Abstract
Background/Aims
This study aims at evaluating the mean eradication rate by a systematic compilation of the studies which involved the standard triple therapy (STT) in first-line Helicobacter pylori (Hp) eradication in Turkey over a period of 10 years between 2004 and 2013 using the meta-analysis method.
Materials and Methods
The systematic compilation and meta-analysis were carried out according to the PRISMA standards defined in the Cochrane handbook. The results of full-text studies published in national and international journals in English and Turkish languages on Turkish population in a period of 10 years, from 2004 to 2013, are included in this study. The studies include open-label trials, controlled trials, treatment arms, and case series that included a triple therapy regimen consisting of standard doses of a proton pump inhibitor (PPI; omeprazole 20 mg BID, lansoprazole 30 mg BID, pantoprazole 40 mg BID, esomeprazole 40 mg BID, or rabeprazole 20 mg BID) along with clarithromycin 500 mg BID and amoxicillin 1 g BID for 7–14 days. They were scanned electronically via the search engines Google Scholar, PubMed, and the Turkish Medicine Index using specific keywords. The related keywords used were Turkey, Helicobacter pylori, infection, standard triple treatment, first-line therapy, eradication, omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, clarithromycin, and amoxicillin. Studies carried out with adults were included in the evaluation. The publication year of the studies and the included number of patients, their age, gender, treatment duration (7, 10, and 14 days), and PPIs used were evaluated by two separate gastroenterologists and biostatisticians. Studies that used at least one reliable method (histology, urea breath test (UBT), or Helicobacter pylori stool antigen (HpSA) test) four weeks after completing the treatment for the control of Hp eradication were included. Only naive patients were accepted, and patients who had previously received eradication treatment were excluded. The effectiveness of the Hp eradication was analyzed using an intention-to-treat (ITT) or per-protocol (PP) analysis.
Results
The STT regime of 45 studies complying with the inclusion criteria was evaluated. A total of 3715 patients were included in the study. Of the 3010 patients whose gender information was available, 55% were women and 45% were men; the weighted age average given explicitly in the studies was 42.14±0.67. The treatment lasted for 14 days in 42 studies, for 7 days in six studies, and for 10 days in 1 study. The eradication rates evaluated according to the ITT and PP analyses were 60% (95% CI: 56%–63%) and 57% (95% CI: 51%–62%), respectively. The rates for 7 days of treatment were 57% (95% CI: 46%–68%) and 60% (95% CI: 51%–67%) and for 14 days of treatment were 60% (95% CI: 56%–63%) and 56% (95% CI: 50%–62%), respectively. The ITT eradication rate of the only 10-day study was 78% (95% CI: 66%–86%). In the meta-regression analysis, the treatment duration, PPI, age, and gender ratio (women/men) used for the ITT analysis had no effect. The gender ratio and age were not considered in this analysis because they were not clearly stated in studies using the PP analysis. The duration of treatment and the PPI used had no effect.
Conclusion
A systematic meta-analysis of studies conducted during the period 2004–2013 in Turkey revealed that the rate of first-line Hp eradication using STT was unacceptably low, and the duration of treatment and PPI used made no difference.
Keywords: Turkey, Helicobacter pylori, infection, first-line therapy, standard triple treatment, eradication
INTRODUCTION
Helicobacter pylori (Hp) infection is the most common bacterial infection in the world. The most recommended treatment for the eradication of Hp is the standard triple therapy (STT) consisting of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin (1–5).
The first handbook for treating Hp infection was published by the National Institutes of Health in 1994. Then, the European Helicobacter pylori Study Group recommended STT as the primary treatment during the first Maastricht conference in 1997 (6). Subsequently, this treatment regimen was proposed and implemented as a general consensus in various countries for 20 years (7–11).
In Turkey, the recommendations of these handbooks were considered for approximately 20 years, and STT was used commonly.
However, the eradication rates using STT have been reported to decrease in studies published in recent years (12). Two previous studies reported an STT eradication rate of 77% (13,14), which was verified by two meta-analysis studies carried out with more than 53,000 patients (15).
As a result of the rising prevalence of antimicrobial resistance of Hp, the success rate of eradication with STT has fallen in many countries including Turkey (16–19).
The third Maastricht consensus conference recommended that the eradication rate should be more than 80% for an intention-to-treat (ITT) analysis for an effective treatment of Hp infection (5).
Kadayıfçı et al. (20) were the first in Turkey to systematically analyze the efficiency of the triple treatment in first-line Hp eradication. In their meta-analysis, the eradication rate of STT regimes in the 10-year period between 1996 and 2005 was found to be 68.8%.
Therefore, the present study aims at evaluating the rate of primary Hp eradication in Turkey from 2004 to 2013 as a continuation of the aforementioned studies. The results of research studies published on the standard triple eradication therapy were compiled to estimate the mean eradication rate of STT for the years 2004–2013 using the meta-analysis method.
MATERIALS AND METHODS
The results of full-text studies published in national and international journals in English and Turkish languages on Turkish population in a period of 10 years, from 2004 to 2013, are included in this study.
The studies were scanned electronically via the search engines Google Scholar, PubMed, and the Turkish Medicine Index using specific keywords: Turkey, Helicobacter pylori, infection, standard triple treatment, first-line therapy, eradication, omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole, clarithromycin, and amoxicillin.
The included studies were carried out with adults using a 7-, 10-, or 14-day STT consisting of a PPI (omeprazole 20 mg BID, lansoprazole 30 mg BID, pantoprazole 40 mg BID, esomeprazole 40 mg BID, or rabeprazole 20 mg BID), clarithromycin (500 mg BID), and amoxicillin (1 g BID) for the eradication of Hp. The publication year of the studies and the included number of patients, their age, gender, treatment duration (7, 10, and 14 days), and PPIs used were evaluated by two separate gastroenterologists and biostatisticians. Only studies published in a full journal format were accepted. Congress abstracts were not included. Studies that used at least one reliable method (histology, UBT, or HpSA) four weeks after the completion of the treatment for the control of the Hp eradication were included in the analysis. Only naive patients were accepted, and patients who had previously received eradication treatment were excluded. The effectiveness of the Hp eradication was analyzed using an ITT or PP analysis. The systematic compilation and meta-analysis were carried out according to the PRISMA standards defined in the Cochrane handbook (the full form of PRISMA is presented in the appendices of the manuscript).
Statistical analysis
The meta-analysis weights of the studies are shown in Table 1. Gender rates were calculated for use in the meta-regression analysis because treatments according to sex were not given separately. These rates were obtained as a ratio of the number of women/the number of men (F/M).
Table 1.
Meta-analysis weights of the studies and general information
| Author (year) | Eradication rate (ITT) | Eradication rate (PP) | Mean age | Type PPI | Treatment duration (days) | F/M |
|---|---|---|---|---|---|---|
| Guliter S (2004) | 1.66 | LAC | 14 | 0.79 | ||
| Horoz M (2004) | 1.58 | 43.00 | LAC | 14 | 1.14 | |
| Horoz M (2004) | 1.54 | 44.00 | LAC | 7 | 1.00 | |
| Altintas E (2004) | 1.80 | 4.97 | 45.70 | LAC | 14 | 1.00 |
| Altintas E (2004) | 1.82 | 47.50 | OAC | 14 | 1.13 | |
| Altintas E (2004) | 1.80 | 44.50 | PAC | 14 | 1.20 | |
| Sivri B (2004) | 1.91 | 5.09 | 42.40 | PAC | 7 | 0.54 |
| Uygun A (2004) | 1.77 | 4.47 | 45.10 | PAC | 14 | 0.90 |
| Uygun A (2004) | 1.78 | 4.47 | 48.30 | LAC | 14 | 1.29 |
| Gumurdulu Y (2004) | 1.69 | 49.00 | OAC | 7 | 1.30 | |
| Gumurdulu Y (2004) | 1.62 | 48.00 | OAC | 14 | 1.46 | |
| Guliter S (2005) | 1.98 | LAC | 14 | |||
| Erzin Y (2005) | 1.71 | 37.33 | LAC | 7 | 2.00 | |
| Ozer B (2005) | 1.92 | 41.00 | LAC | 14 | 2.04 | |
| Goral V (2005) | 1.21 | 34.36 | LAC | 14 | 1.20 | |
| Duman D.G (2005) | 5.71 | OAC | 14 | 1.10 | ||
| Koksal A.S (2006) | 1.71 | 42.00 | OAC | 10 | 2.94 | |
| Sancar M (2006) | 1.03 | 44.00 | LAC | 14 | 0.83 | |
| Sancar M (2006) | 1.03 | 42.00 | OAC | 7 | 1.33 | |
| Uygun A (2007) | 2.01 | 5.45 | 40.70 | LAC | 14 | 0.64 |
| Aydin A (2007) | 1.73 | 4.63 | PAC | 7 | ||
| Aydin A (2007) | 1.66 | 4.13 | PAC | 14 | ||
| Cindoruk M (2007) | 1.87 | 47.56 | LAC | 14 | 2.44 | |
| Uygun A (2008) | 2.04 | 5.56 | 41.20 | PAC | 14 | 0.74 |
| Oztas E (2008) | 1.80 | 4.85 | PAC | 7 | ||
| Oztas E (2008) | 1.71 | 4.37 | PAC | 14 | ||
| Mesut Z (2008) | 1.61 | ES-OM | 14 | |||
| Bektas M (2008) | 1.16 | LAC | 14 | |||
| Bektas M (2009) | 1.42 | 44.60 | LAC | 14 | 3.00 | |
| Songur Y (2009) | 1.97 | 5.40 | 46.22 | LAC | 14 | 1.94 |
| Kebapcilar L (2009) | 1.90 | 32.50 | LAC | 14 | 0.74 | |
| Soylu A (2009) | 2.06 | 43.30 | LAC | 14 | ||
| Demir M (2009) | 1.65 | 49.17 | PAC | 14 | 2.63 | |
| Aydemir S (2010) | 1.48 | LAC | 14 | |||
| Yasar B (2010) | 1.75 | 36.95 | PAC | 14 | 1.71 | |
| Abut E (2010) | 1.76 | 36.90 | PAC | 14 | 1.60 | |
| Ozdil B (2010) | 1.87 | 46.00 | LAC | 14 | 1.50 | |
| Nadir I (2011) | 2.03 | 5.55 | 40.16 | LAC | 14 | 2.07 |
| Erdogan AF (2011) | 2.01 | 44.50 | LAC | 14 | 2.00 | |
| Ermis F (2011) | 1.96 | 5.57 | LAC | 14 | ||
| Alkim H (2011) | 1.87 | 39.60 | OAC | 14 | 0.83 | |
| Alkim H (2011) | 1.85 | 38.90 | LAC | 14 | 0.74 | |
| Alkim H (2011) | 1.64 | 35.30 | RAC | 14 | 0.73 | |
| Alkim H (2011) | 1.87 | 37.60 | PAC | 14 | 0.94 | |
| Alkim H (2011) | 1.89 | 36.40 | ES-OM | 14 | 0.70 | |
| Çetinkaya ZA (2011) | 1.64 | 37.90 | OAC | 14 | 1.31 | |
| Çetinkaya ZA (2011) | 1.66 | 39.23 | PAC | 14 | 1.00 | |
| Çetinkaya ZA (2011) | 1.61 | 40.60 | RAC | 14 | 1.31 | |
| Demir M (2011) | 1.63 | 4.40 | 38.90 | PAC | 14 | 1.07 |
| Sezikli M (2011) | 1.75 | 4.73 | 43.00 | LAC | 14 | 1.67 |
| Gokturk HS (2011) | 1.94 | 5.29 | LAC | 14 | 1.03 | |
| Sezikli M (2012) | 1.76 | 4.75 | 42.70 | LAC | 14 | 2.08 |
| Polat Z (2012) | 1.93 | 5.23 | 41.00 | ES-OM | 14 | 0.92 |
| Balcilar E (2012) | 1.69 | LAC | 14 | 0.74 | ||
| Uyanikoglu A (2012) | 0.95 | 39.98 | LAC | 14 | 1.04 | |
| Onal IK (2013) | 1.88 | PAC | 14 | 3.00 | ||
| Avsar E (2013) | 2.00 | 5.39 | 42.60 | LAC | 14 | 1.36 |
| Ulasoglu C (2013) | 1.79 | 38.20 | LAC | 14 | 1.19 | |
| Ustundag Y (2013) | 1.65 | LAC | 14 |
PPI: proton pump inhibitor
The meta-regression analysis was applied according to the ITT and PP analyses (Table 9). As the age and sex ratios were precisely given in ITT studies, PP was not added to the meta-regression model. Age, sex ratios, treatment days, and PPI varieties were not found as heterogeneity sources for both analyses. However, subgroups were formed for PPIs and treatment days.
Table 9.
Meta-regression analysis results using the ITT analysis
| ITT | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Coefficient | 95% lower | 95% upper | p | p | ||
| Intercept | 0.422 | −1.663 | 2.505 | 0.692 | - | |
| Age | −0.003 | −0.052 | 0.046 | 0.898 | - | |
| Sex ratio (F/M) | −0.099 | −0.458 | 0.259 | 0.588 | - | |
| Days | 7 | Reference | 0.169 | |||
| 10 | 1.182 | −0.306 | 2.670 | 0.120 | ||
| 14 | −0.143 | −0.737 | 0.450 | 0.636 | ||
| PPI | ES-OM | Reference | 0.376 | |||
| LAC | 0.408 | −0.430 | 1.245 | 0.340 | ||
| OAC | 0.076 | −0.875 | 1.027 | 0.876 | ||
| PAC | 0.325 | −0.503 | 1.207 | 0.420 | ||
| RAC | 0.995 | −0.137 | 2.128 | 0.085 | ||
The fixed- or random-effects models were used for combining the calculated eradication rate values according to heterogeneity in the meta-analysis. For model selection, the random-effects model was used when the I2 value was significant or bigger than 25%, and the fixed-effects model was used when it was smaller than 25% and/or insignificant (21). Heterogeneity was not significant in the 7-day PPI, amoxicillin, and clarithromycin (PAC) treatment results using PPI. While the fixed-effects model results were found to be significant, the random-effects model results were found to be insignificant and are added to the table.
In the meta-analysis of the studies, the risk ratio was calculated as eradication rate/noneradication rate. According to this, if the risk ratio was greater than 1, the treatment was considered to be successful.
When applying meta-analysis, the NCSS 11 trial package program was used for calculating the risk ratio and forest plot. The Egger test was used as a significance test for the funnel plot. The study was completed using the PRISMA 2009 check list.
For the calculation of eradication rate, meta-regression results, and funnel plots, the Comprehensive Meta-Analysis V3 trial package program was used.
RESULTS
A total of 45 studies, published between the years 2004 and 2013 in the national and international journals, involving the STT application, were evaluated (Table 1) (22–65). A total of 3715 patients were included in the study. Of the 3010 patients whose gender information was available, 55% were women and 45% were men; the weighted age average given explicitly in the studies was 42.14±0.67. The treatment lasted for 14 days in 42 studies, for 7 days in six studies, and for 10 days in 1 study.
The distribution of the studies by years was as follows: six studies were published in 2004, five in 2005, two in 2006, three in 2007, four in 2008, five in 2009, four in 2010, eight in 2011, four in 2012, and four in 2013. The ITT results were given in 44 of these studies, and 17 studies provided PP results. Table 1 provides the details of these studies.
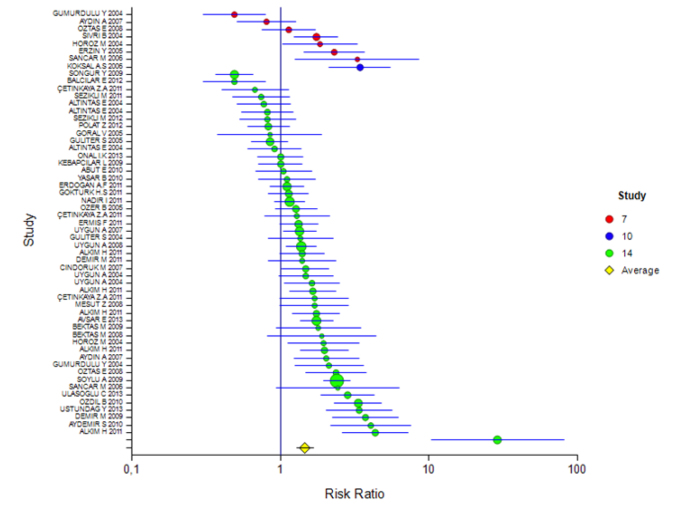
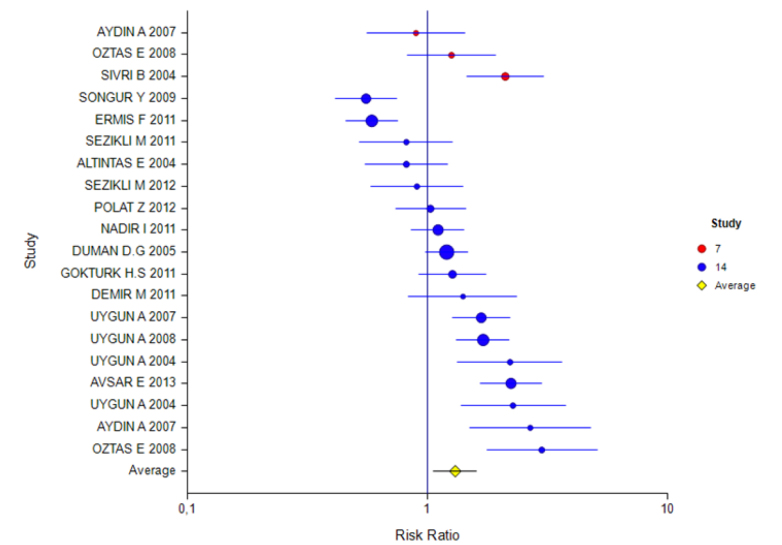
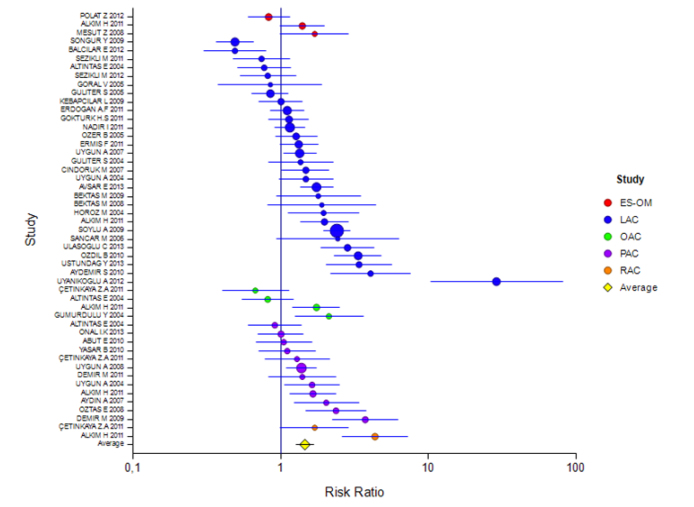
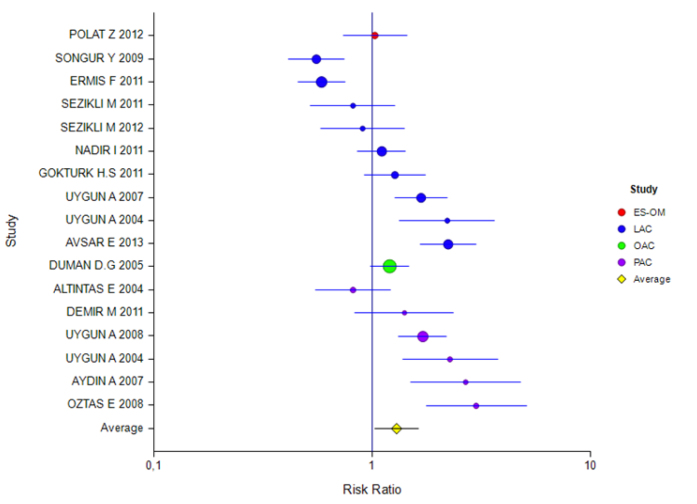
The mean rate of eradication according to the ITT and PP analyses was 60% (95% CI: 56%–63%) and 57% (95% CI: 51%–62%), respectively. The rate for 7 days of treatment was 57% (95% CI: 46%–68%) and 60% (95% CI: 51%–67%) and for 14 days of treatment was 60% (95% CI: 56%–63%) and 56% (95% CI: 50%–62%), respectively (Tables 2 and 3). The ITT eradication rate of the only 10-day study was 78% (95% CI: 66%–86%) (Figure 1, 2). The eradication results according to the PPI used in the 14-day treatment were as follows: based on ITT and PP, respectively, for esomeprazole combination, 54% (95% CI: 46%–61%) and 51% (95% CI: 39%–63%); for lansoprazole, 60% (95% CI: 54%–65%) and 53% (95% CI: 44%–62%); for omeprazole, 55% (95% CI: 41%–68%) and 55% (95% CI: 47%–62%); and for pantoprazole, 60% (95% CI: 56%–63%) and 64% (95% CI: 55%–72%). For rabeprazole, the ITT eradication rate was 75% (95% CI: 65%–83%), and the PP result was not stated (Table 4, 5; Figure 3, 4). The ITT eradication rate of the only 10-day study, in which omeprazole was used, was 78% (95% CI: 66%–86%) (Table 6; Figure 5). The PP eradication rate was not stated. For pantoprazole, the ITT eradication rate for the 7-day treatment was 50% (95% CI: 48%–64%); it was 60% (95% CI: 51%–67%) for the fixed-effects model and 58% (95% CI: 46%–70%) for the random-effects model. The ITT eradication rate was 68% (95% CI: 50%–78%) for lansoprazole and 55% (95% CI: 15%–90%) for omeprazole. No PP results were stated (Tables 7 and 8; Figure 6, 7).
Table 2.
Mean rate of eradication using the ITT analysis
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio, 7-day treatment | 1.34 | 0.87 | 2.07 | ||
| Event rate, 7-day treatment (random-effects model) | 68.95 (p=0.004) | 0.57 | 0.46 | 0.68 | 0.202 |
| Average risk ratio, 10-day treatment | 3.41 | 2.13 | 5.47 | ||
| Event rate, 10-day treatment (fixed-effects model) | 0.00 (p=1.00) | 0.78 | 0.66 | 0.86 | <0.001 |
| Average risk ratio, 14-day treatment | 1.46 | 1.27 | 1.68 | ||
| Event rate, 14-day treatment (random-effects model) | 75.14 (p<0.001) | 0.60 | 0.56 | 0.63 | <0.001 |
| Total average risk ratio | 1.47 | 1.29 | 1.68 | ||
| Event rate (random-effects model) | 74.73 (p<0.001) | 0.60 | 0.56 | 0.63 | <0.001 |
Table 2 shows the results of the meta-analysis in regard to the studies that applied the ITT analysis. According to this, the mean eradication rate of all studies was 0.60 (95% CI: 56%–63%) and the risk ratio was 1.47. Moreover, according to the P value, it was not necessary to add new studies. When subgroups were evaluated according to days, the eradication rate for the 7-day treatment was 0.57 (95% CI: 46%–68%). However, it was not statistically significant. The eradication rate was 0.78 for the 10-day treatment (95% CI: 66%–86%) and 0.60 for the 14-day treatment (95% CI: 56%–63%), and the effects were statistically significant.
Table 3.
Mean rate of eradication using the PP analysis
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio, 7-day treatment | 1.35 | 0.82 | 2.23 | ||
| Event rate, 7-day treatment (fixed-effects model) | 55.97 (p=0.103) | 0.60 | 0.51 | 0.67 | 0.024 |
| Average risk ratio, 14-day treatment | 1.30 | 1.03 | 1.63 | ||
| Event rate, 14-day treatment (random-effects model) | 77.80 (p<0.001) | 0.56 | 0.50 | 0.62 | 0.037 |
| Total average risk ratio | 1.30 | 1.06 | 1.61 | ||
| Event rate (random-effects model) | 75.56 (p<0.001) | 0.57 | 0.51 | 0.62 | 0.018 |
Table 3 shows the results of the meta-analysis in regard to the studies that applied the PP analysis. According to this, the total eradication rate was 0.57 (95% CI: 51%–62%) and the risk ratio was 1.30. This result was found to be statistically significant.
Figure 1.
ITT forest plot for all studies
Figure 2.
PP forest plot for all studies
Table 4.
ITT eradication result for the 14-day treatment
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio [ES-OM] | 1.22 | 0.79 | 1.86 | ||
| Event rate (ES-OM) (fixed-effects model) | 47.14 (p=0.151) | 0.54 | 0.46 | 0.61 | 0.373 |
| Average risk ratio [LAC] | 1.47 | 1.20 | 1.80 | ||
| Event rate (LAC) (random-effects model) | 79.85 (p<0.001) | 0.60 | 0.54 | 0.65 | <0.001 |
| Average risk ratio (OAC) | 1.20 | 0.71 | 2.02 | ||
| Event rate (OAC) (random-effects model) | 81.86 (p<0.001) | 0.55 | 0.41 | 0.68 | 0.487 |
| Average risk ratio (PAC) | 1.47 | 1.20 | 1.81 | ||
| Event rate (PAC) (fixed-effects model) | 43.29 (p=0.054) | 0.60 | 0.56 | 0.63 | <0.001 |
| Average risk ratio (RAC) | 2.73 | 1.08 | 6.88 | ||
| Event rate (RAC) (fixed-effects model) | 73.24 (p=0.053) | 0.75 | 0.65 | 0.83 | <0.001 |
Table 4 shows that studies carried out with ITT were divided into subgroups according to PPIs with a treatment duration of 14 days. Although the effect magnitudes of studies using the ES-OM and OAC combinations were found to be insignificant, the effect of the LAC, PAC, and RAC inhibitors was found to be significant. The highest effect was monitored in the LAC combination.
Table 5.
PP eradication result for the 14-day treatment
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio (ES-OM) | 1.03 | 0.74 | 1.44 | ||
| Event rate (ES-OM) (fixed-effects model) | 0.00 (P=1.00) | 0.51 | 0.39 | 0.63 | 0.903 |
| Average risk ratio (LAC) | 1.11 | 0.78 | 1.58 | ||
| Event rate (LAC) (random-effects model) | 83.22 (p<0.001) | 0.53 | 0.44 | 0.62 | 0.566 |
| Average risk ratio (OAC) | 1.20 | 0.98 | 1.47 | ||
| Event rate (OAC) (fixed-effects model) | 0.00 (p=1.00) | 0.55 | 0.47 | 0.62 | 0.212 |
| Average risk ratio (PAC) | 1.77 | 1.22 | 2.57 | ||
| Event rate (PAC) (random-effects model) | 58.99 (p=0.032) | 0.64 | 0.55 | 0.72 | 0.003 |
Table 5 shows that studies carried out with PP were divided into subgroups according to PPIs with a treatment duration of 14 days. Although the effect magnitudes of studies using the ES-OM, LAC, and OAC combinations were found to be insignificant, the effect of the PAC inhibitors was 0.64 and was found to be significant.
Figure 3.
Forest plot for different inhibitors in the ITT analysis for the 14-day treatment
Figure 4.
Forest plot for different inhibitors in the PP analysis for the 14-day treatment
Table 6.
ITT eradication result for the 10-day treatment
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio (OAC) | 3.41 | 2.13 | 5.47 | ||
| Event rate (OAC) (fixed-effects model) | 0.00 (p=1.00) | 0.78 | 0.66 | 0.86 | <0.001 |
Table 6 shows the results of a study using OAC combination with a treatment duration of 10 days with ITT. For this PPI, the effect was 0.78 (95% CI: 66%–86%) and was found to be significant.
Figure 5.
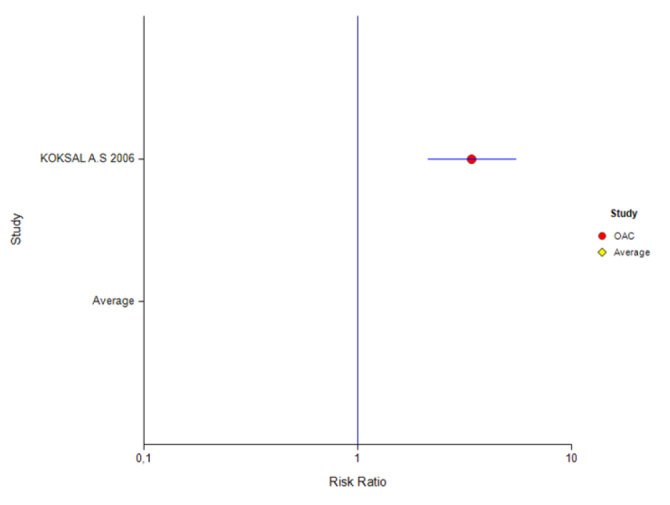
Forest plot for different inhibitors in the ITT analysis for the 10-day treatment
Table 7.
ITT eradication rate for the 7-day treatment
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio (LAC) | 2.11 | 1.47 | 3.05 | ||
| Event rate (LAC) (fixed-effects model) | 0.00 (p=0.671) | 0.68 | 0.50 | 0.78 | 0.002 |
| Average risk ratio (OAC) | 1.21 | 0.19 | 7.79 | ||
| Event rate (OAC) (random-effects model) | 86.94 (p=0.006) | 0.55 | 0.15 | 0.90 | 0.837 |
| Average risk ratio (PAC) | 1.19 | 0.77 | 1.86 | ||
| Event rate (PAC) (fixed-effects model) | 48.05 (p=0.146) | 0.50 | 0.48 | 0.64 | 0.123 |
Table 7 shows that studies carried out using the ITT analysis were divided into subgroups according to PPIs with a treatment duration of 7 days. According to this, although the effect magnitudes of studies using the PAC and OAC combinations were found to be insignificant, the effect for the LAC combination was significant.
Table 8.
PP eradication rate for the 7-day treatment
| I2 | Effectiveness | 0, 95% LCL | 0, 95% UCL | p | |
|---|---|---|---|---|---|
| Average risk ratio (PAC) | 1.35 | 0.82 | 2.23 | ||
| Event rate (PAC) (fixed-effects model) | 55.97 (p=0.103) | 0.60 | 0.51 | 0.67 | 0.024 |
| Event rate (PAC) (random-effects model) | 0.58 | 0.46 | 0.70 | 0.204 |
Table 8 shows that the 7-day treatment duration was evaluated according to PPI using PAC. The I2 value was 55.97, but was not found to be significant (P=0.103). The eradication rate in the case of the fixed-effects model was 0.60% (95% CI: 51%–67%) and was found to be statistically significant (0.024). The risk ratio was 1.35; however, it was not statistically significant as the confidence intervals included a value of 1. Although the risk ratio with the random-effects model was 0.58 (95% CI: 46%–70%), the confidence intervals were wider and statistically insignificant (p=0.204).
Figure 6.
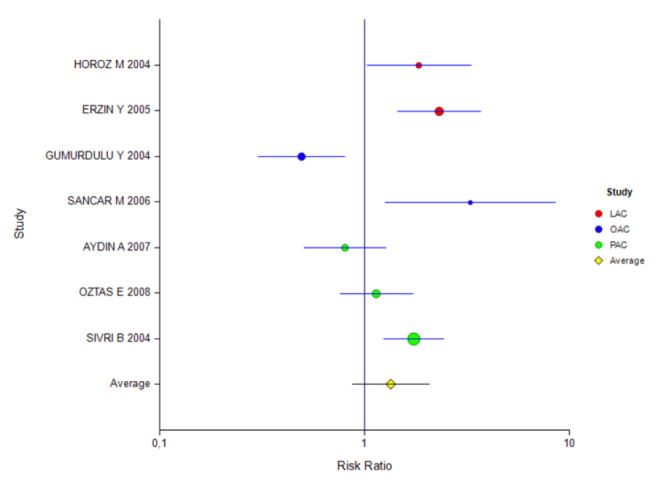
Forest plot for different inhibitors in the ITT analysis for the 7-day treatment
Figure 7.
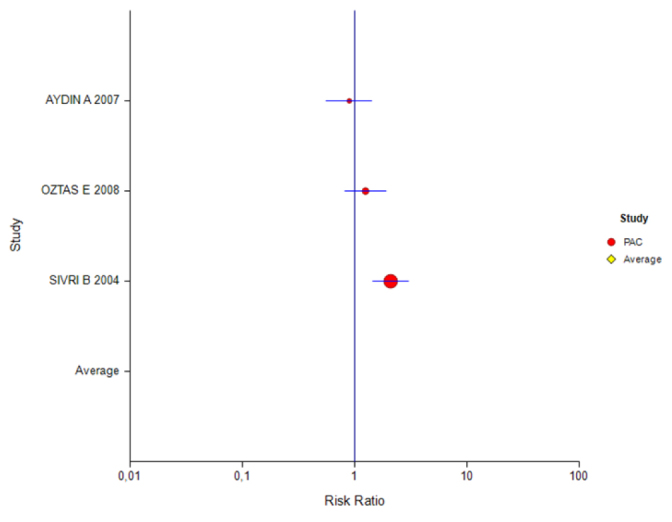
Forest plot for different inhibitors in the PP analysis for the 7-day treatment
In the meta-regression analysis, the treatment duration, PPI, age, and gender ratio (women/men) used for the ITT analysis had no effect. The gender ratio and age were not considered in this analysis because they were not clearly stated in studies using the PP analysis. The duration of treatment and the PPI used had no effect (Tables 9 and 10). The I2 for ITT was 74.73 (p<0.001) and the I2 for PP was 75.56 (p<0.001). As the I2 values were more than 25% and were found to be significant, the meta-analysis was applied to subgroups according to the day and PPIs, assuming heterogeneity (21).
Table 10.
Meta-regression analysis results using the PP analysis
| PP | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Coefficient | 95% lower | 95% upper | p | p | ||
| Intercept | −0.241 | −1.511 | 1.029 | 0.710 | - | |
| Days | 7 | Reference | - | |||
| 14 | 0.271 | −0.420 | 1.034 | 0.486 | ||
| PPI | ES-OM | Reference | 0.375 | |||
| LAC | 0.076 | −0.995 | 1.146 | 0.890 | ||
| OAC | 0.155 | −1.230 | 1.539 | 0.827 | ||
| PAC | 0.559 | −0.550 | 1.668 | 0.324 | ||
As age and gender information were generally given in all studies for patients participating in the study and because ITT covered all randomized patients, the PP analysis could not be used in the data analysis.
The treatment application time for ITT (p=0.169), age (p=0.898), gender ratio (p=0.588), and application of different PPIs (p=0.376) did not affect the success of the treatment.
The treatment application time for PP (p=0.486) and the application of different PPIs (p=0.375) did not affect the success of the treatment.
The eradication rates of the first 5 and the last 5 years were compared to determine if there was a change in the eradication rates during the 10-year period. There was no difference in terms of ITT (59% vs. 61% p: 0.650, respectively), while there was a significant difference in PP results (62% vs. 50%, p: 0.013).
DISCUSSION
STT has been proposed by all authorities in the last two decades as the first option for first-line Hp eradication treatment. However, its effectiveness is gradually diminishing. Studies from different geographical regions of the world have shown a decrease in the success of STT.
In a study published by our group in 2004, the mean eradication rate with a 14-day STT treatment with different PPIs was found to be 45% (18). The type of PPI used did not change the result. Unfortunately, the eradication rates, in studies carried out by us after 2004, with different combinations to increase the eradication success were always less than the optimal limits (50,66–71).
Kadayıfçı et al. (20) were the first in Turkey to systematically analyze the efficiency of the triple treatment in first-line Hp eradication. They evaluated 94 studies involving 3637 patients who underwent an STT regimen during the 10-year period between 1996 and 2005. In the present meta-analysis, the eradication rate was found to be 68.8% (20).
The results of Hp eradication with STT during the 10-year period following this study were examined. As a result of the systematic meta-analysis of STT used within 10 years from 2004 to 2013, the effectiveness of STT against Hp infection was found to be much lower than desired. The ITT analysis showed an eradication rate of 60% (95% CI: 56%–63%) while the PP analysis showed an eradication rate of 57% (95% CI: 51%–62%). Unfortunately, these eradication rates were much less than the 80% required for successful eradication. ITT eradication rates did not show a change over a 10-year period, whereas PP eradication rate decreased in the second 5-year period compared to the previous years. However, the rates of eradication in both periods were unacceptably low.
As is known, the intention-to-treat analysis is a comparison of the treatment groups that includes all patients and it ignores noncompliance, protocol deviations, withdrawal, and anything that happens after randomization. On the other hand, per-protocol analysis is a comparison of treatment groups that includes only those patients who have completed the treatment. Therefore, PP eradication rates are usually higher than those of ITT analysis. But in our study, some PP eradication rates are higher than ITT rates (for example: the mean rate of eradication of all studies according to the ITT and PP analyses was 60% and 57%, respectively; also eradication rates for 14 days of treatment were 60% and 56% for ITT and PP analysis, respectively; a similar trend was observed when different PPIs were used). The reason for this is that the PP results were not stated in some studies.
Another important result of this study was that the type of PPI used and the treatment duration did not affect the eradication result. However, at the third Maastricht consensus conference, the duration of STT was extended to 14 days because the success of treatment increased with an increased duration of treatment (5). Accordingly, the treatments were given for at least 14 days for years (72). This meta-analysis showed that a treatment duration of 7 or 14 days did not change the success of STT. Thus, this became a subject that needed attention. A similar result was obtained by Kadayıfçı et al. (20) in their evaluation carried out in 2004 (20). There has been a consensus for years that different PPIs used for treatment show similar effects (18,73–76). The present study also yielded the same result. The type of PPI used in the combination treatment did not lead to any change in the eradication result.
Although the most important cause of eradication failure is accepted as antibiotic resistance, other reported causes include treatment incompatibility, drug-related side effects, bacterial load, smoking and underlying co-morbidities, and genetic differences in PPI metabolism (77–80). Studies in Turkey and our region (Mediterranean area) showed that, especially, the antibiotic resistance against clarithromycin and metronidazole was very high (16,17). The studies included did not analyze antibiotic resistance. Therefore, high antibiotic resistance rates were believed to be an important factor in the failure of STT regimes, although no definite conclusion was reached in this regard. However, further reduction of Hp eradication success in the second 5-year period may be related to this issue.
The main limitation of this study was its retrospective design. As a limited number of randomized controlled studies were identified, uncontrolled and open-label studies and case series were included. To increase the number of studies and patients included in this analysis, factors such as study design and minimum number of subjects per treatment arm were not strictly defined. The PP results and gender information were not stated in some studies. Patients who underwent STT were selected and evaluated from different study groups.
This systematic meta-analysis of studies conducted between 2004 and 2013 in Turkey indicated that the rate of first-line Hp eradication with STT was unacceptably low. Also, the duration of treatment and PPI used made no difference.
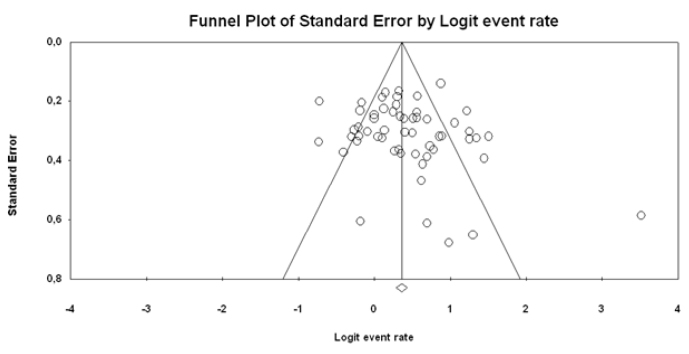
Figure 8.
ITT funnel plot
Figure 8 shows the funnel plot of the studies that used the ITT analysis. The studies were distributed homogeneously. However, there were two studies (43 and 61) outside the funnel plot; the rates of eradication were very high in these two studies (100% and 97%, respectively), but the percentages of the contribution to the meta-analysis were quite low. Therefore, the effect of the image on the funnel plot is negligible. As a result of the Egger test, it does not appear to be a publication bias. Therefore, the publication bias was not detected (p=0.294).
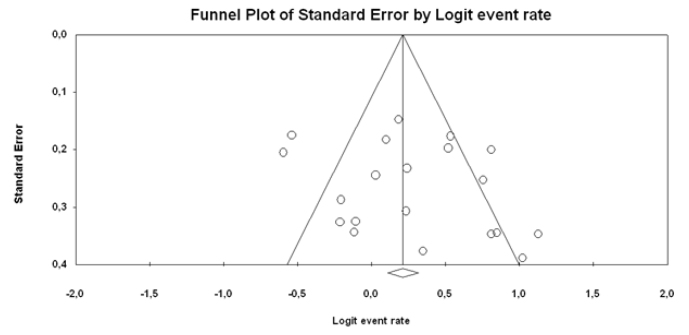
Figure 9.
PP funnel plot
Figure 9 shows the funnel plot of the studies that used the PP analysis. The studies were distributed homogeneously.
The publication bias was not detected (p=0.230).
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: Written informed consent was obtained from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: The author has no conflict of interest to declare.
Financial Disclosure: The author declared that this study has received no financial support.
REFERENCES
- 1.Makola D, Peura DA, Crowe SE. Helicobacter pylori infection and related gastrointestinal diseases. J Clin Gastroenterol. 2007;41:548–58. doi: 10.1097/MCG.0b013e318030e3c3. [DOI] [PubMed] [Google Scholar]
- 2.Franceschi F, Gasbarrini A. Helicobacter pylori and extragastric diseases. Best Pract Res Clin Gastroenterol. 2007;21:325–34. doi: 10.1016/j.bpg.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimer’s disease: preliminary results. Neurobiol Aging. 2012;33:1009.e11–1009.e19. doi: 10.1016/j.neurobiolaging.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Venerito M, Selgrad M, Malfertheiner P. Helicobacter pylori: gastric cancer and extra gastric malignancies - clinical aspects. Helicobacter. 2013;18(Suppl 1):39–43. doi: 10.1111/hel.12078. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Helicobacter Pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gisbert JP, Calvet X, Gomollon F, et al. Treatment for the eradication of Helicobacter pylori. Recommendations of the Spanish Consensus Conference. Med Clin (Barc) 2000;114:185–95. doi: 10.1016/S0025-7753(00)71237-1. [DOI] [PubMed] [Google Scholar]
- 8.Hunt RH, Fallone CA, Thomson AB. Canadian Helicobacter pylori Consensus Conference update: infections in adults. Canadian Helicobacter Study Group. Can J Gastroenterol. 1999;13:213–7. doi: 10.1155/1999/180751. [DOI] [PubMed] [Google Scholar]
- 9.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection. The Maastricht 2–2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 10.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection. The Maastricht IV/ Florence consensus report. Gut. 2012;61:646–64. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 11.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 13.Laine L, Fennerty MB, Osato M, et al. Esomeprazole-based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double-blind trials. Am J Gastroenterol. 2000;95:3393–8. doi: 10.1111/j.1572-0241.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 14.Vakil N, Lanza F, Schwartz H, et al. Seven-day therapy for Helicobacter pylori in the United States. Aliment Pharmacol Ther. 2004;20:99–107. doi: 10.1111/j.1365-2036.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 15.Janssen MJR, Van Oijen AHAM, Verbeek ALM, et al. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Aliment Pharmacol Ther. 2001;15:613–24. doi: 10.1046/j.1365-2036.2001.00974.x. [DOI] [PubMed] [Google Scholar]
- 16.Sezgin O, Aslan G, Altıntaş E, et al. Detection of point mutations on 23S rRNA of Helicobacter pylori and resistance to clarithromycin with PCR-RFLP in gastric biopsy specimens in Mersin, Turkey. Turk J Gastroenterol. 2008;19:163–7. [PubMed] [Google Scholar]
- 17.Çağdaş U, Otağ F, Tezcan S, Sezgin O, Aslan G, Emekdaş G. Detection of Helicobacter pylori and Antimicrobial Resistance in Gastric Biopsy Specimens. Mikrobiyol Bul. 2012;46:398–409. [PubMed] [Google Scholar]
- 18.Altintas E, Sezgin O, Ulu O, et al. Maastricht II treatment scheme and efficacy of different proton pump inhibitors in eradicating Helicobacter pylori. World J Gastroenterol. 2004;10:1656–8. doi: 10.3748/wjg.v10.i11.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadayıfçı A, Büyükhatipoğlu H, Koruk M, et al. Türkiye’de H. pilori eradikasyonunda PPI, amoksisilin ve klaritromisin tedavisinin etkinliği: meta-analiz. Turk J Gastroenterol. 2004;15(Suppl 1):5. [Google Scholar]
- 20.Kadayifci A, Buyukhatipoglu H, Cemil Savas M, Simsek I. Eradication of Helicobacter pylori with triple therapy: an epidemiologic analysis of trends in Turkey over 10 years. Clin Ther. 2006;28:1960–6. doi: 10.1016/j.clinthera.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Güliter S, Kandilci U. The effect of Helicobacter pylori eradication on gastroesophageal reflux disease. J Clin Gastroenterol. 2004;38:750–5. doi: 10.1097/01.mcg.0000139071.30956.30. [DOI] [PubMed] [Google Scholar]
- 23.Horoz M, Bölükbaş C, Bölükbaş FF, Uzunköy A, Soylu A. Klaritromisin- amoksisilin- Lansoprazol Kombinasyonunda optimal Tedavi Süresi”. HrÜ Tıp Fak Der. 2004;1:12–8. [Google Scholar]
- 24.Sivri B, Simsek I, Hulagu S, et al. The efficacy, safety and tolerability of pantoprazole-based one-week triple therapy in H. pylori eradication and duodenal ulcer healing. Curr Med Res Opin. 2004;20:1301–7. doi: 10.1185/030079904125004439. [DOI] [PubMed] [Google Scholar]
- 25.Uygun A, Kadayifçi A, Yeşilova Z, et al. Recent success of pantoprazole -or lansoprazole-based clarithromycin plus amoxicillin treatment in the eradication of Helicobacter pylori. Turk J Gastroenterol. 2004;4:219–24. [PubMed] [Google Scholar]
- 26.Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadriple therapy in Turkey. World J Gastroenterol. 2004;10:668–71. doi: 10.3748/wjg.v10.i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Güliter S, Keleş H, Ozkurt ZN, Cengiz DU, Kolukisa E. Can lansoprazole, amoxicillin, and clarithromycin combination still be used as a first-line therapy for eradication of helicobacter pylori? Turk J Gastroenterol. 2005;16:29–33. [PubMed] [Google Scholar]
- 28.Erzin Y, Altun S, Dobrucali A, et al. Evaluation of two enzyme immunoassays for detecting Helicobacter pylori in stool specimens of dyspeptic patients after eradication therapy. J Med Microbiol. 2005;54:863–6. doi: 10.1099/jmm.0.45914-0. [DOI] [PubMed] [Google Scholar]
- 29.Ozer B, Serin E, Gumurdulu Y, et al. Helicobacter pylori eradication lowers serum homocysteine level in patients without gastric atrophy. World J Gastroenterol. 2005;11:2764–7. doi: 10.3748/wjg.v11.i18.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Göral V, Dönmez M, Temiz H, Şit D. Nonülser dispepside Helikobakter pilori sıklığı ve eradikasyon tedavisine yanıt. Akademik Gastroenteroloji Dergisi. 2006;5:173–8. [Google Scholar]
- 31.Duman DG, Bor S, Ozütemiz O, et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2005;17:1357–61. doi: 10.1097/00042737-200512000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Köksal AŞ, Parlak E, Oğuz D, Çiçek B, Şahin B. Helikobakter pilori eradikasyonunun non-ülser dispepsili hastalarda semptomlar üzerine kısa dönemdeki etkisi. Akademik Gastroenteroloji Dergisi. 2006;5:36–40. [Google Scholar]
- 33.Sancar M, Izzettin FV, Apikoglu-Rabus S, Besisik F, Tozun N, Dulger G. Pharmaco economic comparison of Helicobacter pylori eradication regimens. Pharm World Sci. 2006;28:207–14. doi: 10.1007/s11096-006-9021-y. [DOI] [PubMed] [Google Scholar]
- 34.Uygun A, Kadayifci A, Safali M, Ilgan S, Bagci S. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211–5. doi: 10.1111/j.1751-2980.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 35.Aydin A, Onder G, Akarca U, Tekin F, Tuncyurek M, Ilter T. Comparison of 1 and 2 week pantoprazole-based triple therapies in clarithromycin-sensitive and resistant cases. Eur J Intern Med. 2007;18:496–500. doi: 10.1016/j.ejim.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Cindoruk M, Erkan G, Karakan T, Dursun A, Unal S. Efficacy and safety of Saccharomyces boulardii in the 14-day triple anti-Helicobacter pylori therapy: a prospective randomized placebo-controlled double-blind study. Helicobacter. 2007;12:309–16. doi: 10.1111/j.1523-5378.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 37.Uygun A, Kadayifci A, Yesilova Z, Safali M, Ilgan S, Karaeren N. Comparison of sequential and standard triple-drug regimen for Helicobacter pylori eradication: a 14-day, open-label, randomized, prospective, parallel-arm study in adult patients with nonulcer dyspepsia. Clin Ther. 2008;30:528–34. doi: 10.1016/j.clinthera.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Öztaş E, Bektaş M, İdilman R, Özden A. Helikobakter pilori (+) fonksiyonel dispepside 7 ve 14 günlük pantoprazol temelli üçlü kombinasyon tedavisinin eradikasyona ve eradikasyonun semptomlara olan etkinliğinin değerlendirilmesi. Akademik Gastrroenteroloji Dergisi. 2008;7:160–7. [Google Scholar]
- 39.Kiliç ZM, Köksal AS, Cakal B, et al. Moxifloxacine plus amoxicillin and ranitidine bismuth citrate or esomeprazole triple therapies for Helicobacter pylori infection. Dig Dis Sci. 2008;53:3133–7. doi: 10.1007/s10620-008-0285-z. [DOI] [PubMed] [Google Scholar]
- 40.Bektaş M, Alkan M, Üstün Y, Soykan İ, Özden A. Duodenal ülserli hastalarda Helikobakter pilori eradikasyonunun asit reflüsü üzerine etkisi. Akademik Gastroenteroloji Dergisi. 2008;7:34–8. [Google Scholar]
- 41.Bektas M, Soykan I, Altan M, Alkan M, Özden A. The effect of Helicobacter pylori eradication on dyspeptic symptoms, acid reflux and quality of life in patients with functional dyspepsia. Eur J Int Med. 2009;20:419–23. doi: 10.1016/j.ejim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Songür Y, Senol A, Balkarli A, Baştürk A, Cerçi S. Triple or quadruple tetracycline-based therapies versus standard triple treatment for Helicobacter pylori treatment. Am J Med Sci. 2009;338:50–3. doi: 10.1097/MAJ.0b013e31819c7320. [DOI] [PubMed] [Google Scholar]
- 43.Kebapcilar L, Sari I, Renkal AH, et al. The influence of Helicobacter pylori eradication on leptin, soluble CD40 ligand, oxidative stress and body composition in patients with peptic ulcer disease. Intern Med. 2009;48:2055–9. doi: 10.2169/internalmedicine.48.2562. [DOI] [PubMed] [Google Scholar]
- 44.Soylu A, Dolapcıoğlu C, Yaşar N, Sevindir İ, Sever N. Helikobakter pylori eradikasyonu reflü semptomlarını ve endoskopik özofajiti olumlu etkiliyor. Akademik Gastroenteroloji Dergisi. 2009;8:63–8. [Google Scholar]
- 45.Demir M, Gokturk HS, Ozturk NA, Arslan H, Serin E, Yilmaz U. Clarithromycin Resistance and Efficacy of Clarithromycin-Containing Triple Eradication Therapy for Helicobacter pylori Infection in Type 2 Diabetes Mellitus Patients. South Med J. 2009;102:1116–20. doi: 10.1097/SMJ.0b013e3181bca538. [DOI] [PubMed] [Google Scholar]
- 46.Aydemir S, Eren H, Tekin IO, Harmandar FA, Demircan N, Cabuk M. Helicobacter pylori Eradication Lowers Serum Asymmetric Dimethylarginine Levels. Hindawi. 2010:4. doi: 10.1155/2010/685903. 685903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaşar B, Abut E, Kayadıbı H, et al. Efficacy of probiotics in Helicobacter pylori eradication therapy. Turk J Gastroenterol. 2010;21:212–7. doi: 10.4318/tjg.2010.0090. [DOI] [PubMed] [Google Scholar]
- 48.Abut E, Yaşar B, Güveli H, et al. Effect of the mucolytic erdosteine on the success rate of PPI-based first-line triple therapy for Helicobacter pylori eradication: a prospective, double-blind, randomized, placebo-controlled study. Scand J Gastroenterol. 2010;45:677–83. doi: 10.3109/00365521003702726. [DOI] [PubMed] [Google Scholar]
- 49.Ozdil B, Akkiz H, Bayram S, Bekar A, Akgöllü E, Sandikçi M. Influence of CYP2C19 functional polymorphism on Helicobacter pylori eradication. Turk J Gastroenterol. 2010;21:23–8. doi: 10.4318/tjg.2010.0043. [DOI] [PubMed] [Google Scholar]
- 50.Nadir I, Yonem O, Ozin Y, Kilic ZM, Sezgin O. Comparison of two different treatment protocols in Helicobacter pylori eradication. South Med J. 2011;104:102–5. doi: 10.1097/SMJ.0b013e318200c209. [DOI] [PubMed] [Google Scholar]
- 51.Erdogan AF, Abacı K, Serin E, Özer B, İçer MO. Birinci tercih Helikobakter pilori eradikasyon tedavileri alarm mı veriyor? Akademik Gastroenteroloji Dergisi. 2009;8:59–62. [Google Scholar]
- 52.Ermis F, Akyuz F, Uyanikoglu A, et al. Second-line levofloxacin-based triple therapy’s efficiency for Helicobacter pylori eradication in patients with peptic ulcer. South Med J. 2011;104:579–83. doi: 10.1097/SMJ.0b013e3182249be0. [DOI] [PubMed] [Google Scholar]
- 53.Alkim H, Iscan M, Oz F. Effectiveness of ranitidine bismuth citrate and proton pump inhibitor based triple therapies of Helicobacter pylori in Turkey. Libyan J Med. 2011;6 doi: 10.3402/ljm.v6i0.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Çetinkaya ZA, Sezikli M, Güzelbulut F, et al. Helikobakter pilori eradikasyonunda kullanılan proton pompa inhibitörlerinin etkinliklerinin karşilaştırılması. Akademik gastroenteroloji dergisi. 2011;10:1–4. [Google Scholar]
- 55.Demir M, Ataseven H. The effects of sequential treatment as a first-line therapy for Helicobacter pylori eradication. Turk J Med Sci. 2011;41:427–33. doi: 10.1097/SMJ.0b013e3181eea6cc. [DOI] [PubMed] [Google Scholar]
- 56.Sezikli M, Cetinkaya ZA, Güzelbulut F, et al. Efficacy of vitamins supplementation to therapy on Helicobacter pylori eradication in patients with low antioxidant capacity. Clin Res Hepatol Gastroenterol. 2011;35:745–9. doi: 10.1016/j.clinre.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Gokturk HS, Demir M, Unler GK, Erbayrak M, Sakalli M, Yilmaz U. Does long-term aspirin use have any effect on Helicobacter pylori eradication? Am J Med Sci. 2011;342:15–9. doi: 10.1097/MAJ.0b013e3182174cf1. [DOI] [PubMed] [Google Scholar]
- 58.Sezikli M, Cetinkaya ZA, Güzelbulut F, et al. Efficacy of the Combination of Tetracycline, Amoxicillin, and Lansoprazole in the Eradication of Helicobacter pylori in Treatment-Naïve Patients and in Patients Who Are Not Responsive to Clarithromycin-Based Regimens: A Pilot Study. Gut Liver. 2012;6:41–4. doi: 10.5009/gnl.2012.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polat Z, Kadayifci A, Kantarcioglu M, Ozcan A, Emer O, Uygun A. Comparison of levofloxacin-containing sequential and standard triple therapies for the eradication of Helicobacter pylori. Eur J Intern Med. 2012 Mar;23:165–8. doi: 10.1016/j.ejim.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Balcilar E, Karsidag T, Tüzün S. Helicobacter pylori Taramasinda Gaitada Bakilan Antijenin Güvenilirligi Haseki Tıp Bülteni. 2012;50:89–92. [Google Scholar]
- 61.Uyanıkoğlu A, Coşkun M, Binici DN. Helikobakter pilori eradikasyonunda klasik 3’lü tedavi Doğu Anadolu bölgesinde halen etkilidir. Akademik gastroenteroloji dergisi. 2012;11:24–8. [Google Scholar]
- 62.Onal IK. Diagnostic testing for Helicobacter Pylori in patients with atrophic gastritis. Clin Res Hepatol Gastroenterol. 2013;37:e115–6. doi: 10.1016/j.clinre.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Avşar E, Tiftikçi A, Poturoğlu S, et al. A multicenter, randomized, prospective study of 14-day ranitidine bismuth citrate- vs. lansoprazole-based triple therapy for the eradication of Helicobacter pylori in dyspeptic patients. Turk J Gastroenterol. 2013;24:316–21. doi: 10.4318/tjg.2013.0509. [DOI] [PubMed] [Google Scholar]
- 64.Ulasoglu C, Isbilen B, Doganay L, Ozen F, Kiziltas S, Tuncer I. Effect of Helicobacter pylori eradication on serum ghrelin and obestatin levels. World J Gastroenterol. 2013;19:2388–94. doi: 10.3748/wjg.v19.i15.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ustundag Y, Sahin H, İlikhan S, Dogan BG, Kokturk F, Kar F. Helicobacter pylori eradication does not change circulating insulin-like growth factor 1 and insulin-like growth factor binding protein 3 levels in patients with and without precancerous gastric lesions. Am J Med Sci. 2013;346:381–4. doi: 10.1097/MAJ.0b013e31827beed3. [DOI] [PubMed] [Google Scholar]
- 66.Altıntaş E, Ulu O, Sezgin O, et al. Comparison of ranitidine bismuth citrate, tetracycline and metronidazole with ranitidine bismuth citrate and azithromycin for the eradication of H. pylori in patients resistant to PPI based triple therapy. Turk J Gastroenterol. 2004;15:90–3. [PubMed] [Google Scholar]
- 67.Sezgin O, Altintaş E, Uçbilek E, Tataroğlu C. Bismuth-based therapies for the first step eradication of Helicobacter pylori. Turk J Gastroenterol. 2006;17:90–3. [PubMed] [Google Scholar]
- 68.Sezgin O, Altintaş E, Uçbilek E, Tombak A, Tellioğlu B. Low efficacy rate of moxifloxacin-containing Helicobacter pylori eradication treatment: in an observational study in a Turkish population. Helicobacter. 2007;12:518–22. doi: 10.1111/j.1523-5378.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 69.Sezgin O, Altintaş E, Nayir E, Uçbilek E. A pilot study evaluating sequential administration of a PPI-amoxicillin followed by a PPI-metronidazole-tetracycline in Turkey. Helicobacter. 2007;12:629–32. doi: 10.1111/j.1523-5378.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 70.Sezgin O, Barlas IO, Uçbilek E, Yengel E, Altintaş E. Modified sequential Helicobacter pylori eradication therapy using high dose omeprazole and amoxicillin in the initial phase in the extensive metaboliser Turkish patients for CYP2C19 polymorphism is ineffective. Acta Gastroenterol Belg. 2014;77:3–7. [PubMed] [Google Scholar]
- 71.Sezgin O, Aydın MK, Ateş F, et al. Addition of Rifaximin and N-Acetyl Cysteine to the Standard Helicobacter pylori Treatment Regimens: Is it Possible to Improve Outcomes? J Gastric Disord and Ther. 2016;2:1–5. doi: 10.16966/2381-8689.117. [DOI] [Google Scholar]
- 72.Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxicillin for treating Helicobacter pylori infection. Aliment Pharmacol Ther. 2000;14:603–9. doi: 10.1046/j.1365-2036.2000.00744.x. [DOI] [PubMed] [Google Scholar]
- 73.Vallve M, Vergara M, Gisbert JP, Calvet X. Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta-analysis. Aliment Pharmacol Ther. 2002;16:1149–56. doi: 10.1046/j.1365-2036.2002.01270.x. [DOI] [PubMed] [Google Scholar]
- 74.Wang X, Fang JY, Lu R, Sun DF. A meta-analysis: comparison of esomeprazole and other proton pump inhibitors in eradicating Helicobacter pylori. Digestion. 2006;73:178–86. doi: 10.1159/000094526. [DOI] [PubMed] [Google Scholar]
- 75.Ulmer HJ, Beckerling A, Gatz G. Recent use of proton pump inhibitor-based triple therapies for the eradication of H. pylori: A broad data review. Helicobacter. 2003;8:95–104. doi: 10.1046/j.1523-5378.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 76.Thomson AB. Are the orally administered proton pump inhibitors equivalent? A comparison of lansoprazole, omeprazole, pantoprazole, and rabeprazole. Curr Gastroenterol Rep. 2000;2:482–93. doi: 10.1007/s11894-000-0013-0. [DOI] [PubMed] [Google Scholar]
- 77.Houben MH, van de Beek D, Hensen EF, et al. A systematic review of Helicobacter pylori eradication therapy-the impact of antimicrobial resistance on eradication rates. Aliment Pharmacol Ther. 1999;13:1047–55. doi: 10.1046/j.1365-2036.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 78.Perri F, Villani MR, Festa V, et al. Predictors of failure of Helicobacter pylori eradication with the standard ‘Maastricht triple therapy’. Aliment Pharmacol Ther. 2001;15:1023–9. doi: 10.1046/j.1365-2036.2001.01006.x. [DOI] [PubMed] [Google Scholar]
- 79.Queiroz DM, Dani R, Silva LD, et al. Factors associated with treatment failure of Helicobacter pylori infection in a developing country. J Clin Gastroenterol. 2002;35:315–20. doi: 10.1097/00004836-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Barlas İÖ, Sezgin O, Dandara C, et al. Harnessing Knowledge on Very Important Pharmacogenes CYP2C9 and CYP2C19 Variation for Precision Medicine in Resource-Limited Global Conflict Zones. OMICS. 2016;20:604–9. doi: 10.1089/omi.2016.0133. [DOI] [PubMed] [Google Scholar]