Abstract
Aims
Pelvic lymph node (PLN) radiotherapy for high-risk prostate cancer is limited by late gastrointestinal toxicity. Application of rectal and bowel constraints may reduce risks of side-effects. We evaluated associations between intensity-modulated radiotherapy (IMRT) dose-volume data and long-term gastrointestinal toxicity.
Materials and methods
Data from a single-centre dose-escalation trial of PLN-IMRT were analysed, including conventionally fractionated (CFRT) and hypofractionated (HFRT) radiotherapy schedules. Associations between volumes of rectum and bowel receiving specified doses and clinician- and patient-reported toxicity outcomes were investigated independently. A metric, δ median (δM), was defined as the difference in the medians of a volume between groups with and without toxicity at a specified dose and was used to test for statistically significant differences.
Results
Constraints were respected in most patients and, when exceeded, led to higher rates of gastrointestinal toxicity. Biologically relevant associations between rectum dose-points and toxicity were more numerous with both mild and moderate toxicity thresholds, but statistical significance was limited after correction for false discovery rate. Rectal V50Gy (CFRT) associated with grade 2+ bleeding; bowel V43Gy and V47 (HFRT/4 days/week schedule) associated with patient-reported loose stools and diarrhoea, respectively. Further investigation showed that CFRT patients with rectal bleeding had a mean rectal V50Gy above the treatment planning constraint.
Conclusions
When dose-volume parameters are kept below tight constraints, toxicity is low. Residual dosimetry loses much of its predictive power for gastrointestinal toxicity in the setting of PLN-IMRT for prostate cancer. We have benchmarked dose-volume constraints for safely delivering PLN-IMRT using CFRT or HFRT.
Key words: Dose-volume constraints, dosimetry, IMRT, pelvic lymph nodes, prostate cancer, gastrointestinal side-effects
Highlights
-
•
Dosimetry and toxicity relationships (all endpoints-3 scales) are reported.
-
•
Study includes conventionally-fractionated and hypofractionated IMRT.
-
•
When dose-volume constraints are exceeded, risk for late toxicity increases.
-
•
With constraints, dosimetry loses predictive power for residual toxicities.
-
•
We propose novel benchmark constraints for pelvic lymph node IMRT.
Introduction
Long-term side-effects can become major issues for patients in whom prostate cancer (PCa) is controlled or cured. Late toxicity, starting 3 months to years after treatment, is of particular concern as pelvic lymph node (PLN) radiotherapy may increase gastrointestinal side-effects [1], [2], [3], [4].
The risk of gastrointestinal toxicity is judged with dose-volume histograms. However, there is limited consensus regarding their prognostic value, particularly in the era of dose-volume constraint-based inverse-planned radiotherapy. Although concordant at higher doses, studies show different dose-volume histogram metrics as predictive of rectal bleeding and it is difficult to find consistent correlations with other gastrointestinal symptoms [5], [6], [7], [8]. Treatment planning dose constraints have been developed to minimise risks. Although the QUANTEC meta-analyses on gastrointestinal dose constraints provided evidence-based guidance for radiotherapy planning, they were based on studies using three-dimensional conformal radiotherapy [5], [9]. Intensity-modulated radiotherapy (IMRT) may have changed toxicity patterns and relationships with dosimetry [5], [9]. Other proposed models of risk evaluation have not been introduced in the clinic [10], [11], [12].
We investigated relationships between dose-volume constraints and late gastrointestinal toxicity in patients prospectively treated with conventionally fractionated (CFRT) or hypofractionated (HFRT) prostate and pelvic IMRT for high-risk PCa in a phase I/II study [3].
Materials and methods
Patients and Data
Patients treated in a previously reported phase I/II study of prostate and pelvic IMRT for high-risk PCa (IMRT-PCa trial) were eligible [3]. The trial accrued 473 patients with high-risk PCa between August 2000 and September 2013. CFRT was delivered to the prostate and seminal vesicles (70–74 Gy in 35–37 fractions) and 50, 55 or 60 Gy to the PLN concomitantly over 7–7.4 weeks. Two further cohorts received HFRT, with 60 Gy (20 fractions) delivered to the prostate and seminal vesicles and 47 Gy to the PLN in either 4 (5 days/week, HFRT-5D) or 5 weeks (4 days/week, HFRT-4D). The change to a 5 week schedule (which is an extension of the 4 week schedule to 5 weeks by delivering four instead of five fractions/week) occurred because of documented increases in short-lasting acute gastrointestinal toxicity also observed in other studies [3], [13], [14]. All patients received long-course androgen deprivation therapy.
Only patients with both toxicity and complete dosimetric data were included. Patients with <2 years of follow-up data at the time of analysis were excluded.
Dosimetric Variables
Dosimetric data were obtained from prostate and pelvic node radiotherapy forms, used to assess the clinical suitability of radiotherapy plans according to defined dose constraints (see supplementary Table SUPP-1). Where unavailable, data were obtained from original treatment plans. Optimal and mandatory bowel constraints were derived from dose levels related to Radiation Therapy Oncology Group (RTOG) grade ≥1 and ≥2 in previous reports [15]. Rectal dose constraints were derived by pragmatically adapting available evidence at the time of study design [16], [17]. Constraints were modified for HFRT in proportion to the prescribed dose.
Rectum and bowel were contoured as solid organs according to a previously published protocol (see supplementary text) [3]. Data consisted of absolute rectum and bowel volumes (cm3), and volumes of bowel (cm3) and rectum (% of volume) receiving the constraint dose or higher, henceforth referred to as (a)V(x), where (x) is the respective dose (Gy) and (a) the identifier for rectum (r) or bowel (b).
Radiation-induced Gastrointestinal Toxicity Data
Clinical data were collected at 6-month intervals up to 5 years after radiotherapy and yearly thereafter. Only data collected ≥6 months after radiotherapy were used. In order to capture a comprehensive record of toxicity, three internationally accepted toxicity scales were used [18]. They were the European Organization for Research and Treatment of Cancer (EORTC)/RTOG and the Late Effects Normal Tissue Task Force-Subjective, Objective, Management, Analytic (LENT-SOMA) clinician-reported late toxicity scales and the UCLA Prostate Cancer Index (UCLA-PCI) patient-reported outcomes scale–bowel subset (questions 17–21) [19], [20], [21].
Analysis was undertaken endpoint-by-endpoint. Patients with positive baseline side-effect scores were excluded from analysis for that specific endpoint only. For example, if a patient had rectal bleeding before radiotherapy, he was excluded from the rectal bleeding analysis but not from tenesmus and other endpoints. We also excluded toxicity endpoints that had an incidence of ≤5% in the entire cohort.
Patients were divided by peak toxicity using mild and moderate/severe thresholds for each endpoint (see supplementary Table SUPP-2) [21].
Associations were studied between dose-points and all toxicity outcomes in three different scales by cohort. We do not report amalgamated results of all cohorts given that constraints were not equivalent radiobiologically and because this strategy enables the study of differential relationships between CFRT and HFRT schedules.
Statistical Considerations
Dose-volume metrics (dose-points) where ≥80% of patients had a value of 0 were excluded. They were rV75/bV65 (CFRT) and rV63/bV55 (HFRT). Bowel metrics where the mean volume was <2 cm3 were also excluded. They were bV60 (CFRT) and bV51 (HFRT).
To assess biological significance in this large dataset, a measure of inter-group difference was created. This metric, henceforth named ‘δ median’ (δM), is defined as:
where V(x) is the dose-point of interest. Therefore, δM indicates if volumes appear greater in the group with or without toxicity (see supplementary Figure SUPP-1). Statistical significance was assessed using the Wilcoxon rank sum test.
Associations between dose-points and toxicity were sought by testing all dose-points against each endpoint in all toxicity scales, leading to the use of multiple statistical tests (see supplementary Table SUPP-3). The Holm method was used to correct for false discovery rate (FDR) as per the following equation [22]:
where n is the number of tested hypotheses (number of tests done by toxicity scale per cohort, including both toxicity thresholds) and the target alpha level is 0.05. It follows that all P values reported in this article are ‘uncorrected’, as the Holm method uses the corrected alpha level of significance as a threshold to accept or reject the null hypothesis. A positive result is defined as a positive δM (>0) where statistical significance was reached.
Where positive associations resilient to FDR correction were found, new constraints were derived using receiver operating characteristic (ROC) curves [23]. Odds ratios of toxicity were calculated for protocol and new constraints and significance assessed with Fisher's exact test.
Analysis was carried out in Excel and R [24].
Results
Patient Characteristics
Of 473 patients recruited in the trial, 26 had been recruited <2 years before data lock and thus did not have long-term (≥2 years) toxicity data available and were excluded. Of the remaining 447 patients, 426 had been treated according to protocol and long-term toxicity data were available for 421. Dosimetric data were not retrievable for seven patients and thus 414 (98% of patients with ≥2 year toxicity follow-up data) were included for analysis. Proportions of patients with and without toxicity were comparable with those observed in the main analysis of the IMRT for Prostate Cancer trial, except for UCLA-PCI bowel problem (see supplementary Table SUPP-4). This discrepancy is explained by our methodology, as patients with a moderate/severe bowel problem at baseline (i.e. before the start of radiotherapy) were excluded in an endpoint-by-endpoint basis to obtain an accurate representation of gastrointestinal problems caused by radiotherapy. However, all patient- and clinician-reported gastrointestinal toxicity rates are comparable to previously reported analogous studies [25].
Groups were reported by prostate radiotherapy fractionation (Table 1) because dose-volume constraints were not equivalent radiobiologically [26]. This approach allows a comparison of differences between CFRT and HFRT in terms of relationships between dosimetry and toxicity, which was one of our objectives.
Table 1.
Patient demographics
| All cohorts | CFRT | HFRT-4D | HFRT-5D | |
|---|---|---|---|---|
| No. patients with toxicity follow-up data ≥2 years (no. excluded due to follow-up data <2 years) | LENT-SOMA: 414 (54) RTOG: 414 (63) UCLA-PCI: 369 (73) |
LENT-SOMA: 234 (42) RTOG:234 (51) UCLA-PCI: 213 (43) |
LENT-SOMA: 122 (7) RTOG: 122 (7) UCLA-PCI: 103 (20) |
LENT-SOMA: 58 (5) RTOG: 58 (5) UCLA-PCI: 53 (10) |
| No. patients analysed (no. excluded due to incomplete dosimetric data) | LENT-SOMA: 368 (46) RTOG: 368 (46) UCLA-PCI: 325 (44) |
LENT-SOMA: 205 (29) RTOG: 205 (29) UCLA-PCI: 185 (28) |
LENT-SOMA: 114 (8) RTOG: 114 (8) UCLA-PCI: 95 (8) |
LENT-SOMA: 49 (9) RTOG: 49 (9) UCLA-PCI: 45 (8) |
| No. patients by prescription dose to the PLN n (%) | N/A | 50 Gy: 15 (7.3%) 55 Gy: 52 (25.4%) 60 Gy: 138 (67.3%) |
47 Gy: 103 (90.3%) 43 Gy∗: 11 (9.7%) |
47 Gy: 46 (93.9%) 43 Gy∗: 3 (6.1%) |
| Age at treatment Mean (range) |
65 (45–82) | 64 (45–82) | 67 (47–80) | 67 (47–79) |
| Follow-up (years) Median (IQR) |
5 (4–7) | 7 (5–9) | 4 (3–4) | 5 (5–5.5) |
| Rectum volume (cm3) Median (IQR) |
66.7 (50.6–86.1) | 64.7 (51.1–87.5) | 63.5 (44.3–77.0) | 72.9 (58.1–94.3) |
| Bowel volume (cm3) Median (IQR) |
478.2 (347.7–640.6) | 463.2 (343.9–649.0) | 478.3 (369.9–632.4) | 519.1 (371.3–616.9) |
CFRT, conventionally fractionated radiotherapy; HFRT-4D, hypofractionated radiotherapy (4 days/week); HFRT-5D, hypofractionated radiotherapy (5 days/week); RTOG, Radiation Therapy Oncology Group; PLN, pelvic lymph nodes; IQR, interquartile range.
LENT-SOMA.
UCLA-PCI.
Patients had planned dose reductions due to difficulties in meeting dose-volume constraints.
Number of Patients Failing Constraints and Relationships with Toxicity
We previously published encouraging toxicity outcomes of dose-escalated and hypofractionated PLN-IMRT in the IMRT for Prostate Cancer trial [3]. The trial protocol used strict dose-volume constraints for rectum and bowel (see supplementary Table SUPP-1). Application of dose-volume constraints may reduce gastrointestinal toxicity [27]. We therefore first hypothesised that rates of patients failing constraints were low in our dataset. We also explored relationships between the number of failed constraints and RTOG-scored maximum peak toxicity.
Rectum
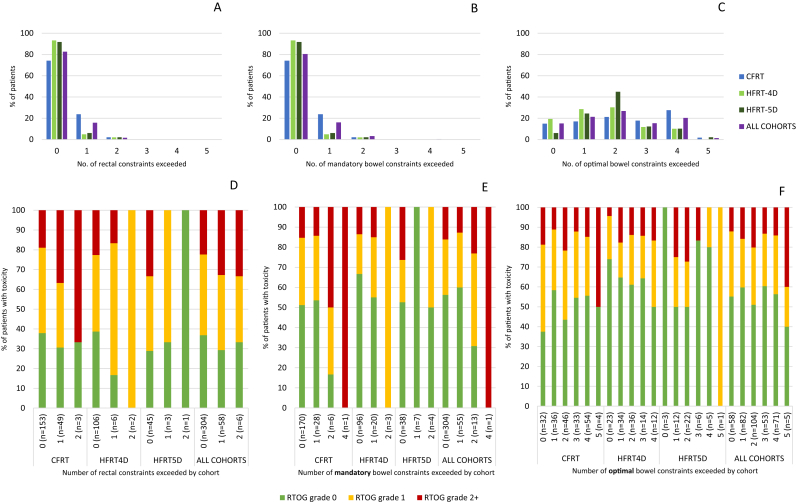
Most patients met all constraints in all cohorts (Figure 1A, supplementary Figure SUPP-2A, Tables SUPP-5–SUPP-7). However, the rV50 constraint was exceeded in 26% in the CFRT cohort. Patients exceeding rectal constraints experienced more toxicity, particularly with CFRT, but this pattern was also reproduced in other cohorts (Figure 1D).
Fig 1.
Rectum and bowel constraints exceeded and relationships with gastrointestinal toxicity. (A–C) Proportion of patients by number of exceeded rectal (A), bowel mandatory (B) and optimal (C) constraints; by cohort. (D–F) Proportions of patients with toxicity by number of rectal (D), bowel mandatory (E) and optimal (F) constraints exceeded; by cohort. Gastrointestinal toxicity was scored with maximum peak cumulative Radiation Therapy Oncology Group (RTOG) grade (rectum: maximum score of bowel frequency, bleeding, diarrhoea, proctitis, rectal stricture, rectal ulcer; bowel: maximum score of bowel frequency, diarrhoea, bowel obstruction).
Bowel
Optimal/mandatory bowel constraints were defined by average volumes correlating with no/mild diarrhoea, respectively, in previous reports [15]. Although optimal constraints were exceeded frequently, mandatory constraints were generally respected (Figure 1B, C, supplementary Figure SUPP-2B, Tables SUPP-8–SUPP-10). As with rectum, a pattern whereby patients exceeding bowel constraints experience more toxicity was observed. This pattern was most evident in the CFRT cohort and mandatory constraints (Figure 1E, F).
Associations between Dosimetric Parameters and Toxicity
We used the δM measure to assess differences in volumes between groups of patients with and without toxicity and assessed their statistical significance stringently. For most endpoints, δM was small: 79% and 74.6% of δM were ≤±3% of rectum or ≤10 cm3 of bowel volumes, respectively (supplementary Table SUPP-11), reflecting small differences in median dose-point values between patients with and without toxicity. Five of 63 (8%) relationships suggested had negative δM values, but were not robust to FDR correction. All relevant results are described in Table 2 (rectum) and 3 (bowel). Supplementary Tables R1–9 (rectum) and B1–9 (bowel) summarise all findings.
Table 2.
Positive associations between rectum dosimetry/toxicity
| Clinician-reported toxicity | |||||
|---|---|---|---|---|---|
| Cohort | Threshold | LENT-SOMA | % patients with toxicity | RTOG | % of patients with toxicity |
| CFRT | M | rV60 and mucosal loss (P = 0.005, δM = 3.3) | 21% | rV60 and bowel frequency (P = 0.01, δM = 3.85) | 38% |
| rV65 and mucosal loss (P = 0.003, δM = 4.3) | 21% | ||||
| rV50 and bleeding (P = 0.04, δM = 20.25) | 22% | ||||
| rV60 and sphincter control (P = 0.008, δM = 3.21) | 18% | ||||
| rV65 and sphincter control (P = 0.04, δM = 4.1) | 18% | ||||
| MS | rV50 and bleeding (P = 0.00004, δM = 8.5) | 8% | rV50 and rectal bleeding (P = 0.00008, δM = 7.1) | 10% | |
| rV60 and mucosal loss (P = 0.003, δM = 4.95) | 6% | ||||
| rV65 and mucosal loss (P = 0.007, δM = 5.54) | 6% | ||||
| rV60 and bleeding (P = 0.04, δM = 5.23) | 8% | ||||
| HFRT-4D | M | rV51 and mucosal loss (P = 0.04, δM = 2.83) | 22% | rV51 and rectal bleeding (P = 0.02, δM = 3.13) | 44% |
| rV55 and mucosal loss (P = 0.03, δM = 3.9) | 22% | rV55 and rectal bleeding (P = 0.005, δM = 4.15) | 44% | ||
| rV59 and bleeding management (P = 0.03, δM = 2.43) | 14% | ||||
| MS | N/A | N/A | |||
| HFRT-5D | M | rV51 and tenesmus (P = 0.01, δM = 5.91) | 39% | rV59 and proctitis (P = 0.002, δM = 2.3) | 55% |
| rV55 and tenesmus (P = 0.01, δM = 3.74) | 39% | ||||
| rV59 and tenesmus (P = 0.01, δM = 2.25) | 39% | ||||
| rV59 and frequency (P = 0.05, δM = 1.86) | 41% | ||||
| MS | rV59 and bleeding (P = 0.04, δM = 2.66) | 26% | N/A | ||
| rV59 and mucosal loss (P = 0.02, δM = 3.9) | 6% | ||||
| Patient-reported toxicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urgency |
Loose stools |
Distress |
Crampy pain |
Problem |
||||||
| Threshold → |
M | MS | M | MS | M | MS | M | MS | M | MS |
| Cohort ↓ | ||||||||||
| CFRT | rV50 (P = 0 .003, δM = 4.5) | rV50 (P = 0.04, δM = 2.75) | N/A | N/A | N/A | N/A | rV50 (P = 0.03, δM = 3.2) | N/A | rV50 (P = 0.03, δM = 2.35) | N/A |
| % patients with toxicity | 38% | 27% | – | – | – | – | 19% | – | 44% | – |
| HFRT-4D | N/A | N/A | rV43 (P = 0.03, δM = 4.56) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| % patients with toxicity | – | – | 43% | – | – | – | – | – | – | – |
| HFRT-5D | N/A | N/A | N/A | rV59 (P = 0.04, δM = 4) | N/A | N/A | N/A | rV43 (P = 0.01, δM = 5.2) | N/A | N/A |
| % patients with toxicity | – | – | – | 20% | – | – | – | 15% | – | – |
Where N/A is reported, no positive associations were found. Where P ≤ 0.01, results are in bold. Where results were significant after Holm correction, results are bold and italicized. M = mild threshold (symptomatic patients are defined as having mild or worse symptoms). MS = moderate/severe (symptomatic patients are defined as having moderate or worse symptoms). % of patients with toxicity events excludes patients where data was unavailable (for full results see supplementary Tables R1–R9).
Rectum
In the CFRT cohort, rV50 was higher in patients with moderate/severe (grade 2+) rectal bleeding in both RTOG (P = 0.00008) and LENT-SOMA (P = 0.00004) scales. These were the only results for rectum that were resilient to Holm correction (Table 2). rV50 was also higher in patients with mild (grade 1+) LENT-SOMA bleeding and bowel problem, albeit non-significantly. In HFRT cohorts, rV51/rV55 (HFRT-4D) and rV59 (HFRT-5D) associated with bleeding, albeit non-significantly.
Bowel
In the HFRT-4D cohort, bV43 and bV47 were higher in patients with mild self-reported loose stools (P = 0.0005) and RTOG-scored diarrhoea (P = 0.002), respectively. These were the only results resilient to FDR correction (Table 3). However, bV39 also associated non-significantly with mild self-reported loose stools and bV43 also associated non-significantly with diarrhoea-related toxicity endpoints, as did bV39/bV43 and bV45/bV55/bV60 in the HFRT-5D and CFRT cohorts, respectively.
Table 3.
Positive associations between bowel dosimetry/toxicity
| Clinician-reported toxicity | |||||
|---|---|---|---|---|---|
| Cohort | Threshold | LENT-SOMA | % patients with toxicity | RTOG | % patients with toxicity |
| CFRT | M | N/A | N/A | ||
| MS | bV45 and consistency/frequency management (P = 0.01, δM = 52.7) | 6% | bV45 and bowel frequency (P = 0.04, δM = 16.96) | 10% | |
| bV55 and bowel frequency (P = 0.03, δM = 3.8) | 10% | ||||
| HFRT-4D | M | bV39 and frequency (P = 0.03, δM = 17.66) | 30% | bV39 and diarrhoea (P = 0.03, δM = 17.32) | 25% |
| bV43 and diarrhoea (P = 0.01, δM = 7.39) | 25% | ||||
| bV47 and diarrhoea (P = 0.002, δM = 2.15) | 25% | ||||
| MS | bV39 and frequency (P = 0.02, δM = 27.5) | 13% | bV39 and bowel frequency (P = 0.04, δM = 27.17) | 11% | |
| bV43 and consistency (P = 0.04, δM = 8.33) | 10% | bV43 and diarrhoea (P = 0.03, δM = 24.50) | 9% | ||
| HFRT-5D | M | bV39 and consistency (P = 0.05, δM = 32.59) | 26% | N/A | |
| bV43 and consistency (P = 0.03, δM = 15.99) | 26% | ||||
| bV43 and frequency (P = 0.05, δM = 13.48) | 31% | ||||
| MS | bV43 and consistency (P = 0.04, δM = 18.76) | 17% | N/A | ||
| Patient-reported toxicity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urgency |
Loose stools |
Distress |
Crampy pain |
Problem |
||||||
| Threshold → |
M | MS | M | MS | M | MS | M | MS | M | MS |
| Cohort ↓ | ||||||||||
| CFRT | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| % patients with toxicity | – | – | – | – | – | – | – | – | – | – |
| HFRT-4D | bV43 (P = 0.006, δM = 7.04) |
bV43 (P = 0.009, δM = 8.8) bV47 (P = 0.03, δM = 1.55) |
bV39 (P = 0.009, δM = 24.78) bV43 (P = 0.0005, δM = 11.74) bV47 (P = 0.05, δM = 0.07) |
N/A | bV43 (P = 0.04, δM = 4.10) | N/A | N/A | N/A | bV39 (P = 0.04, δM = 10.50) bV43 (P = 0.002, δM = 11.37) |
bV39 (P = 0.04, δM = 14.20) bV43 (P = 0.003, δM = 12.22) bV47 (P = 0.03, δM = 2.01) |
| % patients with toxicity | 41% | 33% | 43% | – | 34% | – | – | – | 54% | 39% |
| HFRT-5D | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| % patients with toxicity | – | – | – | – | – | – | – | – | – | – |
Where N/A is reported, no positive associations were found. Where P ≤ 0.01, results are in bold. Where results were significant after Holm correction, results are bold and italicized. M = mild threshold (symptomatic patients are defined as having mild or worse symptoms). MS = moderate/severe (symptomatic patients are defined as having moderate or worse symptoms). % of patients with toxicity events excludes patients where data was unavailable (for full results see supplementary Tables R1–R9).
Relationships between Significant Associations and Dose-volume Constraints
Three significant associations were detected in our dataset: rV50 (CFRT) and rectal bleeding, as well as bV43/bV47 (HFRT4D) with loose stools/diarrhoea. We examined if relationships with constraints were present and explored whether different constraints could be derived from our dataset.
CFRT/rV50 and rectal bleeding
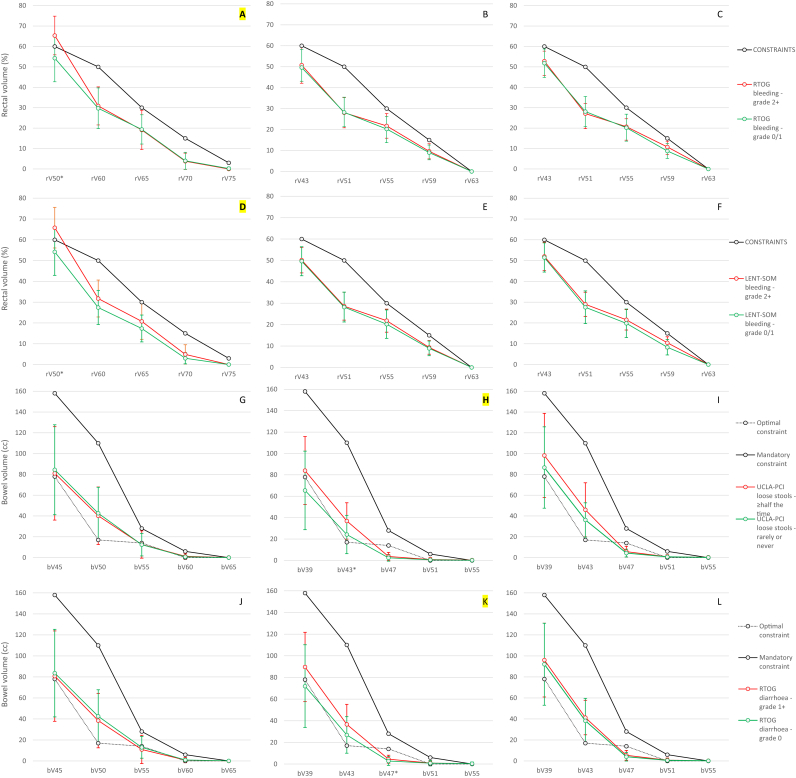
Mean rV50 was 65%/54% and 66%/54% for patients treated with CFRT with/without grade 2+ RTOG and LENT-SOMA bleeding, respectively. Interestingly, in both cases, mean rV50 for patients with bleeding was above the rV50 constraint (Figure 2A–F). Such a relationship was not observed for any other rectal endpoint in relation to bleeding in any cohort. The rV50-CFRT constraint derived using this dataset was 59% (AUC: 0.79; 95% confidence interval 0.69–0.89; supplementary Table SUPP-12). Odds ratios for toxicity using protocol or new constraints were similar (supplementary Table SUPP-13), indicating that the protocol constraint was adequate for preventing toxicity if respected.
Fig 2.
Mean volumes per dose-point by cohort and relationship with dose-volume constraints for Radiation Therapy Oncology Group (RTOG) (A–C) and LENT-SOMA (D–F) bleeding – moderate/severe threshold (rectum); UCLA-PCI loose stools – mild threshold (G–I; bowel); and RTOG diarrhoea – mild threshold (J–L; bowel). Significant results are marked with asterisks and their curve identifier is highlighted. Error bars represent standard deviations.
HFRT-4D/bV43 and loose stools
Although mean bV43 was higher in patients with mild self-reported loose stools (36.9 cm3) than in patients without loose stools (34.3 cm3), they were both under the mandatory (110 cm3) and over the optimal (17 cm3) constraints (Figure 2G–I). bV39 was non-significantly higher than mandatory constraints in patients with loose stools, unlike patients without loose stools. Mean volumes for bV47–bV55 overlapped between groups, similar to the HFRT-5D cohort. With CFRT, there were no differences between groups. The bV43-HFRT constraint derived using this dataset was 24 cm3 (AUC: 0.73; 95% confidence interval 0.62–0.84; see supplementary Table SUPP-12). Odds ratios using protocol constraints could not be calculated, as all patients met the constraint. However, for the new constraint, the odds ratio was 3.81 (1.59–9.80), indicating that it may be useful (see supplementary Table SUPP-13).
HFRT-4D/bV47 and diarrhoea
With HFRT-4D, the mean bV47 was 4.49 cm3 in patients with and 2.80 cm3 in patients without mild (grade 1+) RTOG diarrhoea. These values are below the mandatory (28 cm3) and optimal (14 cm3) constraints for bV47 (Figure 2J–L). Similar differences between these groups were also detected for bV39 and bV43, albeit non-significantly. Such patterns were not observed in other cohorts. The bV47 constraint derived using this dataset was 2.15 cm3 (AUC: 0.69; 95% confidence interval 0.59–0.79; see supplementary Table SUPP-12). Odds ratios using protocol and new constraints were 5.51 (1.79–21.49) and 9.40 (3.24–32.59). This result indicates that failing to meet either constraint predicts for higher toxicity levels, although the new constraint may be more robust (see supplementary Table SUPP-13).
Discussion
Late gastrointestinal toxicity limits high-dose pelvic radiotherapy. Rectum and bowel dose-volume constraints, designed to minimise gastrointestinal side-effects, are increasingly important as inverse-planned IMRT becomes the primary radiotherapy technique for PCa. Several efforts have been made to detect thresholds below which probabilities of gastrointestinal toxicity are low [5], [9], [16], [17], [27], [28], [29].
We acknowledge limitations in our study. Planning computed tomography is a snapshot of a moving organ, especially for non-rectal bowel. By contouring bowel loops proximal to the rectum, different organs are included (sigmoid, colon, jejunum/ileum). However, although segmented bowel outlining is increasingly used in research and has been adopted by some centres, RTOG guidelines recommend that whole bowel (i.e. unsegmented) should be outlined for genitourinary tumours, reflecting general clinical practice [30]. We also recognise that the pelvic doses studied are non-standard, as we used data derived from an IMRT dose-escalation trial [3]. However, PLN-IMRT may become a standard option pending definitive results of ongoing phase III trials. Irrespective of this, our analysis allows an understanding of modern relationships between dosimetry and toxicity in a mature dataset.
Our approach makes comparisons between fractionation schedules possible, which was one of our objectives. However, in practice, it would be imprecise to pool all cohorts into a single dose-equivalent dataset, due to three factors: (1) α/β ratios are not available for most of the symptoms we studied, (2) there could be imprecisions related to time factors related to repair/recovery, (3) dose-points were not radiobiologically equivalent if using a ‘one-fits-all’ late effect α/β ratio of 3 Gy [31].
Numbers of patients with late grade 2+ toxicity were low. For completeness, comparisons using mild toxicity thresholds were carried out in parallel. Multiple statistical tests were used, therefore requiring FDR correction. Holm correction was chosen for its robustness. We nevertheless pragmatically report different levels of significance. Overall, few results were found to be robustly significant.
We show a relationship between the number of exceeded rectal constraints and late toxicity. Our results confirm previous reports showing rV50 to predict grade 2+ rectal bleeding. However, robust relationships of higher doses (≥rV65) with bleeding were not found, as might have been expected from previous reports [16], [27], [29]. Rectal dosimetry was mostly kept under constraints and specific attention was given to higher dose-points, reflecting QUANTEC guidance [5]. The fact that the lower rectal constraint (rV50) was exceeded more often with CFRT is probably due to the superior rectum receiving dose from pelvic irradiation, which was 60 Gy for the majority (67%) of patients. HFRT cohorts received 47 Gy to the PLN, which is equivalent to 55 Gy for an α/β ratio typical of late reactions (3 Gy). This aspect may also explain lower incidences of patients failing constraints with HFRT.
bV43 and bV47 were significantly predictive of diarrhoeal endpoints in the HFRT-4D cohort. Although non-significant after FDR correction, similar trends were observed in other cohorts with clinician-reported diarrhoea. The number of mandatory and/or optimal bowel constraints exceeded showed a relationship with toxicity. A study analysing the prognostic value of bowel dose-volume metrics for acute bowel toxicity in patients undergoing prostate and pelvic radiotherapy for high-risk PCa showed bV40-bV50 to be predictive with CFRT, whereas higher (bV55) and lower (bV15) dose-points were less predictive. Interestingly, derived constraints were similar to the ones we propose, although a whole intestinal cavity contouring method was used [32]. Other studies also showed similar results [33], [34]. Robust dosimetric relationships with late toxicity have proved more elusive, but, to our knowledge, we report the largest prospectively collected patient series to date [35], [36]. Taken together, these data support the mandatory threshold as a relevant dose limit when planning pelvic radiotherapy to avoid grade 2+ diarrhoea. However, we also observed a relationship between exceeding optimal constraints and toxicity. We therefore recommend using optimal constraints as target doses and planning objectives for pelvic IMRT to ensure minimal gastrointestinal toxicity and that mandatory constraints should not be compromised to ensure acceptable gastrointestinal side-effect rates. We propose that the optimal bV50-CFRT/bV43-HFRT constraint should be updated to 24 cm3 instead of 17 cm3 given our results showing this level to be more appropriate for predicting gastrointestinal side-effects. These new findings may usefully inform clinical trials and non-trial clinical practice.
We previously showed that moderate/severe gastrointestinal toxicity was reported in about a third of patients meeting rectal constraints, whereas no gastrointestinal side-effects were reported in 10% of patients failing six or more constraints [27]. We observed similar relationships in this dataset. In addition to uncertainties in reporting dosimetry and toxicity, this result may be attributed to patient-related and environmental factors [37], [38], [39], [40], [41]. Moreover, as many as 50% of patients have multiple gastrointestinal diagnoses when symptoms, which may be misattributed to radiotherapy, are investigated [18].
Our data support that, when dose-volume parameters are kept below tight constraints, gastrointestinal toxicity is low and planning dosimetry loses predictive power for residual moderate/severe gastrointestinal toxicities in the setting of PLN-IMRT. This conclusion suggests that if not escalating radiation dose, further technical development of radiotherapy for PCa may only achieve marginal improvements in gastrointestinal toxicity outcomes unless it reduces the rates of patients where constraints are exceeded. Future research needs to also incorporate contributing biological mechanisms of radiation-induced toxicity, which may help achieve superior clinical stratification of patients at risk of significant side-effects, as suggested by other authors.
To conclude, we provide an evidence base for safely delivering PLN-IMRT using CFRT or HFRT. We propose the constraints summarised in Table 4 as benchmarks for CFRT and HFRT and that the planning goal should be to achieve the target (optimal) bowel constraints whenever possible.
Table 4.
Proposed dose-volume constraints for rectum and bowel
| Rectum |
Bowel |
||||||
|---|---|---|---|---|---|---|---|
| 2 Gy/fraction |
3 Gy/fraction∗ |
2 Gy/fraction |
3 Gy/fraction∗ |
||||
| Dose constraint (Gy) | Volume required (%) | Dose constraint (Gy) | Volume required (%) | Dose constraint (Gy) | Volume required (cm3) Mandatory (target) |
Dose constraint (Gy) | Volume required (cm3) Mandatory (target) |
| rV50 | 60 | rV43 | 60 | bV45 | 158 (78) | bV39 | 158 (78) |
| rV60 | 50 | rV51 | 50 | bV50 | 110 (24) | bV43 | 110 (24) |
| rV65 | 30 | rV55 | 30 | bV55 | 28 (14) | bV47 | 28 (14) |
| rV70 | 15 | rV59 | 15 | bV60 | 6 (0) | bV51 | 6 (0) |
| rV75 | 3 | rV63 | 0 | bV65 | 0 (0) | bV55 | 0 (0) |
Constraints for the 3Gy cohorts were simply extrapolated from the 2Gy cohort in a ratio of 60/70 (reflecting the initial prostate treatment dose of 70Gy) with no implicit radiobiological assumptions.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
We thank the patients and all research support staff. Recognition goes to all the trials unit staff at the Bob Champion Unit and RMH Trial Unit who contributed to the central coordination of the study. We acknowledge support of Cancer Research UK, United Kingdom (C8262/A7253, C1491/A9895, C1491/A15955, SP2312/021), the Department of Health, United Kingdom, the National Institute for Health Research (NIHR) Cancer Research Network, United Kingdom and NHS funding to the NIHR Biomedical Research Centre at the Royal Marsden NHS Foundation Trust, United Kingdom and The Institute of Cancer Research, Portugal. MRF acknowledges support from the Calouste Gulbenkian Foundation, Portugal, the Fundação para a Ciência e a Tecnologia, Portugal and the Champalimaud Foundation, Portugal.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2019.02.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mohler J., Armstrong A., Bahnson R., D’Amico A. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2016;14:19–30. [Google Scholar]
- 2.Mohiuddin J.J., Baker B.R., Chen R.C. Radiotherapy for high-risk prostate cancer. Nat Rev Urol. 2015;12:145–154. doi: 10.1038/nrurol.2015.25. [DOI] [PubMed] [Google Scholar]
- 3.Reis Ferreira M., Khan A., Thomas K., Truelove L., McNair H., Gao A. A phase 1/2 dose escalation study of the use of intensity modulated radiotherapy (IMRT) to treat the prostate and pelvic nodes in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2017;99:1234–1242. doi: 10.1016/j.ijrobp.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreyev J. Gastrointestinal complications of pelvic radiotherapy: are they of any importance? Gut. 2005;54:1051–1054. doi: 10.1136/gut.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michalski J.M., Gay H., Jackson A., Tucker S.L., Deasy J.O. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(Suppl):S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thor M., Apte A., Deasy J.O., Karlsdóttir À., Moiseenko V., Liu M. Dose/volume-response relations for rectal morbidity using planned and simulated motion-inclusive dose distributions. Radiother Oncol. 2013;109:388–393. doi: 10.1016/j.radonc.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald A.M., Baker C.B., Popple R., Shekar K., Yang E.S., Jacob R. Different rectal toxicity tolerance with and without simultaneous conventionally-fractionated pelvic lymph node treatment in patients receiving hypofractionated prostate radiotherapy. Radiat Oncol. 2014;9:129. doi: 10.1186/1748-717X-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Someya M., Hori M., Tateoka K., Nakata K., Takagi M., Saito M. Results and DVH analysis of late rectal bleeding in patients treated with 3D-CRT or IMRT for localized prostate cancer. J Radiat Res. 2014;56:122–127. doi: 10.1093/jrr/rru080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanagh B.D., Pan C.C., Dawson L.A., Das S.K., Li X.A., Ten Haken R.K. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(Suppl):S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 10.Tomatis S., Rancati T., Fiorino C., Vavassori V., Fellin G., Cagna E. Late rectal bleeding after 3D-CRT for prostate cancer: development of a neural-network-based predictive model. Phys Med Biol. 2012;57:1399–1412. doi: 10.1088/0031-9155/57/5/1399. [DOI] [PubMed] [Google Scholar]
- 11.Acosta O., Drean G., Ospina J.D., Simon A., Haigron P., Lafond C. Voxel-based population analysis for correlating local dose and rectal toxicity in prostate cancer radiotherapy. Phys Med Biol. 2013;58:2581–2595. doi: 10.1088/0031-9155/58/8/2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett G.C., Coles C.E., Elliott R.M., Baynes C., Luccarini C., Conroy D. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: a prospective analysis study. Lancet Oncol. 2012;13:65–77. doi: 10.1016/S1470-2045(11)70302-3. [DOI] [PubMed] [Google Scholar]
- 13.Dearnaley D., Syndikus I., Mossop H., Khoo V., Birtle A., Bloomfield D. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catton C.N., Lukka H., Gu C.-S., Martin J.M., Supiot S., Chung P.W.M. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35:1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher M.J., Brereton H.D., Rostock R.A., Zero J.M., Zekoski D.A., Poyss L.F. A prospective study of treatment techniques to minimize the volume of pelvic small bowel with reduction of acute and late effects associated with pelvic irradiation. Int J Radiat Oncol Biol Phys. 1986;12:1565–1573. doi: 10.1016/0360-3016(86)90279-8. [DOI] [PubMed] [Google Scholar]
- 16.Jackson A., Skwarchuk M.W., Zelefsky M.J., Cowen D.M., Venkatraman E.S., Levegrun S. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int J Radiat Oncol Biol Phys. 2001;49:685–698. doi: 10.1016/s0360-3016(00)01414-0. [DOI] [PubMed] [Google Scholar]
- 17.Fiorino C., Cozzarini C., Vavassori V., Sanguineti G., Bianchi C., Mauro Cattaneo G. Relationships between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of a large group of patients pooled from three institutions. Radiother Oncol. 2002;64:1–12. doi: 10.1016/s0167-8140(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 18.Andreyev H.J.N., Vlavianos P., Blake P., Dearnaley D., Norman A.R., Tait D. Gastrointestinal symptoms after pelvic radiotherapy: role for the gastroenterologist? Int J Radiat Oncol Biol Phys. 2005;62:1464–1471. doi: 10.1016/j.ijrobp.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 19.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 20.Lent soma scales for all anatomic sites. Int J Radiat Oncol. 1995;31:1049–1091. doi: 10.1016/0360-3016(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 21.Litwin M.S., Hays R.D., Fink A., Ganz P.A., Leake B., Brook R.H. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 23.Otter S., Schick U., Gulliford S., Lal P., Franceschini D., Newbold K. Evaluation of the risk of grade 3 oral and pharyngeal dysphagia using atlas-based method and multivariate analyses of individual patient dose distributions. Int J Radiat Oncol Biol Phys. 2015;93:507–515. doi: 10.1016/j.ijrobp.2015.07.2263. [DOI] [PubMed] [Google Scholar]
- 24.R Development Core Team R . vol. 1. 2011. (R: a language and environment for statistical computing). [Google Scholar]
- 25.Holch P., Henry A.M., Davidson S., Gilbert A., Routledge J., Shearsmith L. Acute and late adverse events associated with radical radiation therapy prostate cancer treatment: a systematic review of clinician and patient toxicity reporting in randomized controlled trials. Int J Radiat Oncol Biol Phys. 2017;97:495–510. doi: 10.1016/j.ijrobp.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Bentzen S.M., Joiner M.C. The linear-quadratic approach in clinical practice. In: Joiner M.C., Van der Kogel A., editors. Basic clinical radiobiology. 4th ed. Hodder Arnold; London: 2009. [Google Scholar]
- 27.Gulliford S.L., Foo K., Morgan R.C., Aird E.G., Bidmead A.M., Critchley H. Dose-volume constraints to reduce rectal side effects from prostate radiotherapy: evidence from MRC RT01 Trial ISRCTN 47772397. Int J Radiat Oncol Biol Phys. 2010;76:747–754. doi: 10.1016/j.ijrobp.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Emami B., Lyman J., Brown A., Cola L., Goitein M., Munzenrider J.E. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 29.Fiorino C., Fellin G., Rancati T., Vavassori V., Bianchi C., Borca V.C. Clinical and dosimetric predictors of late rectal syndrome after 3D-CRT for localized prostate cancer: preliminary results of a multicenter prospective study. Int J Radiat Oncol Biol Phys. 2008;70:1130–1137. doi: 10.1016/j.ijrobp.2007.07.2354. [DOI] [PubMed] [Google Scholar]
- 30.Gay H.A., Barthold H.J., O’Meara E., Bosch W.R., El Naqa I., Al-Lozi R. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83:e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joiner M., Van Der Koegel A., editors. Basic clinical radiobiology. 4th ed. Hodder Arnold; London: 2009. [Google Scholar]
- 32.Fiorino C., Alongi F., Perna L., Broggi S., Cattaneo G.M., Cozzarini C. Dose-volume relationships for acute bowel toxicity in patients treated with pelvic nodal irradiation for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;75:29–35. doi: 10.1016/j.ijrobp.2008.10.086. [DOI] [PubMed] [Google Scholar]
- 33.Perna L., Alongi F., Fiorino C., Broggi S., Cattaneo Giovanni M., Cozzarini C. Predictors of acute bowel toxicity in patients treated with IMRT whole pelvis irradiation after prostatectomy. Radiother Oncol. 2010;97:71–75. doi: 10.1016/j.radonc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Lee T.F., Huang E.Y. The different dose-volume effects of normal tissue complication probability using lasso for acute small-bowel toxicity during radiotherapy in gynecological patients with or without prior abdominal surgery. Biomed Res Int. 2014;2014:143020. doi: 10.1155/2014/143020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling A., Furhang E., Ryemon S.N., Ennis R.D. Late small bowel toxicity after aggressive abdominopelvic intensity modulated radiation therapy. Adv Radiat Oncol. 2017;2:615–623. doi: 10.1016/j.adro.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green G., Lin A.J., Zhang J., Ramsinghani N., Azawi S. Dose-volume relationship of acute and late small bowel toxicity from radiation therapy for prostate cancer: a Veterans Affairs study. J Radiat Oncol. 2015;4:411–415. [Google Scholar]
- 37.Rancati T., Fiorino C., Fellin G., Vavassori V., Cagna E., Casanova Borca V. Inclusion of clinical risk factors into NTCP modelling of late rectal toxicity after high dose radiotherapy for prostate cancer. Radiother Oncol. 2011;100:124–130. doi: 10.1016/j.radonc.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Barnett G.C., Thompson D., Fachal L., Kerns S., Talbot C., Elliott R.M. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol. 2014;111:178–185. doi: 10.1016/j.radonc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Reis Ferreira M., Muls A., Dearnaley D.P., Andreyev H.J.N. Microbiota and radiation-induced bowel toxicity: lessons from inflammatory bowel disease for the radiation oncologist. Lancet Oncol. 2014;15:PE139–PE147. doi: 10.1016/S1470-2045(13)70504-7. [DOI] [PubMed] [Google Scholar]
- 40.Russell R.M. Changes in gastrointestinal function attributed to aging. Am J Clin Nutr. 1992;55(6 Suppl):1203S–1207S. doi: 10.1093/ajcn/55.6.1203S. [DOI] [PubMed] [Google Scholar]
- 41.Bannister J.J., Abouzekry L., Read N.W. Effect of aging on anorectal function. Gut. 1987;28:353–357. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.