Abstract
Introduction
9-valent human papillomavirus vaccine (9vHPV) was approved by the Food and Drug Administration (FDA) in December 2014. 9vHPV is not recommended during pregnancy but it is possible that some women of childbearing age might be inadvertently exposed prior to knowing they are pregnant. This study aims to assess the safety of 9vHPV administration during pregnancy.
Methods
We searched the Vaccine Adverse Event Reporting System (VAERS) database, a national post-licensure vaccine safety surveillance system, for reports of pregnant women vaccinated with 9vHPV in the United States between December 10, 2014 and December 31, 2017. Disproportionate reporting of adverse events (AEs) was assessed using proportional reporting ratios (PRRs).
Results
A total of 82 pregnancy reports were identified. Sixty reports (73.2%) did not describe an AE and were submitted only to report the vaccine exposure during pregnancy. The most frequently reported pregnancy-specific AE was spontaneous abortion (n = 3; 3.7%), followed by vaginal bleeding (n = 2; 2.4%). Among non-pregnancy-specific AEs, injection site reaction (n = 3; 3.7%) was most common. No disproportional reporting for any AE was found.
Discussion
No unexpected AEs were observed among these pregnancy reports.
Introduction
9-valent human papillomavirus vaccine (9vHPV) was approved by the Food and Drug Administration (FDA) in December 2014 and recommended by the Advisory Committee on Immunization Practices (ACIP) in February 2015 (1, 2). Previously, quadrivalent (4vHPV) and bivalent (2vHPV) forms of the vaccine had been licensed for use (3). 9vHPV has been the only human papillomavirus vaccine distributed in the United States since late 2016 (4). Pre-licensure studies showed that the most common local and system adverse events (AEs) were mild in nature and consisted in injection site pain and headache (5).
9vHPV is not recommended for use during pregnancy due to limited data on its safety in pregnancy (5–7). However, some women of childbearing age might be inadvertently exposed during catchup vaccination (6, 8, 9). Studies following 2vHPV and 4vHPV administration during pregnancy did not reveal concerning patterns of pregnancy-specific or infant/neonatal outcomes following vaccine administration (8–12). Initial pre-licensure clinical study data did not indicate an overall increased risk of spontaneous abortion, stillbirth or major birth defects when 9vHPV was inadvertently administered to pregnant women, compared to 4vHPV (5, 13). However, these data were limited by insufficient power to study less common conditions. This study aims to assess the safety of pregnant women exposed to 9vHPV in the Vaccine Adverse Event Reporting System (VAERS).
Methods
Data source
The Vaccine Adverse Event Reporting System (VAERS) is a national post-licensure vaccine safety surveillance system jointly operated by the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) (14). One of its main purposes is to identify possible vaccine safety signals, such as rare AEs that may be missed in pre-licensure clinical trials (14–16). Manufacturers are required to report all post-vaccination AEs of which they become aware, while healthcare providers are required to report those AEs from the vaccine injury table and are encouraged to report any AE they believe may be associated with the vaccine (14, 17, 18). Others, such as parents of patients and patients themselves, are encouraged to report voluntarily (14). AEs described in VAERS reports are often temporarily associated with vaccination and may or may not include conditions caused by vaccination (14, 19). VAERS is not designed to assess for causality between an AE and a vaccine (14, 19). Additionally, not all reports describe AEs, and some may describe a vaccination error (e.g., vaccine administered to patient of inappropriate age) (20). The signs, symptoms, and vaccination errors described in each report are coded with one or more terms using a clinically validated international medical terminology dictionary known as the Medical Dictionary for Regulatory Activities (MedDRA) (21). Report severity is determined on the basis of criteria established by the Code of Federal Regulations (18). AEs that result in death, life-threatening illness, hospitalization, prolongation of hospitalization, disability or permanent damage, congenital anomaly, or other medically important condition are categorized as serious (18). In this study, reports were classified as serious if AEs described met any of these criteria except for other medically important condition.
Report search
We searched the VAERS database for reports of pregnant women vaccinated with 9vHPV in the United States between December 10, 2014 and December 31, 2017 (received by January 12, 2018). The following strategies were used: (1) searched for any of the following MedDRA preferred terms: “drug exposure during pregnancy,” “exposure during pregnancy,” and “maternal exposure during pregnancy,” (2) performed a text string search for the term “preg” within the symptom description, illness at time of vaccination, and pre-existing illness variables, and (3) searched for reports for which the reporter had answered “yes” to the question “Is the report about vaccine(s) given to a pregnant woman?” (8).
Report review
All VAERS forms and medical records (when available) were reviewed to extract information on pregnancy status at time of vaccination, AEs, AE severity, vaccination errors, and date of last menstrual period or expected date of delivery (8). Our search strategy yielded information on vaccination date, maternal age at time of vaccination, vaccines administered concomitantly, and reporter type. Reports that indicated a patient was not pregnant at the time of vaccination or received 9vHPV more than a month prior to her last menstrual period were excluded (8).
AEs were characterized as primary maternal, secondary maternal, primary infant, or secondary infant AEs. Primary AEs were the main diagnoses determined by the reviewer based on information in the VAERS report and/or medical records (when available). If multiple maternal and/or infant AEs were reported for the same person, the one with the greatest clinical significance was selected as primary and the others were listed as secondary (8). If a VAERS report described AEs in more than one person (e.g., mother and exposed infant), we treated each person as a separate report. Reports of women experiencing AEs prior to receiving 9vHPV or that described AEs associated with administration of an HPV vaccine other than 9vHPV were excluded. Primary maternal AEs were further classified as pregnancy-specific or non-pregnancy-specific. Pregnancy-specific AEs of interest included spontaneous abortion (fetal demise <20 weeks gestation), stillbirth (fetal demise ≥20 weeks gestation), and preterm delivery (live birth ≤37 weeks gestation) (22, 23). No specific assessment was made to investigate causality between reported AEs and 9vHPV (8).
Vaccination errors were noted when explicitly mentioned by the reporter or after review of the VAERS report. These errors were defined as: wrong drug administered (patient was administered 9vHPV when she should have been administered a different vaccine), drug administered to a patient of inappropriate age (patient was over the age of 26 when administered her first dose of HPV vaccine), inappropriate schedule of drug administration (doses of HPV vaccine did not follow the recommended 0, 1–2, and 6 month schedule or 0 and 6–12 month schedule, depending on the age of the patient), and extra dose administered (patient had already received three doses of HPV vaccine) (4, 7, 20).
Gestational age at the time of vaccination was calculated based on the date of a woman’s last menstrual period, expected date of delivery, or gestational age noted by the reporter if neither of the other dates were given (24). This value was used to determine trimester of pregnancy, which was defined as follows: first (0–13 weeks), second (14–27 weeks), third (28+ weeks) (25).
Analysis
Frequencies and percentages of AEs and vaccination errors were calculated using SAS version 9.4 (SAS Institute Inc., Cary, NC). We assessed disproportionate reporting of AEs using proportional reporting ratios (PRRs) whereby the proportion of MedDRA preferred terms after 9vHPV was compared to the proportion of the same MedDRA preferred terms after 4vHPV (26, 27). This method was chosen due to its relative simplicity and straightforward interpretation (26). Reports that indicated vaccination with both HPV vaccines were excluded. 4vHPV is the precursor of 9vHPV, and these vaccines are similar in terms of recommended vaccination ages, recommendation that pregnant women do not receive the vaccine, and likelihood that most inadvertent vaccination would occur during the first trimester, when women may be unaware of their pregnancy status (3, 8). Given their similar composition and indications, 4vHPV was the natural comparison vaccine to use for 9vHPV (3, 8).
For the 4vHPV vaccine comparison, we used VAERS reports identified from a previous study (8). We ran two separate analyses, one of which included, and one of which excluded, manufacturer reports, for the sake of comparability with this similar previous study (8). MedDRA terms with disproportionately higher reporting after 9vHPV compared to 4vHPV were assessed using the criteria of Evans et al. (PRR ≥2, Yates χ2 ≥4, and ≥3 reports in the 9vHPV group) (26). Finally, we graphed trends of pregnancy reports following administration of 9vHPV and 4vHPV to assess how reporting changed over time.
VAERS is a routine, government-sponsored surveillance system that does not meet the definition of research as stipulated in 45 CFR 46.102(d) (8, 28). Therefore, this investigation was not subject to institutional review board review or informed consent requirements (8).
Results
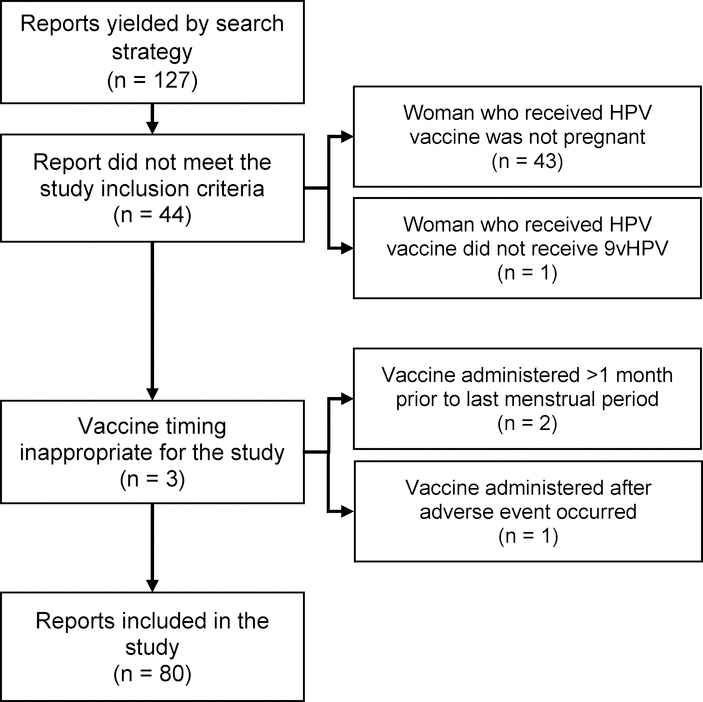
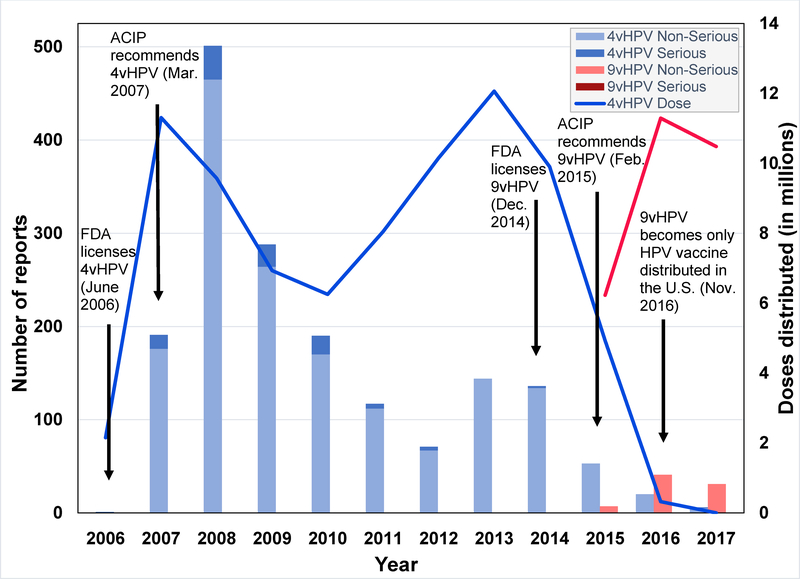
Our search strategy yielded a total of 127 reports of pregnant women vaccinated with 9vHPV in the United States between December 10, 2014 and December 31, 2017. Forty-seven reports were excluded because either the report did not meet study criteria (n = 44) or because the vaccine timing was inappropriate for the study (n = 3) (Figure 1). A total of 80 true pregnancy reports were identified and reviewed, two of which described both maternal and infant AEs, which were considered separately, therefore there were a total of 82 reports. No serious reports were identified. The greatest number of reports was received in 2016 (Figure 2). Nearly all reports were submitted by the vaccine manufacturer (n = 62; 77.5%) or healthcare provider (n = 16; 20.0%) (Table 1). In three-fourths of the reports, with known gestational age, 9vHPV was administered during the first trimester of pregnancy (n = 45; 75%) (Table 1). Median age of gestation was 6.1 weeks and median maternal age at the time of vaccination was 21.5 years (Table 1). Additional demographic and report characteristics are described in Table 1.
Figure 1.
Flow Diagram of VAERS Reports Included and Excluded in this Study
Figure 2.
Number of 9vHPV and 4vHPV Serious and Non-Serious Reports Received in VAERS and Doses of Vaccine Distributed, 2006–2017
Table 1.
| Characteristic | ||
|---|---|---|
| Maternal age in years, median (range)c | 21.5 | (12–38) |
| Gestational age at time of vaccination in weeks, median (range)d | 6.1 | (0–37.1) |
| Trimester of pregnancy at time of vaccination (n = 60)e | ||
| First (0–13 weeks), n (%) | 45 | (75.0) |
| Second (14–27 weeks), n (%) | 7 | (11.7) |
| Third (28+ weeks), n (%) | 8 | (13.3) |
| Reports of 9vHPV given with other vaccines, n (%)f | 20 | (25.0) |
| Type of reporter | ||
| Manufacturer, n (%) | 62 | (77.5) |
| Healthcare provider, n (%) | 16 | (20.0) |
| Other, n (%) | 2 | (2.5) |
Vaccine Adverse Event Reporting System
No serious reports were submitted
Maternal age was missing for 18 reports
Gestational age at time of vaccination was either not reported or unknown for 25 reports
Trimester of pregnancy at time of vaccination was either not reported or unknown for 20 reports
Most common vaccines given concomitantly with 9vHPV were meningitis (Menactra) in 8 (15.4%) reports, hepatitis A in 7 (13.5%) reports, measles, mumps, and rubella in 7 (13.5%) reports, varicella in 7 (13.5%) reports, and inactivated polio in 5 (9.6%) reports
Sixty reports (73.2%) did not describe an AE and were submitted due to vaccine exposure during pregnancy (Table 2). The most frequently reported pregnancy-specific AE was spontaneous abortion (n = 3; 3.7%), followed by vaginal bleeding (n = 2; 2.4%) (Table 2). Among non-pregnancy-specific AEs, injection site reaction (n = 3; 3.7%) was most common (Table 2). Three infant AEs were reported (Table 2); however, it is important to note that not all reports included follow-up through infant delivery.
Table 2.
Primary Adverse Events in Pregnant Women and Infants Following Maternal 9vHPV Administration, VAERSa 2014–2017 (n = 82)b
| Adverse Events | n | % |
|---|---|---|
| Pregnancy-specific outcomes | ||
| Spontaneous abortion | 3 | 3.7 |
| Vaginal bleeding | 2 | 2.4 |
| Elective abortion | 1 | 1.2 |
| Placenta previa | 1 | 1.2 |
| Polyhydramnios | 1 | 1.2 |
| Nausea/vomiting | 1 | 1.2 |
| Total | 9 | 11.0 |
| Nonpregnancy-specific outcomes | ||
| Injection site reaction | 3 | 3.7 |
| Malaise | 1 | 1.2 |
| Elevated BMI during pregnancy | 1 | 1.2 |
| Proteinuria | 1 | 1.2 |
| Urinary tract infection | 1 | 1.2 |
| Total | 7 | 8.5 |
| Infant/neonatal outcomes | ||
| Excessive weight loss | 1 | 1.2 |
| Renal impairment and manifestations | 1 | 1.2 |
| Shoulder dystocia | 1 | 1.2 |
| Total | 3 | 3.7 |
| Unspecified adverse event | 3 | 3.7 |
| No adverse event | 60 | 73.2 |
Vaccine Adverse Event Reporting System
Two reports included both maternal and infant adverse events
Just over one-fifth of reports described a vaccination error (n = 17; 20.7%): Five reports (29.4%) each indicated incorrect vaccine administration, vaccine administration to a patient of inappropriate age, or inappropriate schedule of vaccine administration and two reports (11.8%) of extra dose of vaccine administered. Among reports for which an incorrect vaccine was administered, four indicated that the patient should have received Tdap and one indicated the patient should have received influenza vaccine. Among reports for which the sole vaccination error was vaccine administration to a patient of inappropriate age, two indicated 9vHPV was administered to a patient age 27–30 years, two indicated a patient age 31–35 years, and one indicated a patient age 36–40 years. Among reports for which an extra dose of vaccine was administered, one indicated the patient had previously received three doses of 4vHPV and one indicated the patient had previously received three doses of 9vHPV. Of the 17 vaccination errors, only six reported an AE, which included: injection site reaction (n = 2), spontaneous abortion (n = 1), polyhydramnios (n = 1), proteinuria (n = 1), and an unspecified AE (n = 1).
The PRR analyses comparing 9vHPV and 4vHPV vaccines did not reveal disproportionate reporting for any AE.
Discussion
During the period of this review, VAERS received 2,068 reports of females who were administered 9vHPV, of which 4% were pregnant. No AE clusters or patterns of concern were observed among these pregnancy reports. Nearly three-fourths of reports (60 of 82) did not describe an AE and were likely submitted because 9vHPV is not recommended in pregnant women (6). Most 9vHPV pregnancy reports were received during 2016 and 2017, and, when considered alongside 4vHPV reporting trends, illustrate a Weber-like effect, likely due to reaction to media attention surrounding 4vHPV (Figure 2) (29–31). The Weber effect is an epidemiologic phenomenon whereby reporting rates peak in the second year following the marketing of a new product (or product perceived to be new) and then decline, despite constant prescribing rates (29). Similar phenomena have been observed with other vaccines (32).
Among reports describing an AE, the pregnancy-specific conditions most frequently reported were spontaneous abortion (3.7% of reports) and vaginal bleeding (2.4% of reports), both of which are relatively common during pregnancy generally. Estimates suggest that between 10% and 20% of pregnancies result in spontaneous abortion and approximately 12% of pregnant women experience vaginal bleeding (33–35). A previous review of 4vHPV pregnancy reports in VAERS also found that spontaneous abortion was the most commonly reported pregnancy-specific AE (8). Additionally, data from the Merck pregnancy registry for 4vHPV indicated that rates of spontaneous abortion were not higher than background rates in the general pregnant population (9).
Among non-pregnancy-specific AEs, the most commonly reported condition was injection site reaction (3.7% of reports), which is a known side effect of 9vHPV (5). A recent review of the VAERS database found that the proportion of reports describing injection site reactions following 9vHPV among nonpregnant women varied from 6.3–8.8%, depending on the type of local reaction (36).
All other reported AEs in this study were diverse in nature and only affected a single mother or infant. While vaccination errors were not uncommon, only about a third of reports indicating a vaccination error also described an AE, the most common of which was of minimal concern (injection site reaction). The PRR analyses did not yield any clinically important safety signals.
The results of this study are in line with those of initial pre-licensure 9vHPV clinical study data, which did not indicate overall increased risks of spontaneous abortion, stillbirth, or major birth defect when 9vHPV was inadvertently administered to pregnant women, compared to 4vHPV, although risk of spontaneous abortion was higher among women administered 9vHPV compared to 4vHPV (20.0% vs. 9.2%) within 30 days of pregnancy initiation (5, 13). They are also in line with prior studies assessing AEs following 4vHPV administration during pregnancy that have not revealed any concerning patterns of pregnancy-specific or infant/neonatal outcomes following vaccine administration (8–12). An analysis of prospective reports of vaccine exposure during pregnancy from the United States, Canada, and France submitted to the vaccine manufacturer (Merck and Co., Inc.) registry did not find any concerning patterns of spontaneous abortions, fetal deaths, or birth defects following vaccine administration (9). An analysis of data from the Danish Childhood Vaccine Database, Danish National Prescription Registry, and other Danish health and demographic registries did not indicate a statistically significant difference in risk of spontaneous abortion, preterm birth, major birth defects, small size for gestational age, or low birth weight between women who did and did not receive 4vHPV during pregnancy (10). Finally, two Vaccine Safety Datalink studies assessing the safety of 4vHPV during pregnancy did not find cause for concern (11, 12). One study, which compared outcomes of women who received 4vHPV during pregnancy or during the periconceptional period to those who received at least one dose of 4vHPV 4 to 18 months prior to their last menstrual period but not while pregnant or in the periconceptional period, found that the risks of preterm birth, major birth defects, small size for gestational age, and adverse maternal obstetric outcomes did not differ statistically significantly between groups (11). The other study, which compared outcomes of women who received 4vHPV during pregnancy to those who received at least one dose of 4vHPV 16 to 22 weeks before the last menstrual period but not while pregnant or in the peripregnancy period, found that the risk of spontaneous abortion did not differ significantly between groups (12). To our knowledge, only one study has found substantial differences in percentages of spontaneous abortions between African-American women administered 4vHPV and a placebo (20.0% vs. 6.4%) (37). However, it is possible that differences in baseline characteristics between the two groups may have contributed to this finding (37). Overall, studies of 4vHPV have failed to demonstrate higher risks of AEs following vaccination during pregnancy, and the current study replicates this finding with 9vHPV.
VAERS strengths lie in its flexibility and ability to quickly detect rare and/or previously unrecognized AEs (38). This national system can provide near real-time information on the safety of vaccines (39). However, it has a number of limitations, which in this study included lack of complete data and accuracy of reports (14). For example, some individuals who submitted the VAERS form did not supply all information relevant to the study (e.g., last menstrual period, which would have allowed for the calculation of gestational age at time of vaccination and trimester of pregnancy). VAERS does not collect information on the number of 9vHPV pregnant vaccinees; therefore, it is not possible to calculate rates of AEs among pregnant women receiving 9vHPV (39). Underreporting or overreporting can be problematic, the first due to the voluntary nature of report submission and the second due to media attention and/or the severity of the AE; serious reports are thought to be reported more frequently than non-serious reports (14, 39). In our study, the relatively small number of spontaneous abortion reports submitted likely represents substantial underreporting of this event, given the relative frequency of this event during pregnancy (33, 34).
Conclusion
The findings of this study are reassuring as no unexpected AEs were observed among pregnant women exposed to 9vHPV. 9vHPV is not recommended during pregnancy; however, because of the age group in which it is indicated, it may be inadvertently administered to pregnant women who are unaware they are pregnant (6). Therefore, monitoring the safety of the vaccine in this subpopulation is important – including in active surveillance systems such as CDC’s Vaccine Safety Datalink. CDC routinely monitors the safety of 9vHPV in the United States and will continue to monitor the safety of this vaccine in pregnancy (40).
Acknowledgments
We thank Dr. Frank DeStefano, Dr. Lakshmi Sukumaran, Dr. Jorge Arana, Dr. Maria Cano, Ms. Carmen Ng, and Mrs. Elaine Miller for their helpful comments and advice.
References
- 1.Petrosky E, Bocchini JA Jr., Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morbidity and mortality weekly report. 2015;64(11):300–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Gruber MF. December 10, 2014 Approval letter—GARDASIL 9. Silver Spring, MD: U.S. Department of Health and Human Services, Food and Drug Administration; 2014. [Google Scholar]
- 3.Centers for Disease Control and Prevention. HPV Vaccine Information for Clinicians. U.S. Department of Health and Human Services; https://www.cdc.gov/hpv/hcp/need-to-know.pdf. Published December 20, 2016. Accessed January 2018. [Google Scholar]
- 4.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination - Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and mortality weekly report. 2016;65(49):1405–8. [DOI] [PubMed] [Google Scholar]
- 5.Merck Sharp & Dohme Corp. Highlights of Prescribing Information Gardasil®9 (Human Papillomavirus 9-valent Vaccine, Recombinant). Merck & Co., Inc; https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM426457.pdf. Published 2014. Updated February 2018. Accessed April 2018. [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2014;63(Rr-05):1–30. [PubMed] [Google Scholar]
- 7.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2007;56(Rr-2):1–24. [PubMed] [Google Scholar]
- 8.Moro PL, Zheteyeva Y, Lewis P, et al. Safety of quadrivalent human papillomavirus vaccine (Gardasil) in pregnancy: adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006–2013. Vaccine. 2015;33(4):519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goss MA, Lievano F, Buchanan KM, et al. Final report on exposure during pregnancy from a pregnancy registry for quadrivalent human papillomavirus vaccine. Vaccine. 2015;33(29):3422–8. [DOI] [PubMed] [Google Scholar]
- 10.Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV Vaccination and the Risk of Adverse Pregnancy Outcomes. The New England journal of medicine. 2017;376(13):1223–33. [DOI] [PubMed] [Google Scholar]
- 11.Lipkind HS, Vazquez-Benitez G, Nordin JD, et al. Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy. Obstetrics and gynecology. 2017;130(3):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Risk of Spontaneous Abortion After Inadvertent Human Papillomavirus Vaccination in Pregnancy. Obstetrics and gynecology. 2018;132(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira ED, Block SL, Ferris D, et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics. 2016;138(2). [DOI] [PubMed] [Google Scholar]
- 14.Varricchio F, Iskander J, Destefano F, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. The Pediatric infectious disease journal. 2004;23(4):287–94. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg SS. Safety considerations for new vaccine development. Pharmacoepidemiology and drug safety. 2001;10(5):411–5. [DOI] [PubMed] [Google Scholar]
- 16.Fritzell B Detection of adverse events: what are the current sensitivity limits during clinical development? Vaccine. 2001;20 Suppl 1:S47–8. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine (US) Committee on Review of Priorities in the National Vaccine Plan. Priorities for the National Vaccine Plan. Washington (DC): National Academies Press (US), 2010. [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration. U.S. Code of Federal Regulations, 21 CFR 600.80. Silver Spring, MD: U.S. Department of Health and Human Services; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.80. Updated August 14, 2017. Accessed April 2018. [Google Scholar]
- 19.Centers for Disease Control and Prevention. Understanding the Vaccine Adverse Event Reporting System (VAERS). U.S. Department of Health and Human Services; https://www.cdc.gov/vaccines/hcp/patient-ed/conversations/downloads/vacsafe-vaers-color-office.pdf. Updated February 2013 Accessed March 2018. [Google Scholar]
- 20.Hibbs BF, Moro PL, Lewis P, et al. Vaccination errors reported to the Vaccine Adverse Event Reporting System, (VAERS) United States, 2000–2013. Vaccine. 2015;33(28):3171–8. [DOI] [PubMed] [Google Scholar]
- 21.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. MedDRA. http://www.ich.org/products/meddra.html. Accessed April 2018.
- 22.Chapter 1 - Overview of Obstetrics In: Cunningham F, Leveno K, Bloom S, et al. , eds. Williams Obstetrics. 23 ed. New York, NY: McGraw-Hill Companies, Inc.; 2010. [Google Scholar]
- 23.Chapter 9 - Abortion In: Cunningham F, Leveno K, Bloom S, et al. , eds. Williams Obstetrics. 23 ed. New York, NY: McGraw-Hill Companies, Inc.; 2010. [Google Scholar]
- 24.Medical College of Wisconsin. Pregnancy Date Calculator. https://www.mcw.edu/calculators/pregnancydate.htm. Accessed January 2018.
- 25.Chapter 8 - Prenatal Care In: Cunningham F, Leveno K, Bloom S, et al. , eds. Williams Obstetrics. 23 ed. New York, NY: McGraw-Hill Companies, Inc.; 2010. [Google Scholar]
- 26.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiology and drug safety. 2001;10(6):483–6. [DOI] [PubMed] [Google Scholar]
- 27.Banks D, Woo EJ, Burwen DR, et al. Comparing data mining methods on the VAERS database. Pharmacoepidemiology and drug safety. 2005;14(9):601–9. [DOI] [PubMed] [Google Scholar]
- 28.Office for Human Research Protections (OHRP). U.S. Code of Federal Regulations, 45 CFR 46.102. U.S. Department of Health and Human Services; https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.102. Updated January 15, 2010. Accessed April 2018. [Google Scholar]
- 29.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24(6):743–9. [DOI] [PubMed] [Google Scholar]
- 30.Eberth JM, Kline KN, Moskowitz D, et al. The role of media and the Internet on vaccine adverse event reporting: a case study of HPV vaccination. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2014;54(3):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gollust SE, LoRusso SM, Nagler RH, et al. Understanding the role of the news media in HPV vaccine uptake in the United States: Synthesis and commentary. Human vaccines & immunotherapeutics. 2016;12(6):1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moro PL, Broder K, Zheteyeva Y, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. American journal of obstetrics and gynecology. 2011;205(5):473.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldhaber MK, Fireman BH. The fetal life table revisited: spontaneous abortion rates in three Kaiser Permanente cohorts. Epidemiology (Cambridge, Mass). 1991;2(1):33–9. [PubMed] [Google Scholar]
- 34.Wilcox AJ, Treloar AE, Sandler DP. Spontaneous abortion over time: comparing occurrence in two cohorts of women a generation apart. American journal of epidemiology. 1981;114(4):548–53. [DOI] [PubMed] [Google Scholar]
- 35.Ananth CV, Savitz DA. Vaginal bleeding and adverse reproductive outcomes: a meta-analysis. Paediatric and perinatal epidemiology. 1994;8(1):62–78. [DOI] [PubMed] [Google Scholar]
- 36.Arana J Adverse events following 9-valent human papillomavirus vaccine (9vHPV) reported to the Vaccine Adverse Event Reporting System (VAERS). Presented at the Meeting of the Advisory Committee on Immunization Practices (ACIP), Atlanta, GA, February 21–22, 2018. [Google Scholar]
- 37.Clark LR, Myers ER, Huh W, et al. Clinical trial experience with prophylactic human papillomavirus 6/11/16/18 vaccine in young black women. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2013;52(3):322–9. [DOI] [PubMed] [Google Scholar]
- 38.Singleton JA, Lloyd JC, Mootrey GT, et al. An overview of the vaccine adverse event reporting system (VAERS) as a surveillance system. VAERS Working Group. Vaccine. 1999;17(22):2908–17. [DOI] [PubMed] [Google Scholar]
- 39.Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Human Papillomavirus (HPV) Vaccine Safety. U.S. Department of Health and Human Services; https://www.cdc.gov/vaccinesafety/vaccines/hpv-vaccine.html. January 30, 2018. Accessed March 2018. [Google Scholar]
- 41.Markowitz L Update: Recommendations for HPV Vaccination. Centers for Disease Control and Prevention; https://www.cdc.gov/vaccines/ed/ciinc/downloads/2016-10-26/recommendations-hpv-2-doses-2016.pdf. Accessed Apil 2018. [Google Scholar]