Highlights
-
•
African swine fever virus replicates predominanly in macrophages or monocytes.
-
•
Understanding hoe virus modulation of these cells is important to understand the outcomes of infection.
-
•
Deletion of interferon inhibitory genes can attenuate virus and induce a protective immune response.
-
•
Severeal apoptosis inhibitors are encoded by ASFV.
-
•
Genes coding for these inhibitory proteins provide targets to delete to construct rationally attenuated vaccine strains.
Abbreviations: ASFV, African swine fever virus; AP endonuclease, Apurinic/apyrimidinic (AP) endonuclease; BER, base excision repair; BIR, baculovirus inhibitor of apoptosis region; Bcl-2, B-cell lymphoma 2; BH3, Bcl-2 homology domain; c-GAS, cyclic GMP-AMP Synthase; c-GAMP, cyclic GMP-AMP; CDN, cyclic dinucleotides; CHOP, C/EBP homologous protein, an inducer of apoptosis; cDCs, conventional dendritic cells; c-FLIP, cellular FLICE inhibitor protein; dNTP, deoxy nucleotide triphosphate; dUTPase, deoxyuridine-triphosphatase; ER, endoplasmic reticulum; eIF-2α, eukaryotic translation initiation factor 2 alpha; ER, endoplasmic reticulum; IFN, Interferon; ISG, Interferon stimulated gene; IRF3, interferon regulatory factor 3; iDCs, interdigitating dendritic cells; IAP, inhibitor of apoptosis protein; JAK STAT, janus kinases, signal transducer and activator of transcription proteins; MAVs, mitochondrial antiviral-signaling protein; MDA5, melanoma differentiation-associated protein 5; MoDCs, monocyte derived dendritic cells; MHC Class I, major histocompatibility protein class I; i Met-tRNAi, methionine initiator transfer RNA; NF-kB, nuclear factor kappa B transcription factor; NF-kB, nuclear factor kappa B transcription factor; PRRs, pattern recognition receptors; PAMPS, pathogen associated molecular patterns; pDCs, plasmacytoid dendritic cells; RIG-I, retinoic acid-inducible gene I; STING, stimulator of Interferon Genes; TBK1, tank binding kinase; TLR, toll-like receptor; TRIF, TIR-domain-containing adapter-inducing interferon-β; TNF-α, tumour necrosis factor alpha
Keywords: African swine fever, Immune evasion, Interferon, Apoptosis, CD2v, Pathogenesis
Abstract
African swine fever virus causes a haemorrhagic fever in domestic pigs and wild boar. The continuing spread in Africa, Europe and Asia threatens the global pig industry. The lack of a vaccine limits disease control. To underpin rational strategies for vaccine development improved knowledge is needed of how the virus interacts with and modulates the host’s responses to infection. The virus long double-stranded DNA genome codes for more than 160 proteins of which many are non-essential for replication in cells but can have important roles in evading the host’s defences. Here we review knowledge of the pathways targeted by ASFV and the mechanisms by which these are inhibited. The impact of deleting single or multiple ASFV genes on virus replication in cells and infection in pigs is summarised providing information on strategies for rational development of modified live vaccines.
1. Introduction
The outcome of infection with African swine fever virus varies dramatically, depending on complex interactions involving both virus and host factors. The infection of the natural reservoir hosts, including warthogs and bush pigs in E. Africa, results in few clinical signs, although animals can remain persistently infected. This reflects a long term adaptation to maintain a virus reservoir without elimination of either the virus or host. A species of soft tick, Ornithodoros moubata, that inhabits warthog burrows, acts as a biological vector and reservoir of the virus and plays a key role in maintaining infection in this transmission cycle (Jori and Bastos, 2009; Jori et al., 2013). In contrast, infection of domestic pigs or wild boar with similar ASFV isolates, results in an acute haemorrhagic fever with very high case fatality (Blome et al., 2013). Although most ASFV isolates cause this acute form of disease in domestic pigs, some less virulent isolates have evolved and these can result in a sub-acute form of disease from which 30–50% of pigs survive (Sanchez-Cordon et al., 2018; Sanchez-Vizcaino et al., 2015). Some low virulence isolates cause few clinical signs and very low fatality (Boinas et al., 2004; Leitao et al., 2001; Zani et al., 2018).
Increasing knowledge of ASFV host interactions at the molecular, cellular and whole animal level provides fresh insights into the processes governing different disease outcomes. This knowledge will also drive development of improved control strategies, including vaccines. In this review we summarise current knowledge and identify knowledge gaps.
2. ASFV cellular tropism
ASFV has a restricted cellular tropism. The primary target cells for replication are macrophages and monocytes, although replication in dendritic cells has also been reported. These cell types play a key role in induction of innate immune responses, responding to specific pathogen associated signals. These cells also present antigen to T cells in order to activate the adaptive immune response, which is vital for development of a protective immune response (Correia et al., 2013; Haig, 2001; Janeway and Medzhitov, 2002). Macrophages are specialised in recognition and destruction of pathogens and initiation of an inflammatory response. Dendritic cells respond to inflammatory responses to become efficient antigen presenting cells and migrate to T cell areas in secondary lymphoid tissues.
2.1. Replication in macrophages and monocytes
Cells typical of monocytes and macrophages, at an intermediate to late stage of differentiation, are the main cells infected both in vitro and in vivo in pigs (McCullough et al., 1999). The virus enters these cells by either receptor-mediated endocytosis, into clathrin-coated pits, or by macropinocytosis, a less specific mechanism (Hernaez and Alonso, 2010; Hernaez et al., 2016; Sanchez et al., 2012). The restricted cellular tropism suggests that receptor-mediated endocytosis is the main mechanism of entry, although the cellular receptor(s) for binding and entry are unknown. Earlier reports suggested that CD163 may be a receptor for ASFV (Sanchez-Torres et al., 2003) but, results showed that deleting the CD163 gene from the pig genome did not restrict virus replication in macrophage cultures and did not result in reduced virulence in pigs (Popescu et al., 2017). The complex ASF virion multi-layered structure adds further complexity to these questions. Both the intracellular mature and the extracellular enveloped forms of the virus are infectious. The outer envelope, which is gained as the virus buds through the plasma membrane, is lost when the virus particles move to the acidic environment of late endosomes (Hernaez et al., 2016). The inner virus envelope fuses with the endosomal membrane releasing the virus core particle into the cytoplasm to initiate the replication cycle (Hernaez et al., 2016). Several virus proteins have been identified that are important in the binding and entry process including p54/pE183 L, p30/pCP204 L and p12/pO61R but the cellular receptors are not known (Alcami et al., 1992; Gomez-Puertas et al., 1998; GomezPuertas et al., 1996).
2.2. Replication in dendritic cells
Porcine dendritic cells can be split into two main populations: conventional DCs (cDCs) and plasmacytoid DCs (pDCs). It is cDCs that are classified as professional antigen presenting cells. The pDCs are specialist type I IFN producing cells, which is key in the maturation of DCs through upregulating MHC class I and II expression and initiation on the adaptive immune response (Summerfield and McCullough, 2009).
There is some evidence that dendritic cells are susceptible to ASFV infection. Initially it was shown that skin-derived DCs were susceptible in vitro (Gregg et al., 1995b), followed by the identification of ASFV antigens in interdigitating DCs (iDCs) in the mandibular lymph nodes at 3 days post-infection (Gregg et al., 1995a). More recently it has been shown that monocyte derived dendritic cells (MoDCs) are susceptible to infection with both virulent and attenuated strains of ASFV. However, upon maturation with IFNα, there is a decreased susceptibility to infection with attenuated strains. In contrast to this, maturation with TNFα led to an increased susceptibility to infection with virulent isolates (Franzoni et al., 2018). It was also indicated that ASFV infected pDCs could be a source of type I interferon in in vitro infections. This is a potential explanation of the source of high levels of type I interferon in the serum during acute ASFV infections (Golding et al., 2016). The impact of ASFV infection on dendritic cell function has been little studied.
3. Macrophage responses to ASFV
Macrophages have extraordinary plasticity and can adopt different phenotypes and functions in response to intercellular signals (Mosser and Edwards, 2008). This cascade of new adaptation includes activation of phagocytosis, increased cell size and subsequently activation of different secretory signals, including cytokines and chemokines (Kawai and Akira, 2009; Mogensen, 2009).
3.1. Overcoming barriers to replication in the monocyte/macrophage
To replicate in the hostile, highly-oxidising, environment of the macrophage cytoplasm, ASFV codes for enzymes involved in a DNA base excision repair (BER) pathway (Chapman et al., 2008; Dixon et al., 2013). DNA damage can result in introduction of potentially lethal mutations in the virus genome or, inhibit activity of the virus DNA or RNA polymerases to reduce virus replication. Components of this BER pathway, including the repair DNA polymerase X, AP endonuclease and DNA ligase have been shown to be required for replication in macrophages, but not in tissue culture cells, highlighting the critical role of the BER pathway in macrophages (Table 1) (Redrejo-Rodriguez et al., 2009, 2013; Redrejo-Rodriguez and Salas, 2014). Components of the BER pathway are packaged in virus particles, ready for use early during replication when the virus core particles enter cells.
Table 1.
Non-essential genes identified on the African swine fever virus genome.
| A Gene Deleted | B Function | C Isolate | D Growth in cells | E Virulence in pigs | F Ref. |
|---|---|---|---|---|---|
| DNA repair pathway, genome integrity and nucleotide metabolism | |||||
| Q174L | DNA repair polymerase X |
BA71 V (a) | Required for efficient macrophage growth | ND | (Redrejo-Rodriguez et al., 2013) |
| E296R | AP endonuclease | BA71 V (a) | Required for macrophage growth | ND | (Redrejo-Rodriguez et al., 2006) |
| E165R | dUTPase | BA71 V (a) | Required for macrophage growth | ND | (Oliveros et al., 1999) |
| A240L | Thymidine kinase | Malawi (v) Haiti (v) |
Required for macrophage growth | Attenuated Partial protection |
(Sanford et al., 2016) |
| Type I Interferon Response | |||||
| MGF360 MGF 505/530 |
Type I Interferon Inhibitors |
Benin 97/1(v) | No effect | Attenuated Good protection |
(Reis et al., 2016) |
| MGF360 MGF 505/530 |
Type I Interferon Inhibitors |
Pr4 (v) | No effect | Attenuated Good protection |
(Zsak et al., 2001) |
| MGF360 MGF 505/530 |
Type I Interferon Inhibitors |
Georgia (v) | No effect | Attenuated Good protection |
(O’Donnell et al. (2015b)) |
| DP96R(UK) | IFN inhibitor | Malawi (v) | No effect | Attenuated Induced protection |
(Zsak et al., 1998) |
| EP402R/CD2v | Binding to red blood cells | BA71 (v) | No effect | Attenuated Good protection |
(Monteagudo et al., 2017) |
| EP402R/CD2v/8-DR | Binding to red blood cells | Malawi LIL20/1(v) | No effect | Delay in clinical signs | (Borca et al., 1998) |
| B119 L(9 G L) | morphogenesis | Malawi (v) | Reduced replication | Attenuated Induced protection |
(Lewis et al., 2000) |
| B119 L(9 G L) | morphogenesis | Georgia (v) | Reduced replication | At low doses attenuated and Induced protection | (O’Donnell et al. (2015c)) |
| DP96R(UK) B119 L (9 G L) |
Georgia (v) | Reduced replication | Attenuated Induced protection |
(O’Donnell et al. (2017)) | |
| MGF 360 MGF 505/530 9 G L |
IFN inhibitor morphogenesis |
Georgia (v) | Reduced replication | Attenuated No protection |
(O’Donnell et al. (2016)) |
| A224 L/4CL | IAP apoptosis inhibitor | Malawi (v) | No effect | No reduction in virulence | (Neilan et al., 1997a; Reis et al., 2017) |
| DP71 L/NL | Malawi (v) Pr4 (v) |
(Afonso et al., 1998a) | |||
| DP71 L/NL | E70 (v) | Attenuated Induced protection |
(Afonso et al., 1998a) | ||
| L83L | IL-1beta binding protein | Georgia (v) | No effect | No reduction in virulence | (Borca et al., 2018) |
| DP148R | Benin 97/1 (v) | No effect | Attenuated Induced protection |
(Reis et al., 2017) | |
| EP153R | C-type lectin | Malawi (v) | No effect | No reduction in virulence | (Neilan et al., 1999) |
| A238L | Inhibitor of inflammatory responses | Malawi (v) | No effect | No reduction in virulence | (Neilan et al., 1997b) |
| L11 L | Transmembrane | Malawi (v) | No effect | No reduction in virulence | (Kleiboeker et al., 1998) |
Column A shows the name of the gene(s) deleted and column B the function of the gene if known. Column C shows the isolate from which the gene has been deleted and v) or (a) shows if this is a virulent or attenuated isolate. The effect of the gene deletion on virus replication in macrophages or tissue culture cells is shown in column D and the effect on virus virulence in pigs indicated in column E. Column F gives the reference to the research.
The virus encoded nucleotide metabolism enzyme, thymidine kinase, is also important for efficient replication in macrophages, but not in tissue culture cells. This is presumably to increase pools of dNTPs that are required for virus genome replication, but present at relatively low levels in non-dividing macrophages. Deletion of this gene from the virulent Georgia isolate attenuated the virus in pigs, most likely due to the restricted ability of the virus to replicate in macrophages (Table 1) (Sanford et al., 2016).
4. Modulation of the type I interferon response
The induction of the type I interferon response involves recognition of pathogen associated molecular patterns (PAMPs), including viral RNA or DNA, by pattern recognition receptors (PRRs), in the cytoplasm, or on cellular or endosomal membranes. Sensing of viral DNA is mediated by the cyclic GMP-AMP synthase (cGAS) binding to the viral DNA to catalyse the generation of cyclic dinucleotides (CDN) known as cyclic GMP-AMP (cGAMP) in the presence of ATP and GTP (Danilchanka and Mekalanos, 2013). The CDN or cGAMP, binds to the stimulator of interferon gene encoded protein (STING), which is associated with the ER. This interaction between CDN and STING results in conformational changes in STING, allowing it to form a complex with TANK-binding kinase 1 (TBK1)(Cai et al., 2014; Ishikawa and Barber, 2008; Saitoh et al., 2009). This leads to the transport of the complex to the perinuclear space, via pre- autophagosomal like structures (STING related autophagy), which are potentially derived from the ER (Ishikawa et al., 2009). This re-localization of the STING, CDN and TBK1 complex is essential for delivering the TBK1 to the endolysosomal chamber, where it phosphorylates transcription factors nuclear factor-kappa B (NF-κB) and interferon regulatory gene 3 (IRF3)(Cai et al., 2014; Ishikawa and Barber, 2008; Saitoh et al., 2009). These transcription factors are translocated to the nucleus to initiate transcription of type I interferon (Konno et al., 2013). Viral RNA activates cytoplasmic sensors RIG-I and MDA-5, which leads to relocation of MAVS protein from the mitochondria and activation of IRF3. Toll-like receptors, present on the endosomal membrane, sense exogenous nucleic acids. In particular TLR3 sensing of double-stranded RNA, leads to activation of TRAF3 and TBK1, which are upstream of IRF3 activation (Randall and Goodbourn, 2008).
As a cytoplasmic DNA virus, it is likely that the DNA sensing pathway is key to the sensing of ASFV infection and induction of type I IFN. However, recognition of double stranded RNA, either transcribed from viral DNA by cytoplasmic host RNA polymerase III, or by the viral RNA polymerase, is also likely to have a role in sensing infection and induction of type I interferon. Currently the relative importance of different PAMPs and PRRs involved in induction of type I IFN in ASFV infected macrophages is unknown.
ASFV encodes multiple genes that inhibit type I interferon responses. In macrophages infected with virulent ASFV, induction of type I IFN is suppressed. However deletion of multiple genes belonging to Multigene families 360 (MGF360) and MGF530/505 results in increased induction of type I IFN and genes stimulated by type I IFN in infected macrophages (Table 1) (Afonso et al., 2004; Reis et al., 2017). These results show that at least some members of these gene families suppress the production of type I interferon. MGF360-15R (A276R) inhibits the induction of type I IFN via both TLR3 and the cytosolic sensing pathways by inhibiting IRF3 through a mechanism independent of IRF7 and NF-κB transcription factors. Hence, a limited cellular type I IFN response is generated (Correia et al., 2013). MGF505-7R (A528R) inhibits the type I IFN induction through the inhibition of both IRF3 and NF-κB transcription factors. This protein also inhibits the type I and II IFN signalling pathways (Correia et al., 2013).
The ASFV pI329 L protein is similar to cellular TLR3; it is a highly glycosylated protein expressed in the cell membrane and inhibits TLR3-mediated induction of IFN- β and activation of both NF-κB and IRF3. The pI329 L protein was indicated to target TRIF, an adaptor protein in this pathway. Overexpression of TRIF reversed the inhibition of NF-κB and IRF3 activation (de Oliveira et al., 2011; Henriques et al., 2011).
4.1. Modulation of the IFN response pathway
In response to the binding of secreted IFN l (α/β) to their respective receptors, infected or neighbouring cells initiate the Janus kinase/signal transducer and activator of transcription JAKs STATs pathway. This leads to activation of STAT 1 and 2, by phosphorylation, resulting in dimerization and association with IRF9, forming a complex known as "interferon stimulated gene factor 3″ (ISGF3). This complex translocates to the nucleus to activate transcription of several hundred interferon stimulated genes (ISGs). These have a variety of antiviral activities, which can restrict virus replication. The ISGs also include cytokines, chemokines and other proteins involved in activating the innate and adaptive responses to control infection (Randall and Goodbourn, 2008).
Pre-treatment of cells with type I IFN, to activate ISGs and create an antiviral state, did not reduce replication of virulent ASFV isolates. This indicates that the virus has mechanisms to inhibit type I IFN responses and the antiviral state. Gene-deleted viruses lacking members of MGFs 360 and 505/530 were partially sensitive to type I IFN pre-treatment suggesting these have a role in inhibiting the IFN responses or antiviral state (Golding et al., 2016).
As described above, the ASFV MGF 505-7R (A528R) gene encodes a protein that can inhibit both the type I and type II IFN signalling pathways, hence limiting the impact of IFN l and IFN ll on induction of the JAK-STAT pathway and expression of ISGs. The mechanism by which MGF 505-7R A528R supress the induction of IFNs is not well described and other proteins with this function have not yet been described (Correia et al., 2013).
The impact of deleting members of the MGF 360 and 505/530 genes, from genomes of virulent ASFV isolates, has established that multiple copies of these genes can be deleted without reducing the ability of the virus to replicate in macrophages (Table 1). Immunisation of pigs with deletion mutants of virulent viruses, lacking several different members of MGFs 360 and 505/530, confirmed that they were attenuated in pigs (Afonso et al., 2004; O’Donnell et al. (2015b); Reis et al., 2016). Moreover, an immune response was induced that protected pigs against challenge with virulent viruses. The levels of protection varied depending on dose, the parental virus and the genes deleted (Afonso et al., 2004; O’Donnell et al. (2015a); Reis et al., 2016). These results confirm the importance of the type I IFN system in controlling ASFV replication and in activating a protective immune response in infected pigs. An increased induction of type I IFN in infected macrophages would lead to amplification of this signal in neighbouring cells and induction of ISGs to activate innate responses and reduce virus replication (Afonso et al., 2004). Activation of the antiviral state in cells may have a limited effect on controlling ASFV replication however activation and recruitment of innate response cells could be an important factor in controlling levels of virus replication (Golding et al., 2016). This would allow further time for activation and direction of the adaptive response to clear infection.
4.2. Modulation of interferon responses in vivo
As described above, ASFV has been shown to inhibit expression of type I IFN in cell culture. However, in vivo data has shown the presence of IFN in serum of animals infected with virulent isolates (Karalyan et al., 2012). Both IFNα and IFNβ were detected in serum of animals infected with virulent ASFV, and this coincided with viraemia (Golding et al., 2016).
5. Inhibition of apoptosis
Induction of cell death by apoptosis is a common cellular response to viral infection. This has the effect of limiting replication and spread of viral progeny. As a result many viruses have developed mechanisms to inhibit apoptosis in the infected cells. ASFV encodes a number of anti-apoptotic proteins that act via several pathways, as described below.
5.1. Bcl-2 family member A179L
The A179L protein is a member of the B-cell lymphoma-2 (Bcl-2) family (Brun et al., 1996; Revilla et al., 1997). Members of this family can be either pro-apoptotic or anti-apoptotic as determined by the presence of up to 4 Bcl-2 homology regions (BH1- BH4) and their interactions with other proteins from the Bcl-2 family. The apoptosis inducers include BH3-only proteins, which sense cellular damage and initiate the death process. The Bax and Bak proteins are activated downstream of BH3-only proteins. Bak and Bax are the gatekeepers of mitochondrial integrity and their activation results in loss of mitochondrial membrane potential and release of cytochrome C, formation of the APAF1/cytochrome C complex and activation of the caspase cascade, including caspase 9 and downstream caspases 3 and 7 (Kvansakul et al., 2017; Youle and Strasser, 2008).
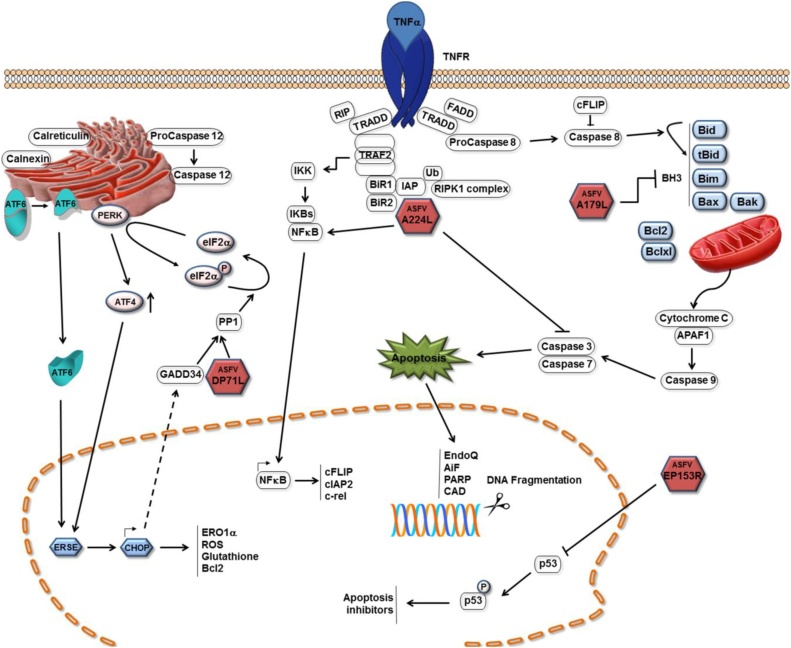
The BH3-only proteins include Bim, Bid, Puma, Noxa, Bmf, Bik, Bad and Hrk, and function either by directly activating Bak and Bax, or sequestering and neutralizing the pro-survival Bcl-2 members. The A179 L protein contains domains similar to all BH domains and interacts with a number of pro-apoptotic BH3 only domain proteins. The binding affinity of A179 L protein, with a spectrum of peptides coding for BH3 domains from these proteins, has been determined as well as the structure of A179 L bound to BH3 domain peptides from Bid and Bax (Banjara et al., 2017). This analysis has shown that A179 L binds with highest affinity to the truncated Bid, Bim, Puma and also with the downstream Bak and Bax proteins. The broad spectrum of interactions with both upstream pro-apoptotic BH3 domain proteins and the downstream Bak and Bax proteins is potentially to ensure a widespread inhibition of apoptosis within infected cells and in the different virus hosts (Fig. 1) (Banjara et al., 2017; Galindo et al., 2008). The A179 L protein also has potential roles in inhibiting autophagy mediated by binding to the BH3 domain of Beclin-1. This has potential to modulate peptides presented to T cells by the swine leucocyte antigen 1 (SLA 1) complex (Fig. 1) (Hernaez et al., 2013). Currently information is lacking on the targets and impact of A179 L in infected cells, particularly macrophages and monocytes, the main target cells for replication in vivo.
Fig. 1.
Mechanisms of apoptosis inhibition by ASFV. Pathways by which ASFV inhibits induction of apoptosis in infected cells are shown as red icosahedra with the name of the protein inside. The ASFV pA179 L Bcl-2 family protein binds to and inhibits several BH3 only domain pro-apoptotic proteins. The pA224 L IAP-family protein binds to and inhibits caspase 3 and activates NF-kB signalling thus increasing expression of anti-apoptotic genes including cFLIP, cIAP2 and c-rel. The pDP71 L protein recruits protein phosphatase 1 to dephosphorylate eIF-2α restoring global protein synthesis and inhibiting transcriptional activation of pro-apoptotic CHOP. The pEP153R protein inhibits activation of the p53 protein. (Dixon et al., 2017).
The A179 L protein is expressed both early and late post-infection of cells with ASFV, but is not packaged into virus particles (Afonso et al., 1996; Alejo et al., 2018). This suggests that A179 L may be involved in inhibiting apoptosis throughout infection but not at the earliest stage when virus cores enter the cytoplasm.
5.2. Inhibitor of apoptosis family member, A224L
The ASFV A224 L protein is a member of the inhibitor of apoptosis (IAP) protein family and contains a BIR motif, a hallmark of this family. Its expression inhibits apoptosis induced by stimuli including TNF-α. In cells infected with ASFV from which A224 L gene was deleted, increased caspase 3 activity was observed, suggesting that A224 L inhibits caspase 3 activation and can thus caspase 3 activated apoptosis. The mechanism by which this is mediated has not been determined but may involve direct binding of A224 L to caspase 3 (Fig. 1). Expression of A224 L has been shown to activate NF-κB, potentially inhibiting apoptosis by activating transcription of a number of anti-apoptotic genes, including IAP and Bcl-2 family members. Activation of NF-kB also drives expression of cFLIP, an inactive caspase 8 homolog that inhibits caspase 8 activity. A224 L is expressed as a late protein and is also packaged into virus particles indicating potential roles both immediately following entry of virus cores in the cytoplasm as well as later during infection (Nogal et al., 2001; Rodriguez et al., 2002).
Deletion of the A224 L gene, from the genome of a virulent isolate, did not reduce ASFV replication in macrophages nor virulence in domestic pigs (Table 1) (Neilan et al., 1997a). This suggests that other ASFV proteins may compensate for loss of A224 L and/or the pathway of apoptosis induction through the TNF-α receptor is less important during in vivo infections.
5.3. Inhibitors of stress-induced apoptosis
Infection of cells leads to activation of cellular stress, for example by inducing endoplasmic reticulum stress to activate the unfolded protein response (UPR). This results in activation of the endoplasmic reticulum resident protein kinase PERK. The double-stranded RNA activated protein kinase PKR is also often activated by virus infection. These activated protein kinases phosphorylate translation initiation factor eIF2-α. During translation initiation eIF2 associates with initiator Met-tRNAi, GTP and the 40S ribosomal subunit. The phosphorylation of eIF2-α, at serine 51, inhibits the exchange of eIF2-GDP for eIF2-GTP blocking delivery of the initiator tRNA to the ribosome and reducing global protein synthesis (Ron and Walter, 2007; Walter and Ron, 2011). Translation of a subset of mRNAs, with short upstream open reading frames, can however continue in the presence of phosphorylated eIF-2α. These mRNAs encode a number of proteins, including the transcription factor ATF4 and its downstream target CHOP, which activate transcription of pro-apoptotic proteins (Hinnebusch et al., 2016; Young and Wek, 2016). The ASFV DP71 L protein interacts with PP1 and eIF-2α to target dephosphorylation of eIF-2α and so restore global protein synthesis (Fig. 1). As a consequence, the activation of the pro-apoptotic transcription factor CHOP is inhibited, thus inhibiting apoptosis induced by this pathway (Rivera et al., 2007; Zhang et al., 2010). The Herpes simplex virus 1, (HSV-1) ICP34.5 and host DNA damage-inducible, GADD34 proteins also target the dephosphorylation of eIF-2α via interaction with PP1. These proteins share a conserved C-terminal domain with DP71 L (Zhang et al., 2008).
In cells infected with ASFV with the DP71 L gene deleted from Malawi Lil20/1 or E70 isolates, no increase in phosphorylation of eIF-2α or CHOP induction was detected, compared with cells infected with wild-type viruses. This suggests that ASFV encodes other inhibitors of this pathway (Zhang et al., 2010). DP71 L may be required for virulence in some isolates, but not others. For example, deletion of the long and short form of DP71 L from Malawi Lil20/1 and Pretoriuskop/96/4 isolates respectively, had no effect on virulence in domestic pigs and resulted in 100% case fatality (Table 1)(Afonso et al., 1998b). In contrast, deletion of the short form of DP71 L from the E70 isolate of ASFV resulted in attenuation of the virus (Zsak et al., 1996). Differences in the gene complement encoded by these viruses may account for the differences observed, for example, E70 may lack genes present in other isolates which can compensate for the loss of DP71 L.
5.4. The ASFV C-type lectin domain containing protein pE153R
pE153R, a C-type lectin domain containing protein, has also been described to inhibit apoptosis, acting through the p53 pathway and caspase 3 activation (Hurtado et al., 2004). As p53 activates the transcription of many apoptosis inhibitors this could explain its mechanism of action (Granja et al., 2004a). Deletion of the EP153R gene did not reduce virulence of virus for pigs.
5.5. Induction of bystander apoptosis in vivo
ASFV has a number of mechanisms to inhibit apoptosis in infected cells and allow virus replication, however, at later stages of infection apoptosis is observed. This may facilitate virus spread and uptake by monocytes/macrophages in apoptotic bodies, thus avoiding inflammatory responses induced by necrotic cell death. Apoptosis of infected monocytes/macrophages is also observed in vivo. A major characteristic of acute ASFV disease is the massive apoptosis of bystander non-infected B and T lymphocytes induced in lymphoid tissue and in blood. Both lymphoid depletion in primary and secondary lymphoid organs and the death of infiltrate lymphocytes in non-lymphoid organs, such as liver and kidney, have been attributed to massive apoptosis of lymphocyte subsets. The mechanisms involved in inducing bystander lymphocyte apoptosis are poorly understood (Carrasco et al., 1996; Gomez-Villamandos et al., 2013; Oura et al., 1998b; RamiroIbanez et al., 1996). The presence of infected macrophages, close to areas with intense apoptosis, suggested that the infected cells may secret or present cell surface factors, which induce apoptosis in bystander uninfected lymphocytes. TNF-α has been suggested as one possible factor involved (Oura et al., 1998a; Salguero et al., 2002). This massive destruction of lymphocytes caused by apoptosis provides a mechanism of immunosuppression.
6. Inhibition of inflammatory responses by ASFV
6.1. Transcriptional regulation of inflammatory responses by the pA238L protein
The pA238 L protein contains ankyrin repeats, similar to those in the I-kB inhibitor of NF-kB transcription factor. The NF-kB transcription factor is required for transcriptional activation of a number of inflammatory responses in addition to its role in activation of the type I IFN promoter. The first described function was inhibition of NF-kB activation (Powell et al., 1996). Subsequently, pA238 L was shown to bind to and inhibit the calcium/calmodulin-regulated phosphatase calcinerurin (CaN), thus inhibiting pathways activated by this phosphatase, including the nuclear factor of activated T cell cytoplasmic (NFAT) family of transcription factors (Miskin et al., 2000, 1998). In resting cells NFAT factors are present as hyper phosphorylated forms in the cytoplasm and when dephosphorylated by calcineurin, translocate to the nucleus to activate transcription of genes dependent on NFAT. Further complexity was added since A238 L was shown to inhibit transcriptional activation mediated by several factors that interact with the N-terminal domain of the transcriptional co-activator p300/CBP. These included NF-ATc2, NF-kappa B, and c-Jun (Granja et al., 2008, 2009). In keeping with these predicted functions, pA238 L was shown to shuttle between the cytoplasm and nucleus of cells and is expressed as an early protein during infection (Silk et al., 2007). Evidence also accumulated, both from analysis of cells expressing pA238 L and deletion mutants lacking the gene, that the pA238 L protein is a potent anti-inflammatory protein and functioned to inhibit induction of TNF-α and pathways activated by TNF-α, inducible nitric oxide synthase and cyclo-oxygenase (Granja et al., 2006, 2004b; Granja et al., 2008). Deletion of the A238 L gene from a virulent ASFV isolate did not reduce virus replication in macrophages or virulence in pigs although levels of TNF-α were increased (Salguero et al., 2008).
6.2. L83L a putative IL-1β binding protein
The pl83 L 83 amino acid protein is encoded close to the left end of the genome. This protein was shown to bind to IL-1β using a yeast two-hybrid assay (Borca et al., 2018). The authors suggested pl83 L protein may have a role in modulating the activity of this proinflammatory cytokine although evidence for this role in vivo is lacking. Deletion of this gene did not reduce virus replication in macrophages or reduce virulence in domestic pigs (Table 1). The functional role of this protein requires further investigation.
7. The role of ASFV adhesion protein CD2v
The ASFV genome contains an ORF, EP402R, which encodes a type I transmembrane protein. Various terminologies have been used in the literature for its identity such as CD2v, CD2- like, 5 H L, pEP402R and 8-DR (Borca et al., 1998; Goatley and Dixon, 2011; Kay-Jackson et al., 2004; Rodriguez et al., 1993). The viral pEP402R/CD2v protein is similar in its extracellular domain to mammalian encoded CD2 and contains two Ig –like domains (Rodriguez et al., 1993). The ASFV pCD2v/pEP402R protein has a role in virus dissemination and immune evasion. It is required for virus-induced rosetting of erythrocytes around the infected cells (haemadsorbtion or HAD) and binding of extracellular virus particles to erythrocytes (Borca et al., 1998). ASFV infection of cultured peripheral blood mononuclear cells (PBMC) reduces the mitogen-dependent proliferation of lymphocytes, which do not become infected. Deletion of the gene encoding pCD2v/pEP402R abrogates this effect, indicating a role for CD2v in immune modulation (Borca et al., 1998). The ability of antibodies, from infected pigs, to inhibit the HAD of red blood cells around infected cells has been shown to correlate with cross-protection (Burmakina et al., 2016), suggesting a possible role for this protein in protection (Malogolovkin et al., 2015). Amongst genotype I isolates the sequence is well conserved (Bastos et al., 2003). The pCD2v/pEP402R protein is the only protein definitively shown to be present on the external envelope of virus particles, suggesting a role in virus entry or spread between cells (Alejo et al., 2018).
The cytoplasmic tail of pCD2v/pEP402R differs from that of mammalian CD2 and contains variable numbers of a proline rich repeat, which binds to a host SH3 domain containing protein, SH3P7/mAbp1. This protein binds to actin and has roles in vesicle transport (Kay-Jackson et al., 2004). The CD2v protein also interacts with the adaptor protein AP-1 (Perez-Nunez et al., 2015). The data indicate a possible role for pCD2v/pEP402R protein in modulating vesicular transport. The observed cleavage of the cytoplasmic tail of pCD2v/pEP402R during infection may disrupt this interaction and have a role in cellular localisation of the protein (Goatley and Dixon, 2011).
The role of the pCD2v/pEP402R protein in virulence in pigs depends on the genetic background of the virus. Most ASFV isolates are HAD positive and express a functional pCD2v/EP402R protein. A few low virulence isolates have been described which are non-HAD (Boinas et al., 2004; Leitao et al., 2001). In one study deletion of the EP402R gene encoding pCD2v/pEP402R did not reduce the lethality of the virus, although a delay in onset of clinical signs and virus dissemination was observed (Borca et al., 1998). A recent study showed that deletion of the EP402R gene from the BA71 virulent isolate resulted in virus attenuation and the induction of protection against challenge with virulent virus (Monteagudo et al., 2017) (Table 1). One study suggested that pCD2v/EP402R is required for efficient viral replication in the tick vector, by increasing uptake of virus across the tick midgut (Rowlands et al., 2009). Since the tick vector plays a key role in the transmission cycle involving warthogs, there is likely to be a selection pressure to retain this function in that cycle.
8. Future research
Progress has been made in understanding ASFV virus host interactions and virus encoded proteins that modulate host responses to infection, but much remains to be investigated. Information is lacking on the phenotype of macrophages/monocytes that are susceptible to infection and the potential role of infection of dendritic cells in pathogenesis and immune responses. The barriers to infection of non-susceptible cells are also not known. Determination of the ASFV proteome map has provided groundwork to further investigate virus and host proteins involved in virus entry. This would provide additional targets for vaccine development or other strategies to control infection.
An important feature of the ASFV genome is the large number of genes encoded that belong to different multigene families (MGFs). Members of two of these families MGF360 and 505/530 have been shown to be virulence determinants for pigs and to act by inhibiting type I IFN induction and/or responses. However, there is no information on the mechanisms by which these proteins act and the role of individual genes in these families. Additional ASFV proteins that inhibit IFN induction or responses have been identified one of which, I329 L, acts as an agonist of TLR3 signalling. Further information on the mechanism of actions and role of these proteins in infected cells and in vivo is needed. Likewise, virus inhibitors of the NF-kB transcription pathway have been identified, but more information is required about their roles in cells and during in vivo infections.
ASFV proteins that inhibit several apoptotic pathways have been identified. The cellular targets of the A179 L Bcl-2 family member have been identified as a broad range of members of the BH3 only domain pro-apoptotic proteins. As yet the preferred binding targets in infected cells haven’t been defined nor the impact of deleting the A179 L gene from the virus genome. The mechanism by which the DP71 L protein acts to prevent global protein synthesis shut-off and stress-induced apoptosis mediated by CHOP has been defined. Evidence from analysis of cells infected with virus gene deletion mutants lacking this gene indicates that other ASFV inhibitors of this pathway remain to be identified.
A full report on gaps in knowledge and priorities for research is available on the website of the Global Alliance for Research on African swine fever virus (GARA).
Acknowledgements
We thank many colleagues for stimulating discussions and suggestions. We acknowledge support from BBSRC Grant References BBS/E/1/00007031, 7034
References
- Afonso C.L., Neilan J.G., Kutish G.F., Rock D.L. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J. Virol. 1996;70(7):4858–4863. doi: 10.1128/jvi.70.7.4858-4863.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso C.L., Zsak L., Carrillo C., Borca M.V., Rock D.L. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 1998;79:2543–2547. doi: 10.1099/0022-1317-79-10-2543. [DOI] [PubMed] [Google Scholar]
- Afonso C.L., Zsak L., Carrillo C., Borca M.V., Rock D.L. African swine fever virus NL gene is not required for virus virulence. J. Gen. Virol. 1998;79(Pt 10):2543–2547. doi: 10.1099/0022-1317-79-10-2543. [DOI] [PubMed] [Google Scholar]
- Afonso C.L., Piccone M.E., Zaffuto K.M., Neilan J., Kutish G.F., Lu Z., Balinsky C.A., Gibb T.R., Bean T.J., Zsak L., Rock D.L. African swine fever virus multigene family 360 and 530 genes affect host interferon response. J. Virol. 2004;78(4):1858–1864. doi: 10.1128/JVI.78.4.1858-1864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcami A., Angulo A., Lopezotin C., Munoz M., Freije J.M.P., Carrascosa A.L., Vinuela E. Amino-acid-Sequence and structural-properties of Protein-P12, an african swine fever virus attachment protein. J. Virol. 1992;66(6):3860–3868. doi: 10.1128/jvi.66.6.3860-3868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejo A., Matamoros T., Guerra M., Andres G. A proteomic atlas of the african swine fever virus particle. J. Virol. 2018;92(23) doi: 10.1128/JVI.01293-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjara S., Caria S., Dixon L.K., Hinds M.G., Kvansakul M. Structural insight into African swine fever virus A179L-mediated inhibition of apoptosis. J. Virol. 2017;91(6) doi: 10.1128/JVI.02228-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos A.D.S., Penrith M.L., Cruciere C., Edrich J.L., Hutchings G., Roger F., Couacy-Hymann E., Thomson G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003;148(4):693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- Blome S., Gabriel C., Beer M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013;173(1):122–130. doi: 10.1016/j.virusres.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Boinas F.S., Hutchings G.H., Dixon L.K., Wilkinson P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004;85:2177–2187. doi: 10.1099/vir.0.80058-0. [DOI] [PubMed] [Google Scholar]
- Borca M.V., Carrillo C., Zsak L., Laegreid W.W., Kutish G.F., Neilan J.G., Burrage T.G., Rock D.L. Deletion of a CD2-like gene, 8-DR, from African swine fever virus affects viral infection in domestic swine. J. Virol. 1998;72(4):2881–2889. doi: 10.1128/jvi.72.4.2881-2889.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borca M.V., O’Donnell V., Holinka L.G., Ramirez-Medina E., Clark B.A., Vuono E.A., Berggren K., Alfano M., Carey L.B., Richt J.A., Risatti G.R., Gladue D.P. The L83L ORF of African swine fever virus strain Georgia encodes for a nonessential gene that interacts with the host protein IL-1 beta. Virus Res. 2018;249:116–123. doi: 10.1016/j.virusres.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Brun A., Rivas C., Esteban M., Escribano J.M., Alonso C. African swine fever virus gene A179L, a viral homologue of bcl- 2, protects cells from programmed cell death. Virology. 1996;225(1):227–230. doi: 10.1006/viro.1996.0592. [DOI] [PubMed] [Google Scholar]
- Burmakina G., Malogolovkin A., Tulman E.R., Zsak L., Delhon G., Diel D.G., Shobogorov N.M., Morgunov Y.P., Morgunov S.Y., Kutish G.F., Kolbasov D., Rock D.L. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J. Gen. Virol. 2016;97:1670–1675. doi: 10.1099/jgv.0.000490. [DOI] [PubMed] [Google Scholar]
- Cai X., Chiu Y.H., Chen Z.J. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol. Cell. 2014;54(2):289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Carrasco L., deLara F.C.M., delasMulas J.M., GomezVillamandos J.C., Perez J., Wilkinson P.J., Sierra M.A. Apoptosis in lymph nodes in acute African swine fever. J. Comp. Pathol. 1996;115(4):415–428. doi: 10.1016/s0021-9975(96)80075-2. [DOI] [PubMed] [Google Scholar]
- Chapman D.A.G., Tcherepanov V., Upton C., Dixon L.K. Comparison of the genome sequences of nonpathogenic and pathogenic African swine fever virus isolates. J. Gen. Virol. 2008;89:397–408. doi: 10.1099/vir.0.83343-0. [DOI] [PubMed] [Google Scholar]
- Correia S., Ventura S., Parkhouse R.M. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 2013;173(1):87–100. doi: 10.1016/j.virusres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Danilchanka O., Mekalanos J.J. Cyclic dinucleotides and the innate immune response. Cell. 2013;154(5):962–970. doi: 10.1016/j.cell.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira V.L., Almeida S.C.P., Soares H.R., Crespo A., Marshall-Clarke S., Parkhouse R.M.E. A novel TLR3 inhibitor encoded by African swine fever virus (ASFV) Arch. Virol. 2011;156(4):597–609. doi: 10.1007/s00705-010-0894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L.K., Chapman D.A.G., Netherton C.L., Upton C. African swine fever virus replication and genomics. Virus Res. 2013;173(1):3–14. doi: 10.1016/j.virusres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Dixon L.K., Sanchez-Cordon P.J., Galindo I., Alonso C. Investigations of Pro- and anti-apoptotic factors affecting african swine fever virus replication and pathogenesis. Viruses-Basel. 2017;9(9) doi: 10.3390/v9090241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni G., Graham S.P., Sanna G., Angioi P., Fiori M.S., Anfossi A., Amadori M., Giudici S.D., Oggiano A. Interaction of porcine monocyte-derived dendritic cells with African swine fever viruses of diverse virulence. Vet. Microbiol. 2018;216:190–197. doi: 10.1016/j.vetmic.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Galindo I., Hernaez B., Diaz-Gil G., Escribano J.M., Alonso C. A 179L, a viral Bcl-2 homologue, targets the core Bcl-2 apoptotic machinery and its upstream BH3 activators with selective binding restrictions for Bid and Noxa. Virology. 2008;375(2):561–572. doi: 10.1016/j.virol.2008.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goatley L.C., Dixon L.K. Processing and localization of the african swine fever virus CD2v transmembrane protein. J. Virol. 2011;85(7):3294–3305. doi: 10.1128/JVI.01994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J.P., Goatley L., Goodbourn S., Dixon L.K., Taylor G., Netherton C.L. Sensitivity of African swine fever virus to type I interferon is linked to genes within multigene families 360 and 505. Virology. 2016;493 doi: 10.1016/j.virol.2016.03.019. 154-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GomezPuertas P., Rodriguez F., Oviedo J.M., RamiroIbanez F., RuizGonzalvo F., Alonso C., Escribano J.M. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 1996;70(8):5689–5694. doi: 10.1128/jvi.70.8.5689-5694.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Puertas P., Rodriguez F., Oviedo J.M., Brun A., Alonso C., Escribano J.M. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology. 1998;243(2):461–471. doi: 10.1006/viro.1998.9068. [DOI] [PubMed] [Google Scholar]
- Gomez-Villamandos J.C., Bautista M.J., Sanchez-Cordon P.J., Carrasco L. Pathology of African swine fever: the role of monocyte-macrophage. Virus Res. 2013;173(1):140–149. doi: 10.1016/j.virusres.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Granja A.G., Nogal M.L., Hurtado C., Salas J., Salas M.L., Carrascosa A.L., Revilla Y. Modulation of p53 cellular function and cell death by African swine fever virus. J. Virol. 2004;78(13):7165–7174. doi: 10.1128/JVI.78.13.7165-7174.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A.G., Nogal M.L., Hurtado C., Vila V., Carrascosa A.L., Salas M.L., Fresno M., Revilla Y. The viral protein A238L inhibits cyclooxygenase-2 expression through a nuclear factor of activated T cell-dependent transactivation pathway. J. Biol. Chem. 2004;279(51):53736–53746. doi: 10.1074/jbc.M406620200. [DOI] [PubMed] [Google Scholar]
- Granja A.G., Nogal M.L., Hurtado C., del Aguila C., Carrascosa A.L., Salas M.L., Fresno M., Revilla Y. The viral protein A238L inhibits TNF-alpha expression through a CBP/p300 transcriptional coactivators pathway. J. Immunol. 2006;176(1):451–462. doi: 10.4049/jimmunol.176.1.451. [DOI] [PubMed] [Google Scholar]
- Granja A.G., Perkins N.D., Revilla Y. A238L inhibits NF-ATc2, NF-kappa B, and c-Jun activation through a novel mechanism involving protein kinase C-theta-mediated up-regulation of the amino-terminal transactivation domain of p300. J. Immunol. 2008;180(4):2429–2442. doi: 10.4049/jimmunol.180.4.2429. [DOI] [PubMed] [Google Scholar]
- Granja A.G., Sanchez E.G., Sabina P., Fresno M., Revilla Y. African swine fever virus blocks the host cell antiviral inflammatory response through a direct inhibition of PKC-theta-Mediated p300 transactivation. J. Virol. 2009;83(2):969–980. doi: 10.1128/JVI.01663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg D.A., Mebus C.A., Schlafer D.H. Early infection of interdigitating dendritic cells in the pig lymph-node with african swine fever viruses of high and low virulence - immunohistochemical and ultrastructural studies. J. Vet. Diagn. Investig. 1995;7(1):23–30. doi: 10.1177/104063879500700104. [DOI] [PubMed] [Google Scholar]
- Gregg D.A., Schlafer D.H., Mebus C.A. African swine fever virus-infection of skin-derived dendritic cells in-vitro causes interference with subsequent foot-and-Mouth-Disease virus-infection. J. Vet. Diagn. Investig. 1995;7(1):44–51. doi: 10.1177/104063879500700106. [DOI] [PubMed] [Google Scholar]
- Haig D.M. Subversion and piracy: DNA viruses and immune evasion. Res. Vet. Sci. 2001;70(3):205–219. doi: 10.1053/rvsc.2001.0462. [DOI] [PubMed] [Google Scholar]
- Henriques E.S., Brito R.M.M., Soares H., Ventura S., de Oliveira V.L., Parkhouse R.M.E. Modeling of the Toll-like receptor 3 and a putative Toll-like receptor 3 antagonist encoded by the African swine fever virus. Protein Sci. 2011;20(2):247–255. doi: 10.1002/pro.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaez B., Alonso C. Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J. Virol. 2010;84(4):2100–2109. doi: 10.1128/JVI.01557-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaez B., Cabezas M., Munoz-Moreno R., Galindo I., Cuesta-Geijo M.A., Alonso C. A179L, a new viral Bcl2 homolog targeting beclin 1 autophagy related protein. Curr. Mol. Med. 2013;13(2):305–316. [PubMed] [Google Scholar]
- Hernaez B., Guerra M., Salas M.L., Andres G. African swine fever virus undergoes outer envelope disruption, capsid disassembly and inner envelope fusion before core release from multivesicular endosomes. PLoS Pathog. 2016;12(4) doi: 10.1371/journal.ppat.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A.G., Ivanov I.P., Sonenberg N. Translational control by 5’ -untranslated regions of eukaryotic mRNAs. Science. 2016;352(6292):1413–1416. doi: 10.1126/science.aad9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado C., Granja A.G., Bustos M.J., Nogal M.L., Gonzalez de Buitrago G., de Yebenes V.G., Salas M.L., Revilla Y., Carrascosa A.L. The C-type lectin homologue gene (EP153R) of African swine fever virus inhibits apoptosis both in virus infection and in heterologous expression. Virology. 2004;326(1):160–170. doi: 10.1016/j.virol.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. 2008. STING Is an Endoplasmic Reticulum Adaptor That Facilitates Innate Immune Signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–U740. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C.A., Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jori F., Bastos A.D.S. Role of wild suids in the epidemiology of african swine fever. Ecohealth. 2009;6(2):296–310. doi: 10.1007/s10393-009-0248-7. [DOI] [PubMed] [Google Scholar]
- Jori F., Vial L., Penrith M.L., Perez-Sanchez R., Etter E., Albina E., Michaud V., Roger F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013;173(1):212–227. doi: 10.1016/j.virusres.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Karalyan Z., Zakaryan H., Sargsyan K., Voskanyan H., Arzumanyan H., Avagyan H., Karalova E. Interferon status and white blood cells during infection with African swine fever virus in vivo. Vet. Immunol. Immunopathol. 2012;145(1-2):551–555. doi: 10.1016/j.vetimm.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay-Jackson P.C., Goatley L.C., Cox L., Miskin J.E., Parkhouse R.M.E., Wienands J., Dixon L.K. The CD2v protein of African swine fever virus interacts with the actin-binding adaptor protein SH3P7. J. Gen. Virol. 2004;85:119–130. doi: 10.1099/vir.0.19435-0. [DOI] [PubMed] [Google Scholar]
- Kleiboeker S.B., Kutish G.F., Neilan J.G., Lu Z., Zsak L., Rock D.L. A consented African swine fever virus right variable region gene, I1 1L, is non-essential for growth in vitro and virulence in domestic swine. J. Gen. Virol. 1998;79:1189–1195. doi: 10.1099/0022-1317-79-5-1189. [DOI] [PubMed] [Google Scholar]
- Konno H., Konno K., Barber G.N. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155(3):688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvansakul M., Caria S., Hinds M.G. The Bcl-2 family in host-virus interactions. Viruses-Basel. 2017;9(10) doi: 10.3390/v9100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitao A., Cartaxeiro C., Coelho R., Cruz B., Parkhouse R.M.E., Portugal F.C., Vigario J.D., Martins C.L.V. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti- virus immune response. J. Gen. Virol. 2001;82:513–523. doi: 10.1099/0022-1317-82-3-513. [DOI] [PubMed] [Google Scholar]
- Lewis T., Zsak L., Burrage T.G., Lu Z., Kutish G.F., Neilan J.G., Rock D.L. An African swine fever virus ERV1-ALR homologue, 9GL, affects virion maturation and viral growth in macrophages and viral virulence in swine. J. Virol. 2000;74(3):1275–1285. doi: 10.1128/jvi.74.3.1275-1285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malogolovkin A., Burmakina G., Tulman E.R., Delhon G., Diel D.G., Salnikov N., Kutish G.F., Kolbasov D., Rock D.L. African swine fever virus CD2v and C-type lectin gene loci mediate serological specificity. J. Gen. Virol. 2015;96:866–873. doi: 10.1099/jgv.0.000024. [DOI] [PubMed] [Google Scholar]
- McCullough K.C., Basta S., Knotig S., Gerber H., Schaffner R., Kim Y.B., Saalmuller A. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98(2):203–212. doi: 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskin J.E., Abrams C.C., Goatley L.C., Dixon L.K. A viral mechanism for inhibition of the cellular phosphatase calcineurin. Science. 1998;281(5376):562–565. doi: 10.1126/science.281.5376.562. [DOI] [PubMed] [Google Scholar]
- Miskin J.E., Abrams C.C., Dixon L.K. African swine fever virus protein A238L interacts with the cellular phosphatase calcineurin via a binding domain similar to that of NFAT. J. Virol. 2000;74(20):9412–9420. doi: 10.1128/jvi.74.20.9412-9420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22(2):240–273. doi: 10.1128/CMR.00046-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo P.L., Lacasta A., Lopez E., Bosch L., Collado J., Pina-Pedrero S., Correa-Fiz F., Accensi F., Navas M.J., Vidal E., Bustos M.J., Rodriguez J.M., Gallei A., Nikolin V., Salas M.L., Rodriguez F. BA71 Delta cd2: a new recombinant live attenuated african swine fever virus with cross-protective capabilities. J. Virol. 2017;91(21) doi: 10.1128/JVI.01058-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilan J.G., Lu Z., Kutish G.F., Zsak L., Burrage T.G., Borca M.V., Carrillo C., Rock D.L. A BIR motif containing gene of African swine fever virus, 4CL, is nonessential for growth in vitro and viral virulence. Virology. 1997;230(2):252–264. doi: 10.1006/viro.1997.8481. [DOI] [PubMed] [Google Scholar]
- Neilan J.G., Lu Z., Kutish G.F., Zsak L., Lewis T.L., Rock D.L. A conserved African swine fever virus I kappa B homolog, 5EL, is nonessential for growth in vitro and virulence in domestic swine. Virology. 1997;235(2):377–385. doi: 10.1006/viro.1997.8693. [DOI] [PubMed] [Google Scholar]
- Neilan J.G., Borca M.V., Lu Z., Kutish G.F., Kleiboeker S.B., Carrillo C., Zsak L., Rock D.L. An African swine fever virus ORF with similarity to C-type lectins is non-essential for growth in swine macrophages in vitro and for virus virulence in domestic swine. J. Gen. Virol. 1999;80:2693–2697. doi: 10.1099/0022-1317-80-10-2693. [DOI] [PubMed] [Google Scholar]
- Nogal M.L., de Buitrago G.G., Rodriguez C., Cubelos B., Carrascosa A.L., Salas M.L., Revilla Y. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J. Virol. 2001;75(6):2535–2543. doi: 10.1128/JVI.75.6.2535-2543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell V., Holinka L.G., Gladue D.P., Sanford B., Krug P.W., Lu X., Arzt J., Reese B., Carrillo C., Risatti G.R., Borca M.V. African swine fever virus georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J. Virol. 2015;89(11):6048–6056. doi: 10.1128/JVI.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell V., Holinka L.G., Gladue D.P., Sanford B., Krug P.W., Lu X.Q., Arzt J., Reese B., Carrillo C., Risatti G.R., Borca M.V. African swine fever virus georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J. Virol. 2015;89(11):6048–6056. doi: 10.1128/JVI.00554-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell V., Holinka L.G., Krug P.W., Gladue D.P., Carlson J., Sanford B., Alfano M., Kramer E., Lu Z.Q., Arzt J., Reese B., Carrillo C., Risatti G.R., Borca M.V. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administered at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J. Virol. 2015;89(16):8556–8566. doi: 10.1128/JVI.00969-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell V., Tiolinka L.G., Sanford B., Krug P.W., Carlson J., Pacheco J.M., Reese B., Risatti G.R., Gladue D.P., Borca M.V. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Res. 2016;221:8–14. doi: 10.1016/j.virusres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- O’Donnell V., Risatti G.R., Holinka L.G., Krug P.W., Carlson J., Velazquez-Salinas L., Azzinaro P.A., Gladue D.P., Borca M.V. Simultaneous deletion of the 9GL and UK genes from the african swine fever virus georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017;91(1) doi: 10.1128/JVI.01760-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveros M., Garcia-Escudero R., Alejo A., Vinuela E., Salas M.L., Salas J. African swine fever virus dUTPase is a highly specific enzyme required for efficient replication in swine macrophages. J. Virol. 1999;73(11):8934–8943. doi: 10.1128/jvi.73.11.8934-8943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oura C.A.L., Powell P.P., Anderson E., Parkhouse R.M.E. The pathogenesis of African swine fever in the resistant bushpig. J. Gen. Virol. 1998;79:1439–1443. doi: 10.1099/0022-1317-79-6-1439. [DOI] [PubMed] [Google Scholar]
- Oura C.A.L., Powell P.P., Parkhouse R.M.E. African swine fever: a disease characterized by apoptosis. J. Gen. Virol. 1998;79:1427–1438. doi: 10.1099/0022-1317-79-6-1427. [DOI] [PubMed] [Google Scholar]
- Perez-Nunez D., Garcia-Urdiales E., Martinez-Bonet M., Nogal M.L., Barroso S., Revilla Y., Madrid R. CD2v interacts with adaptor protein AP-1 during african swine fever infection. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu L., Gaudreault N.N., Whitworth K.M., Murgia M.V., Nietfeld J.C., Mileham A., Samuel M., Wells K.D., Prather R.S., Rowland R.R.R. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology. 2017;501:102–106. doi: 10.1016/j.virol.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Powell P.P., Dixon L.K., Parkhouse R.M.E. An I kappa B homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 1996;70(12):8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RamiroIbanez F., Ortega A., Brun A., Escribano J.M., Alonso C. Apoptosis: a mechanism of cell killing and lymphoid organ impairment during acute African swine fever virus infection. J. Gen. Virol. 1996;77:2209–2219. doi: 10.1099/0022-1317-77-9-2209. [DOI] [PubMed] [Google Scholar]
- Randall R.E., Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 2008;89:1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Redrejo-Rodriguez M., Salas M.L. Repair of base damage and genome maintenance in the nucleo-cytoplasmic large DNA viruses. Virus Res. 2014;179:12–25. doi: 10.1016/j.virusres.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Redrejo-Rodriguez M., Garcia-Escudero R., Yanez-Munoz R.J., Salas M.L., Salas J. African swine fever virus protein pE296R is a DNA repair apurinic/apyrimidinic endonuclease required for virus growth in swine macrophages. J. Virol. 2006;80(10):4847–4857. doi: 10.1128/JVI.80.10.4847-4857.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrejo-Rodriguez M., Ishchenko A.A., Saparbaev M.K., Salas M.L., Salas J. African swine fever virus AP endonuclease is a redox-sensitive enzyme that repairs alkylating and oxidative damage to DNA. Virology. 2009;390(1):102–109. doi: 10.1016/j.virol.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrejo-Rodriguez M., Rodriguez J.M., Suarez C., Salas J., Salas M.L. Involvement of the reparative DNA polymerase pol X of african swine fever virus in the maintenance of viral genome stability in vivo. J. Virol. 2013;87(17):9780–9787. doi: 10.1128/JVI.01173-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A.L., Abrams C.C., Goatley L.C., Netherton C., Chapman D.G., Sanchez-Cordon P., Dixon L.K. Deletion of African swine fever virus interferon inhibitors from the genome of a virulent isolate reduces virulence in domestic pigs and induces a protective response. Vaccine. 2016;34(39):4698–4705. doi: 10.1016/j.vaccine.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A.L., Goatley L.C., Jabbar T., Sanchez-Cordon P.J., Netherton C.L., Chapman D.A.G., Dixon L.K. Deletion of the african swine fever virus gene DP148R does not reduce virus replication in culture but reduces virus virulence in pigs and induces high levels of protection against challenge. J. Virol. 2017;91(24) doi: 10.1128/JVI.01428-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla Y., Cebrian A., Baixeras E., Martinez C., Vinuela E., Salas M.L. Inhibition of apoptosis by the African swine fever virus bcl-2 homologue: role of the BH1 domain. Virology. 1997;228(2):400–404. doi: 10.1006/viro.1996.8395. [DOI] [PubMed] [Google Scholar]
- Rivera J., Abrams C., Hernaez B., Alcazar A., Escribano J.M., Dixon L., Alonso C. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J. Virol. 2007;81(6):2923–2929. doi: 10.1128/JVI.02077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.M., Yanez R.J., Almazan F., Vinuela E., Rodriguez J.F. African swine fever virus encodes a Cd2 homolog responsible for the adhesion of erythrocytes to infected-cells. J. Virol. 1993;67(9):5312–5320. doi: 10.1128/jvi.67.9.5312-5320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C.I., Nogal M.L., Carrascosa A.L., Salas M.L., Fresno M., Revilla Y. African swine fever virus IAP-like protein induces the activation of nuclear factor kappa B. J. Virol. 2002;76(8):3936–3942. doi: 10.1128/JVI.76.8.3936-3942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Rowlands R.J., Duarte M.M., Boinas F., Hutchings G., Dixon L.K. The CD2v protein enhances African swine fever virus replication in the tick vector, Ornithodoros erraticus. Virology. 2009;393(2):319–328. doi: 10.1016/j.virol.2009.07.040. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Fujita N., Hayashi T., Takahara K., Satoh T., Lee H., Matsunaga K., Kageyama S., Omori H., Noda T., Yamamoto N., Kawai T., Ishii K., Takeuchi O., Yoshimori T., Akira S. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl. Acad. Sci. U. S. A. 2009;106(49):20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero F.J., Ruiz-Villamor E., Bautista M.J., Sanchez-Cordon P.J., Carrasco L., Gomez-Villamandos J.C. Changes in macrophages in spleen and lymph nodes during acute African swine fever: expression of cytokines. Vet. Immunol. Immunopathol. 2002;90(1-2):11–22. doi: 10.1016/s0165-2427(02)00225-8. [DOI] [PubMed] [Google Scholar]
- Salguero F.J., Gil S., Revilla Y., Gallardo C., Arias M., Martins C. Cytokine mRNA expression and pathological findings in pigs inoculated with African swine fever virus (E-70) deleted on A238L. Vet. Immunol. Immunopathol. 2008;124(1-2):107–119. doi: 10.1016/j.vetimm.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Sanchez E.G., Quintas A., Perez-Nunez D., Nogal M., Barroso S., Carrascosa A.L., Revilla Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012;8(6) doi: 10.1371/journal.ppat.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cordon P.J., Montoya M., Reis A.L., Dixon L.K. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet. J. 2018;233:41–48. doi: 10.1016/j.tvjl.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Torres C., Gomez-Puertas P., Gomez-del-Moral M., Alonso F., Escribano J.M., Ezquerra A., Dominguez J. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 2003;148(12):2307–2323. doi: 10.1007/s00705-003-0188-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vizcaino J.M., Mur L., Gomez-Villamandos J.C., Carrasco L. An update on the epidemiology and pathology of african swine fever. J. Comp. Pathol. 2015;152(1):9–21. doi: 10.1016/j.jcpa.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Sanford B., Holinka L.G., O’Donnell V., Krug P.W., Carlson J., Alfano M., Carrillo C., Wu P., Lowe A., Risatti G.R., Gladue D.P., Borca M.V. Deletion of the thymidine kinase gene induces complete attenuation of the Georgia isolate of African swine fever virus. Virus Res. 2016;213:165–171. doi: 10.1016/j.virusres.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Silk R.N., Bowick G.C., Abrams C.C., Dixon L.K. African swine fever virus A238L inhibitor of NF-kappa B and of calcineurin phosphatase is imported actively into the nucleus and exported by a CRM1-mediated pathway. J. Gen. Virol. 2007;88:411–419. doi: 10.1099/vir.0.82358-0. [DOI] [PubMed] [Google Scholar]
- Summerfield A., McCullough K.C. Dendritic cells in innate and adaptive immune responses against influenza virus. Viruses-Basel. 2009;1(3):1022–1034. doi: 10.3390/v1031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Young S.K., Wek R.C. Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J. Biol. Chem. 2016;291(33):16927–16935. doi: 10.1074/jbc.R116.733899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L., Forth J.H., Forth L., Nurmoja I., Leidenberger S., Henke J., Carlson J., Breidenstein C., Viltrop A., Hoper D., Sauter-Louis C., Beer M., Blome S. Deletion at the 5’ -end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 2018:8. doi: 10.1038/s41598-018-24740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Tang J., Xie J., Zhang H., Li Y., Zhang J., Verpooten D., He B., Cao Y. A conserved domain of herpes simplex virus ICP34.5 regulates protein phosphatase complex in mammalian cells. FEBS Lett. 2008;582(2):171–176. doi: 10.1016/j.febslet.2007.11.082. [DOI] [PubMed] [Google Scholar]
- Zhang F., Moon A., Childs K., Goodbourn S., Dixon L.K. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J. Virol. 2010;84(20):10681–10689. doi: 10.1128/JVI.01027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Lu Z., Kutish G.F., Neilan J.G., Rock D.L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J. Virol. 1996;70(12):8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Caler E., Lu Z., Kutish G.F., Neiland J.G., Rock D.L. A nonessential African swine fever virus gene UK is a significant virulence determinant in domestic swine. J. Virol. 1998;72(2):1028–1035. doi: 10.1128/jvi.72.2.1028-1035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Lu Z., Burrage T.G., Neilan J.G., Kutish G.F., Moore D.M., Rock D.L. African swine fever virus multigene family 360 and 530 genes are novel macrophage host range determinants. J. Virol. 2001;75(7):3066–3076. doi: 10.1128/JVI.75.7.3066-3076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]