 10.1007/BF00705403. [DOI] [PubMed] [Google Scholar]
- 75.Marsh MN, Crowe P, Moriarty K, Ensari A. Morphometric analysis of intestinal mucosa: The measurement of volume compartments and cell volumes in human intestinal mucosa. In: Marsh MN, editor. Coeliac Disease: Methods and Protocols [Methods in Molecular Medicine #41] Totowa [NJ]: Humana Press; 2002. p. 125. [DOI] [PubMed] [Google Scholar]
- 76.Marsh MN, Hinde J. Inflammatory component of celiac sprue mucosa: I - Mast cells, basophils, and eosinophils. Gastroenterology. 1985;89:92–101. doi: 10.1016/0016-5085(85)90749-8. [DOI] [PubMed] [Google Scholar]
- 77.Brown I, Mino-Kenudson M, Deshpande V, Lauwers G. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa. Arch Pathol Lab Med. 2006;130:1020–5. doi: 10.5858/2006-130-1020-ILIAPP. [DOI] [PubMed] [Google Scholar]
- 78.Goldman H, Antonioli D. Mucosal biopsy of the esophagus, stomach and proximal duodenum. Hum Pathol. 1982;13:423–48. doi: 10.1016/S0046-8177(82)80026-9. [DOI] [PubMed] [Google Scholar]
- 79.Hindryckx P, Levesque B, Holvoet T, et al. Disease activity indices in coeliac disease: systematic review and recommendations for clinical trials. Gut. 2018;67:61–7. doi: 10.1136/gutjnl-2016-312762. [DOI] [PubMed] [Google Scholar]
- 80.Ludvigsson J, Ciacci C, Green P, et al. Outcome measures in coeliac disease trials: the Tampere recommendations. Gut. 2018;67:1410–4. doi: 10.1136/gutjnl-2017-314853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman B, Henry K, Paice F, Coghill N, Stewart J. Measuring the response of the jejunal mucosa in adult coeliac disease to treatment with a gluten-free diet. Gut. 1974;15:870–4. doi: 10.1136/gut.15.11.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weibel ER. Stereological Methods. Vol. 1. New York [NY]: Academic Press; 1979. [Google Scholar]
- 83.Piscaglia AC, Rutella S, Laterza L, et al. Circulating hematopoietic stem cells and putative intestinal stem cells in coeliac disease. J Transl Med. 2015;13:220–9. doi: 10.1186/s12967-015-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


