Abstract
The visualisation and quantitative analysis of the native drug distribution in a pre-clinical or clinical setting are desirable for evaluating drug effects and optimising drug design. Here, using matrix-assisted laser desorption ionisation imaging mass spectrometry (MALDI-IMS) with enhanced resolution and sensitivity, we compared the distribution of a paclitaxel (PTX)-incorporating micelle (NK105) with that of PTX alone after injection into tumour-bearing mice. We demonstrated optically and quantitatively that NK105 delivered more PTX to the tumour, including the centre of the tumour, while delivering less PTX to normal neural tissue, compared with injection with PTX alone. NK105 treatment yielded a greater antitumour effect and less neural toxicity in mice than did PTX treatment. The use of high-resolution MALDI-IMS may be an innovative approach for pharmacological evaluation and drug design support.
Advances in our understanding of cancer at the cellular and molecular levels have promoted the development of new drugs1,2. Pharmacokinetic (PK) and pharmacodynamic (PD) studies are very important to evaluate the efficacy and toxicity of new drugs as well as to optimise drug design. For these purposes, tissue homogenate samples are generally analysed by high-performance liquid chromatography (HPLC) or liquid chromatography mass spectrometry (LC-MS)3. For the development of anticancer drugs (ACAs), including molecular targeting agents, precise chemical modulation is needed because the small differences between cancer cells and their host cells creates a narrow therapeutic window. In addition, clinical human cancer tissues generally exhibit abundant and versatile stroma, which is the result of the process of tumour cell invasion into tumour vessels, haemorrhage, fibrin clot formation, and replacement with collagen tissues and non-malignant stromal cells. Therefore, it is very important to consider the delivery of ACAs to cancer tissues and their distribution to target cancer cells within this heterogeneous tumour microenvironment. Furthermore, the drug distribution within normal tissues, particularly vital organs, should also be evaluated because ACAs frequently cause adverse effects4. A large body of clinical evidence has revealed that neoadjuvant chemotherapy is useful for a variety of solid tumours. The tissue excised during surgery or endoscopic biopsy can be used to investigate drug distribution5,6. Thus, a convenient method for evaluating the distribution of clinically used native (non-radiolabeled or non-chemically modified) drugs is urgently required.
Matrix-assisted laser desorption ionisation imaging mass spectrometry (MALDI-IMS) has been developed for the investigation of the distribution of molecules such as small peptides, drugs, and their metabolites7,8,9,10,11,12. Moreover, MALDI-IMS can be used to evaluate numerous molecules in a single measurement without a specialised probe7,8,9,10,11,12. Therefore, this method enables the observation of a drug directly within tissue with the distinction between the original compound and its metabolites.
We have developed a mass microscopy method in which a microscope is coupled with a high-resolution atmospheric pressure-laser desorption/ionisation and quadruple ion trap time-of-flight (TOF) analyser. In this study, we investigated the ability of our mass microscopy technique to visualise the tissue distribution of unlabelled ACA and its micellar formulation and obtain precise regional information about the drug distribution in a specific anatomical area.
Results
The drug imaging system and its application in PTX analysis on the MALDI target
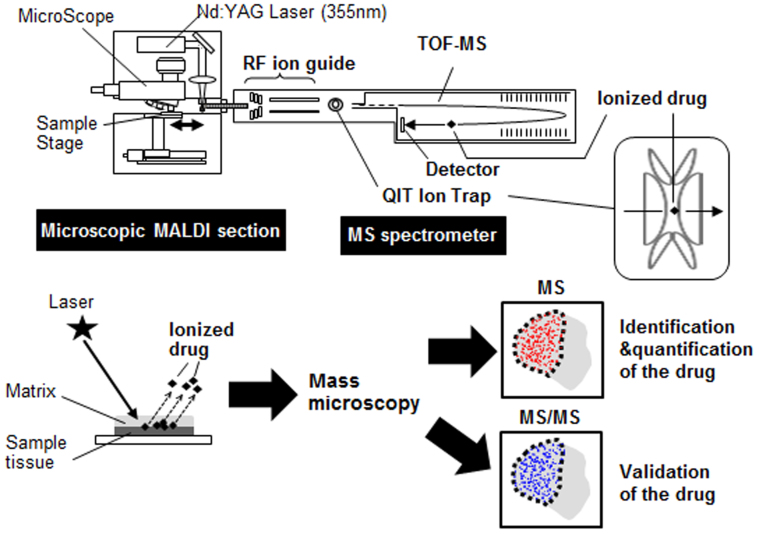
A schematic representation of our drug imaging system is shown in Fig. 1. Imaging data were acquired using a mass microscope. In the analysis, mass spectrometry (MS) and tandem mass spectrometry (MS/MS) were used for quantification and validation, respectively (Fig. 1).
Figure 1. Drug imaging system.
A schematic illustration of the drug imaging system. The matrix-coated drug sample is ionised and then separated on the basis of its m/z. Images from MS or MS/MS analysis are recorded.
Paclitaxel (PTX) is a mitotic inhibitor and an ACA that is used to treat various cancers. However, PTX is associated with peripheral neuropathy, a serious adverse affect13. NK105, a PTX-incorporating micelle, was developed to address this limitation of PTX14,15,16,17. On the basis of the enhanced permeability and retention (EPR) effect17,18,19,20, NK105 can be selectively delivered to a tumour, resulting in an enhanced antitumour effect and the minimisation of adverse effects, including peripheral neuropathy. The high efficacy and low toxicity of NK105 have been demonstrated in both preclinical and clinical studies14,15,16,17. Although the administered drug content per tissue weight can be determined by conventional HPLC or LC-MS, the detailed drug distribution within the tumour and normal tissue has not been examined. Therefore, we used our drug imaging system to evaluate the difference in the distribution of NK105 and free PTX within tumour and normal peripheral neuronal tissue.
Antitumour activity and visualisation of PTX and NK105 distribution within the tumour with MS analysis
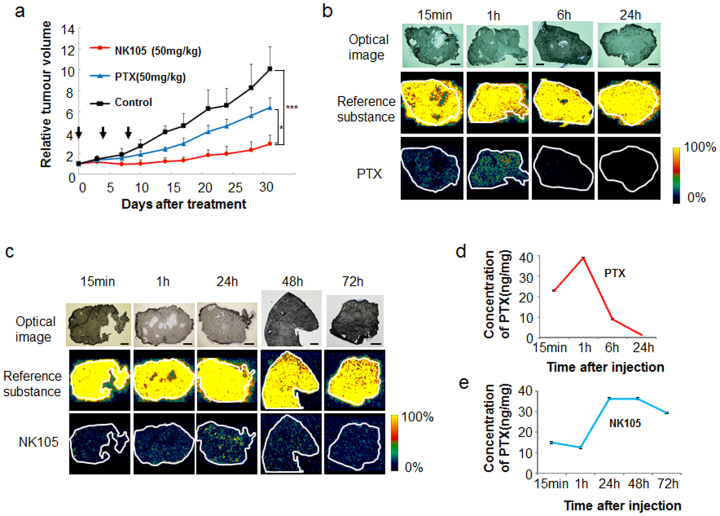
NK105 or PTX was administered at a PTX equivalent dose of 50 mg/kg/day to mice bearing BxPC3 pancreatic cancer xenografts on days 0, 4, and 8. NK105 showed significantly higher antitumour activity than the control (saline) and free PTX (Fig. 2a). To confirm the correlation of the distribution with the antitumour effect, the corresponding tumour sections were subjected to MALDI-IMS. A drug signal originating from PTX was detected in the tumours at 15 min and 1 h after the administration of PTX, but this signal decreased at 6 h and was below the limit of detection by 24 h (Fig. 2b). By contrast, the signal originating from the PTX released from NK105 (rPTX) following the accumulation of NK105 in the tumour was detected at 15 min as well as at 1, 24, 48, and 72 h after the administration of NK105. The signal intensity was greatest at 24 h (Fig. 2c). Tissue sections serial to those for MALDI-MS were also quantified by LC-MS (Fig. 2d, e), the results of which correlated with the drug imaging results (Fig. 2b–e). The data did not contradict previous data obtained by HPLC14. The results of the MALDI-MS analysis demonstrate that significant levels of PTX were present in the tumour clusters, including within the centre of the tumour tissue.
Figure 2. Antitumour activity and visualisation of PTX and NK105 distribution with MS analysis.
(a) Antitumour activity was examined in an animal model with BXPC3 xenografts. NK105, PTX, or saline (as a control) was administered at a PTX equivalent dose of 50 mg/kg on days 0, 4, and 8. *P < 0.05 (PTX vs. NK105), ***P < 0.001 (saline vs. NK105). Bar = SD. (b)(c) Imaging of PTX within the tumour was performed after PTX (b) or NK105 (c) administration at a dose of 100 mg/kg. The upper, middle, and lower columns display the optical images, reference substance (an arbitrary signal of m/z 824.6), and PTX (specific signal of m/z 892.3 [M + K]+), respectively. Bar, 1 mm. (d)(e) LC-MS analysis of the PTX concentration in the tumours treated by PTX (d) or NK105 (e). Tissue sections serial to those shown in (b) and (c).
Validation of the PTX and NK105 distribution within tumour tissue by MS/MS analysis
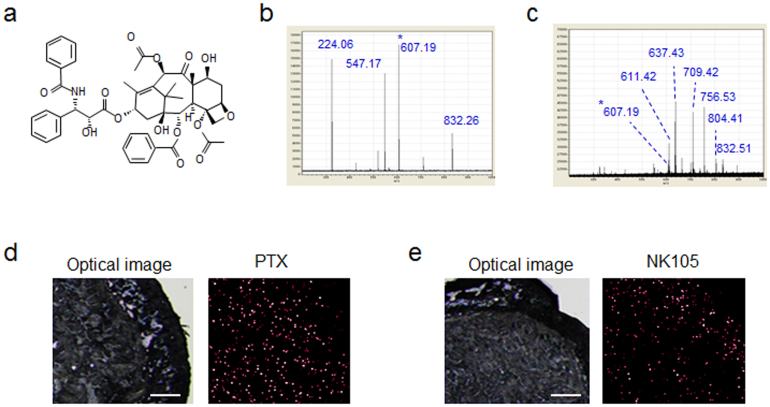
Validation of the PTX content in each sample was performed in MS/MS mode. A structural diagram and the MS/MS fragmentation pattern (FP) of PTX are shown in Fig. 3a and b, respectively. According to the MS/MS-FP, m/z 607.19, which was selected as a PTX-specific fragment peak (Fig. 3c), was observed at a higher level in the tumour tissue sample at 1 h after PTX injection than at 1 h after NK105 injection (Fig. 3d, e).
Figure 3. Validation of the PTX and NK105 distribution within the tumour tissues by MS/MS analysis.
(a) The structure of PTX is shown. (b) (c) The PTX-specific MS/MS fragments at m/z 224.06, 547.17, 607.19, and 832.26 are shown in (b). The fragment pattern from MS/MS analysis of the tumour tissue sample is shown in (c). (d) (e) Images obtained by optical microscopy (left) and by MS/MS analysis of PTX (m/z 607.19*) (right) in tumours treated with PTX (d) or NK105 (e) at 1 h after injection. Bar, 500 μm.
Peripheral neurotoxicity and visualisation of the PTX and NK105 distribution by MS analysis
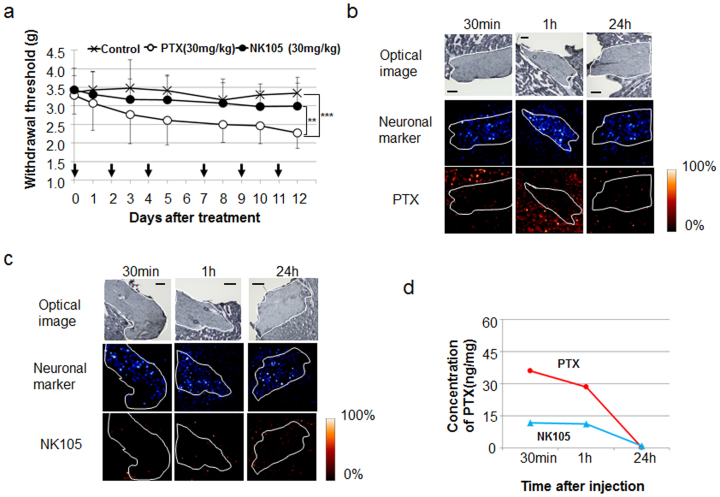
Next, a mechanical stress test that measured the degree of peripheral neurotoxicity demonstrated that the mice in the PTX treatment group exhibited a significantly stronger hypersensitive reaction to the mechanical stress test than those in the control and NK105 treatment groups (Fig. 4a). To confirm the correlation of the distribution with the abnormal neurological reaction, we also applied MALDI-IMS and examined the distribution of PTX in peripheral neural tissue at 30 min, 1 h, and 24 h after administration. The signals surrounding and inside the nerve were lower after NK105 injection than after PTX injection (Fig. 4b, c). LC-MS analysis of the neural samples revealed that the concentration of rPTX after NK105 injection was also lower than that after PTX injection (Fig. 4d).
Figure 4. Peripheral neurotoxicity and visualisation of PTX and NK105 distribution by MS analysis.
(a) Mechanical sensory stress was assayed in an animal model of PTX-induced peripheral neuropathy. NK105, PTX, or saline was administered at 30 mg/kg on days 0, 2, 4, 7, 9, and 11. **P < 0.01 (PTX vs. NK105), ***P < 0.001 (saline vs. PTX). Bar = SD. (b) (c) PTX within neuronal tissue was imaged after PTX (b) or NK105 (c) administration at a dose of 50 mg/kg. The upper, middle, and lower columns show the optical images, a neuronal marker (sphingomyelin-specific signal of 851.6 m/z), and PTX (specific signal of m/z 892.3 [M + K]+), respectively. The neuronal area is delineated by a white line. Bar, 200 μm. (d) Analysis of the PTX concentration by LC-MS. Tissue sections serial to those shown in (b) and (c).
Discussion
Conventional MALDI-IMS was expected to aid in the analysis of the global distribution of drugs within tissue. However, its application has been limited for a variety of reasons, including its limited resolution7,8. Recent progress in MALDI-IMS analysis, including the new features of our instrument, have achieved a MALDI-IMS resolution of 10 μm or less, which is advantageous for evaluating the drug distribution in specific cells or areas of interest within tissues9,10,11,12. The improved resolution also allows an IMS image to be overlaid on an optical image of the same sample. In fact, we were able to distinguish the nerve component from the surrounding tissue and evaluate the specific distribution of PTX in the region.
Tissue samples should be frozen without liquid solution to avoid the diffusion or loss of the drug from the tissue to the solution. For efficient ionisation in the present study, the sample was coated with a sufficient quantity of matrix by spraying. 2,5-Dihydroxybenzoic acid (DHB) was selected as the matrix to facilitate the efficient ionisation of the drug. We are now attempting to use several other matrix materials to enhance the sensitivity of our MALDI-IMS technique. Moreover, we used a combination of MS and MS/MS for the imaging analysis. In the MS analysis, accurate quantification of PTX was demonstrated in vivo. In the MS/MS analysis, the presence of PTX was validated by a fragment-specific signal at 607.19 m/z, which does not overlap with any other signals. The combination of MS and MS/MS thus facilitates the accurate evaluation of the drug-originated signal by distinguishing the drug signal from endogenous metabolites with a similar m/z.
In this report, MALDI-IMS demonstrated that NK105 successfully delivered a large amount of the PTX payload within the tumour tissue after NK105 injection compared with PTX injection alone. More importantly, the precise localisation and levels of PTX within the tumour tissue were visualised and quantified due to the high resolution of the MALDI-IMS technique. The obtained data did not contradict our MS data or previous data obtained by conventional pharmacological analysis using HPLC14. Thus, our data demonstrated that the antitumour activity of NK105 is superior to that of PTX alone. In addition, the peripheral neurotoxicity of NK105 was significantly lower than that of PTX, consistent with the MALDI-IMS data. In fact, in a phase 2 clinical trial of NK105 in patients with previously treated advanced stomach cancer, only one of the 56 patients (1.8%) who entered the trial experienced grade 3 peripheral neuropathy16. Phase 2 trials of other PTX formulations, including Abraxane and conventional PTX, have demonstrated that the incidence of grade 3 or 4 peripheral neuropathy is greater than 10%21,22. A phase 3 clinical trial of NK105 vs. PTX is now underway, which may elucidate the clinical significance of this micellar drug delivery system (DDS).
Although many studies have indicated that NK105 accumulates selectively in tumour tissue compared to PTX by HPLC or LC-MS analysis, whether NK105 could deliver PTX to cancer-cell clusters within the tumour tissue was unknown. Cancer tissue is heterogeneous and consists not only of cancer cells but also of abundant tumour stroma, the latter of which can act as a barrier against macromolecules, including NK10523,24. In the present study, significant levels of PTX, even in the core of the tumour tissue, were observed following NK105 administration, and the NK105 was retained for a long period of time.
Low molecular weight (LMW) ACAs, including molecular targeting agents, can easily extravasate from normal blood vessels and cause various adverse effects. DDS drugs such as NK105, which exhibit low short-term accumulation in normal tissues that lack the EPR effect, can minimise this drug toxicity. Our data clearly demonstrate that the distribution of rPTX from NK105 in the peripheral nerve and surrounding tissues was quite low compared with PTX alone. These observations support the low incidence of peripheral neuropathy when PTX is administered as NK105.
This is the first report describing the precise distribution of a DDS drug by MSI, a new technique developed by our lab and others. Notably, we successfully visualise and quantified the distribution of a non-radiolabeled and non-chemically modified drug in various frozen tissue slices microscopically. In addition to PTX, we have successfully visualised other anticancer agents, including SN-38, epirubicin, and monomethyl auristatin E (MMAE) (data not shown). This success indicates that the MALDI-IMS technique can be applied to clinical biopsy specimens or surgically resected tissues after neo-adjuvant chemotherapy. In addition, the data obtained by MALDI-IMS can be utilised to facilitate drug design.
Methods
Cells and reagents
The human pancreatic cancer cell line BxPC3 was purchased from the American Type Culture Collection and maintained in DMEM (Sigma, St. Louis, MO) supplemented with 10% foetal bovine serum (Tissue Culture Biologicals, CA), penicillin, streptomycin, and amphotericin B (Sigma) at 5% CO2 and 37°C. NK105, a PTX-incorporating ‘core-shell-type’ polymeric micellar nanoparticle, was supplied by Nippon Kayaku Co. Ltd. (Tokyo, Japan). The weight-average diameter of the nanoparticles was approximately 85 nm, ranging from 20 to 430 nm. PTX was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan).
Drug imaging by mass microscopy
IMS analysis was performed using an atmospheric pressure (AP) MALDI-IT-TOF mass spectrometer (prototype Mass Microscope; Shimadzu)25.
To prepare tissue samples, the tumour and sciatic nerve with surrounding tissue were surgically removed from a xenograft model at 15 min, 30 min, 1 h, or 24 h after drug administration (50 or 100 mg/kg). Samples wrapped in gauze were frozen in dry ice powder. The samples were then sectioned at a thickness of 10 μm and transferred to an indium tin oxide-coated glass slide (Sigma). The tissue section was dried (with no washing step) before matrix coating.
For the application of the matrix onto the tissue slide, 30 and 50 mg/ml of DHB in 50% methanol and 0.1% trifluoroacetic acid were used. The 30 mg/ml DHB solution (0.4 ml) was sprayed twice, and then the 50 mg/ml (0.4 ml) solution was sprayed once with a 0.2-mm nozzle calibre airbrush (Procon Boy FWA Platinum; Mr. Hobby, Tokyo, Japan); each spraying step was completed over 5 min. During spraying, the distance between the nozzle and the tissue surface was maintained at 15 cm to keep the surface dry.
IMS analyses were performed in positive-ion mode within a mass range of m/z 720–920 for PTX, with a spatial resolution of 30 μm. The laser was irradiated at 40 shots/spectrum at a frequency of 400 Hz, and the power was set to 60–65% using the Mass Microscope operation software. PTX distribution mapping was performed in BioMap (Novartis, Basel, Switzerland) using the m/z 892.3 ([M + K]+) signal because the ([M + K]+ signal showed more sensitive mass spectra than did the [M + H]+ and [M + Na]+ signals. The uniformly distributed m/z 824.6 ion (corresponding to cerebrosides (42:6) + Na) in tumour sections and m/z 851.6 ion (sphingomyelin (d18:1/24:1) + K) in neuronal tissue were used as internal controls to correct the PTX signal intensity.
MS/MS analysis of PTX (m/z 892.3) was performed with the CID function of the quadruple ion trap cell on the Mass Microscope. The m/z 607.19 fragment ion was generated on the tissue. This ion was also observed for the authentic PTX as the derivative and was used for MS/MS imaging of the drug. The instrument conditions for MS/MS imaging were identical to those used for the MS mapping described above, but the spatial resolution was 15 μm, and the laser power was 50%.
Animal model
Antitumour activity
Female BALB/c nude mice (5 weeks old) and DBA/2N mice (8 weeks old) were purchased from SLC Japan (Shizuoka, Japan). The nude mice were inoculated subcutaneously in the flank with 1 × 106 BxPC3 cells. The length (L) and width (W) of the tumour masses were measured every 3 to 4 days, and the tumour volume was calculated using the following formula: (L × W2)/2. When the mean tumour volume reached approximately 300 mm3, the mice were randomly assigned to groups of five. Drugs (50 mg/kg) were administered on days 0, 4, and 8 by injection into the mouse tail vein.
Peripheral neuropathy
To investigate the neurotoxicity induced by PTX and NK105, we designed the following experimental scheme: The development of nocifensive responses to mechanical stimuli was assessed in the mice (ref). Six-week-old female DBA/2N mice were randomly assigned to one of three groups, and their baseline nocifensive responses were measured. We confirmed that the mean latency was statically identical between the groups. The mice were then administered a dose of 30 mg/kg PTX or 30 mg/kg NK105 on days 0, 2, and 4 every week for 2 weeks, for a total of 6 injections (n = 10). Control mice were injected with 5% dextrose solution on the same schedule. After a total of 6 administrations, the mice were tested for transitional changes in their nocifensive responses. Mechanical allodynia was assessed by measuring the latency of paw withdrawal in response to noxious mechanical stimuli using a Dynamic Plantar Aesthesiometer (Ugo Basile, Varese, Italy). The mice were placed on a wire mesh floor in individual Plexiglas cages and were allowed to acclimate for approximately 1 h, during which exploratory and grooming activity was completed. The mechanical stimulus was applied to the plantar aspect of the hind paw using a 2-mm-diameter metal filament. The force was automatically increased at a fixed rate (0–5 g, 0.25 g/sec) until the mouse withdrew its paw. The analysis of paw withdrawal responses was repeated 4 times at 10-sec intervals. The paw withdrawal threshold (g) was determined from the average of the four measurements. None of the mice in this assay were inoculated with tumour cells.
All animal procedures and experiments were approved by Committee for Animal Experimentation of the National Cancer Centre, Tokyo Japan.
These guidelines meet the ethical standards required by law and comply with the guidelines for the use of experimental animals in Japan.
Statistical analysis was performed using analysis of variance (ANOVA) with Tukey's multiple comparison tests.
LC-MS
For LC-MS, several sections immediately adjacent to the sections for IMS imaging were serially collected into a vial, and the drug was extracted into acetonitrile by vortexing. The samples were analysed with a Liquid Chromatograph Mass Spectrometer LCMS-8040 (Shimadzu Corp.). A Kinetex 2.6 μm C18 100A (100 × 2.1 mm) analytical column was used. The injection volume was 1 μl, and the flow rate was 0.5 ml/min. (A) Acetonitrile and (B) 0.1% (w/v) formic acid solution were used as the mobile phases. The mobile phase was introduced into the spectrometer via electrospray ionisation in positive ion mode under multiple reaction monitoring (MRM) conditions. In terms of the gradient, acetonitrile was conducted at 50% (B) for the first 1.5 min, increased to 100% for 0.25 min, and subsequently decreased back to 50% for 1.25 min. The PTX quantification was performed with the precursor m/z 854.45 ion, and the standard curve generated using the product m/z 104.95 ion was used. The data were collected in triplicate experiments.
Acknowledgments
This work was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) (YM), Third Term Comprehensive Control Research for Cancer from the Ministry of Health, Labour and Welfare of Japan (YM), a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, the National Cancer Center Research and Development Fund (YM and MY), the Kobayashi Foundation Research Grant for Cancer Research (MY), and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (MY). We thank Mrs K. Shiina for her secretarial support.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.M. developed the method. M.Y., M.F., K.O., Y.K., Y.Y. and M.T. performed the experiments and analysed the data. Y.M. and M.Y. wrote the manuscript.
References
- Chin L. & Gray J. W. Translating insights from the cancer genome into clinical practice. Nature 452, 553–563 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dort M. E., Rehemtulla A. & Ross B. D. PET and SPECT Imaging of Tumor Biology: New Approaches towards Oncology Drug Discovery and Development. Curr. Comput. Aided Drug Des. 4, 46–53 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M. D. & Workman P. Discovering novel chemotherapeutic drugs for the third millennium. Eur. J. Cancer 35, 2010–2030 (1999). [DOI] [PubMed] [Google Scholar]
- Abramson R. G. et al. Complications of targeted drug therapies for solid malignancies: manifestations and mechanisms. AJR Am J Roentgenol. 200, 475–483 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak C. E. et al. Biomarker analysis of neoadjuvant doxorubicin/cyclophosphamide followed by ixabepilone or Paclitaxel in early-stage breast cancer. Clin. Cancer Res. 19, 1587–1595 (2013). [DOI] [PubMed] [Google Scholar]
- Waddell T. & Cunningham D. Impact of targeted neoadjuvant therapies in the treatment of solid organ tumours. Br. J. Surg. 100, 5–14 (2013). [DOI] [PubMed] [Google Scholar]
- Cornett D. S., Reyzer M. L., Chaurand P. & Caprioli R. M. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat. Methods 4, 828–833 (2007). [DOI] [PubMed] [Google Scholar]
- Schwamborn K. & Caprioli R. M. Molecular imaging by mass spectrometry--looking beyond classical histology. Nat. Rev. Cancer 10, 639–646 (2010). [DOI] [PubMed] [Google Scholar]
- Castellino S., Groseclose M. R. & Wagner D. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development. Bioanalysis 3, 2427–2441 (2011). [DOI] [PubMed] [Google Scholar]
- Saito Y. et al. Development of imaging mass spectrometry. Biol. Pharm. Bull. 35, 1417–1424 (2012). [DOI] [PubMed] [Google Scholar]
- Lorenz M., Ovchinnikova O. S., Kertesz V. & Van Berkel G. J. Laser microdissection and atmospheric pressure chemical ionisation mass spectrometry coupled for multimodal imaging. Rapid Commun Mass Spectrom. 27, 1429–1436 (2013). [DOI] [PubMed] [Google Scholar]
- Römpp A. & Spengler B. Mass spectrometry imaging with high resolution in mass and space. Histochem Cell Biol. 139, 759–783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky E. K. et al. Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J. Clin. Oncol. 11, 2010–2020 (1993). [DOI] [PubMed] [Google Scholar]
- Hamaguchi T. et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 92, 1240–1246 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T. et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br J Cancer 97, 170–176 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K. et al. Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Invest. New Drugs 30, 1621–1627 (2012). [DOI] [PubMed] [Google Scholar]
- Matsumura Y. & Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 100, 572–579 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y. & Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986). [PubMed] [Google Scholar]
- Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 6, 688–701 (2006). [DOI] [PubMed] [Google Scholar]
- Peer D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007). [DOI] [PubMed] [Google Scholar]
- Ibrahim N. K. et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 23, 6019–26 (2005). [DOI] [PubMed] [Google Scholar]
- Johnson D. H., Chang A. Y. & Ettinger D. S. Taxol (paclitaxel) in the treatment of lung cancer: the Eastern Cooperative Oncology Group experience. Ann Oncol. Suppl 6, S45–50 (1994). [PubMed] [Google Scholar]
- Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev. 64, 710–719 (2012). [DOI] [PubMed] [Google Scholar]
- Dimou A., Syrigos K. N. & Saif M. W. Overcoming the stromal barrier: technologies to optimize drug delivery in pancreatic cancer. Ther Adv Med Oncol. 5, 271–279 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T. et al. Visualization of volatile substances in different organelles with an atmospheric-pressure mass microscope. Anal Chem. 81, 9153–9157 (2009). [DOI] [PubMed] [Google Scholar]