Abstract
Background
Poor outcomes after breech birth might be the result of underlying conditions causing breech presentation or due to factors associated with the delivery.
Objectives
To assess the effects of planned caesarean section for singleton breech presentation at term on measures of pregnancy outcome.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2015).
Selection criteria
Randomised trials comparing planned caesarean section for singleton breech presentation at term with planned vaginal birth.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
Three trials (2396 participants) were included in the review. Caesarean delivery occurred in 550/1227 (45%) of those women allocated to a vaginal delivery protocol and 1060/1169 (91%) of those women allocated to planned caesarean section (average risk ratio (RR) random‐effects, 1.88, 95% confidence interval (CI) 1.60 to 2.20; three studies, 2396 women, evidence graded low quality). Perinatal or neonatal death (excluding fatal anomalies) or severe neonatal morbidity was reduced with a policy of planned caesarean section in settings with a low national perinatal mortality rate (RR 0.07, 95% CI 0.02 to 0.29, one study, 1025 women, evidence graded moderate quality), but not in settings with a high national perinatal mortality rate (RR 0.66, 95% CI 0.35 to 1.24, one study, 1053 women, evidence graded low quality). The difference between subgroups was significant (Test for subgroup differences: Chi² = 8.01, df = 1 (P = 0.005), I² = 87.5%). Due to this significant heterogeneity, a random‐effects analysis was performed. The average overall effect was not statistically significant (RR 0.23, 95% CI 0.02 to 2.44, one study, 2078 infants). Perinatal or neonatal death (excluding fatal anomalies) was reduced with planned caesarean section (RR 0.29, 95% CI 0.10 to 0.86, three studies, 2388 women). The proportional reductions were similar for countries with low and high national perinatal mortality rates.
The numbers studied were too small to satisfactorily address reductions in birth trauma and brachial plexus injury with planned caesarean section. Neither of these outcomes reached statistical significance (birth trauma: RR 0.42, 95% CI 0.16 to 1.10, one study, 2062 infants (20 events),evidence graded low quality; brachial plexus injury: RR 0.35, 95% CI 0.08 to 1.47, three studies, 2375 infants (nine events)).
Planned caesarean section was associated with modestly increased short‐term maternal morbidity (RR 1.29, 95% CI 1.03 to 1.61, three studies, 2396 women,low quality evidence). At three months after delivery, women allocated to the planned caesarean section group reported less urinary incontinence (RR 0.62, 95% CI 0.41 to 0.93, one study, 1595 women); no difference in 'any pain' (RR 1.09, 95% CI 0.93 to 1.29, one study, 1593 women,low quality evidence); more abdominal pain (RR 1.89, 95% CI 1.29 to 2.79, one study, 1593 women); and less perineal pain (RR 0.32, 95% CI 0.18 to 0.58, one study, 1593 women).
At two years, there were no differences in the combined outcome 'death or neurodevelopmental delay' (RR 1.09, 95% CI 0.52 to 2.30, one study, 920 children,evidence graded low quality); more infants who had been allocated to planned caesarean delivery had medical problems at two years (RR 1.41, 95% CI 1.05 to 1.89, one study, 843 children). Maternal outcomes at two years were also similar. In countries with low perinatal mortality rates, the protocol of planned caesarean section was associated with lower healthcare costs, expressed in 2002 Canadian dollars (mean difference ‐$877.00, 95% CI ‐894.89 to ‐859.11, one study, 1027 women).
All of the trials included in this review had design limitations, and the GRADE level of evidence was mostly low. No studies attempted to blind the intervention, and the process of random allocation was suboptimal in two studies. Two of the three trials had serious design limitations, however these studies contributed to fewer outcomes than the large multi‐centre trial with lower risk of bias.
Authors' conclusions
Planned caesarean section compared with planned vaginal birth reduced perinatal or neonatal death as well as the composite outcome death or serious neonatal morbidity, at the expense of somewhat increased maternal morbidity. In a subset with 2‐year follow up, infant medical problems were increased following planned caesarean section and no difference in long‐term neurodevelopmental delay or the outcome "death or neurodevelopmental delay" was found, though the numbers were too small to exclude the possibility of an important difference in either direction.
The benefits need to be weighed against factors such as the mother's preference for vaginal birth and risks such as future pregnancy complications in the woman's specific healthcare setting. The option of external cephalic version is dealt with in separate reviews. The data from this review cannot be generalised to settings where caesarean section is not readily available, or to methods of breech delivery that differ materially from the clinical delivery protocols used in the trials reviewed. The review will help to inform individualised decision‐making regarding breech delivery. Research on strategies to improve the safety of breech delivery and to further investigate the possible association of caesarean section with infant medical problems is needed.
Keywords: Female, Humans, Pregnancy, Breech Presentation, Breech Presentation/mortality, Cesarean Section, Cesarean Section/adverse effects, Cesarean Section/statistics & numerical data, Elective Surgical Procedures, Elective Surgical Procedures/adverse effects, Elective Surgical Procedures/statistics & numerical data, Birth Injuries, Birth Injuries/prevention & control, Brachial Plexus Neuropathies, Brachial Plexus Neuropathies/prevention & control, Odds Ratio, Randomized Controlled Trials as Topic
Plain language summary
Planned caesarean section for term breech delivery
What is the issue?
Babies are usually born head first. If the baby is in another position the birth may be complicated. In a ‘breech presentation’ the unborn baby is bottom‐down instead of head‐down. Babies born bottom‐first are more likely to be harmed during a normal (vaginal) birth than those born head‐first. For instance, the baby might not get enough oxygen during the birth. Having a planned caesarean may reduce these problems. We looked at evidence comparing planned caesarean sections and vaginal births at the normal time of birth.
Why is this important?
Although having a caesarean might reduce some risks to babies who are lying bottom‐first, the operation itself has other risks for the mother and the baby.
What evidence did we find?
We found 3 studies involving 2396 women. (We included studies up to March 2015.) The quality of the studies and therefore the strength of the evidence was mainly low. In the short term, births with a planned caesarean were safer for babies than vaginal births. Fewer babies died or were seriously hurt when they were born by caesarean. However, children who were born by caesarean had more health problems at age two, though the numbers were too small to be certain. Caesareans caused some short‐term problems for mothers such as more abdominal pain. They also had some benefits, such as less urinary incontinence and less perineal pain in one study. The studies did not look at effects on future pregnancies, when having had a previous caesarean may cause complications. The studies only looked at single births (not twins or triplets) and did not study premature babies.
What does this mean? If your baby is in the breech position, it may be safer to have a planned caesarean section. However, caesareans may not be so good for the mother and may make future births less safe. We also do not yet know the effects of caesarean birth on babies’ health when they are older.
Summary of findings
Summary of findings for the main comparison. Planned caesarean section for term breech delivery.
| Planned caesarean section for term breech delivery | ||||||
| Patient or population: women with term breech delivery Settings: 3 studies (2 in the USA, 1 international multicentre trial: 121 centres in 26 countries) Intervention: planned caesarean section | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Planned caesarean section | |||||
| Perinatal/neonatal death or severe neonatal morbidity ‐ Low national perinatal mortality rate | Study population | RR 0.07 (0.02 to 0.29) | 1025 (1 study) | ⊕⊕⊕⊝ moderate1 | ||
| 57 per 1000 | 4 per 1000 (1 to 16) | |||||
| Perinatal/neonatal death or severe neonatal morbidity ‐ High national perinatal mortality rate | Study population | RR 0.66 (0.35 to 1.24) | 1053 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 44 per 1000 | 29 per 1000 (15 to 54) | |||||
| Birth trauma, as defined by trial authors | Study population | RR 0.42 (0.16 to 1.1) | 2062 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 14 per 1000 | 6 per 1000 (2 to 15) | |||||
| Death or neurodevelopmental delay at age 2 years | Study population | RR 1.09 (0.52 to 2.3) | 920 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 28 per 1000 | 31 per 1000 (15 to 65) | |||||
| Caesarean section | Study population | RR 2.04 (1.91 to 2.17) | 2396 (3 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 448 per 1000 | 843 per 1000 (717 to 986) | |||||
| Moderate | ||||||
| 522 per 1000 |
981 per 1000 (835 to 1000) |
|||||
| Short‐term maternal morbidity | Study population | RR 1.29 (1.03 to 1.61) | 2396 (3 studies) | ⊕⊕⊝⊝ low5 | ||
| 86 per 1000 | 111 per 1000 (89 to 139) | |||||
| Moderate | ||||||
| 391 per 1000 | 504 per 1000 (403 to 630) | |||||
| Any pain after at 3 months | Study population | RR 1.09 (0.93 to 1.29) | 1593 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 250 per 1000 | 272 per 1000 (232 to 322) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 One study with design limitations. 2 Wide confidence interval crossing the line of no effect. 3 Statistical Heterogeneity (I2 > 40%). Direction of effect consistent but size of effect variable. 4 Studies contributing data had design limitations. 5 Studies contributing data had design limitations, with more than 40% of weight from studies with serious design limitations (‐2).
Background
Description of the condition
Breech presentation occurs in 3% to 5% of all pregnancies at term, accounting for the greatest proportion of non‐cephalic presentations. Sixty‐five to seventy per cent of breech babies are in the frank breech position, in which the baby's legs are flexed at the hip and extended at the knees (with feet near the ears). Non‐frank breech position include complete breech (both the baby's hips and knees are flexed) and footling (presenting one or both feet first, which is more common with premature fetuses than at term). Some babies will spontaneously turn to a cephalic position before birth, and others can be rotated using external cephalic version. However, for those persisting in the breech position, a decision will need to be taken to deliver the baby vaginally or by caesarean section.
Factors which have been associated with breech presentation include: nulliparity; previous breech birth; uterine anomaly; contracted pelvis; use of anticonvulsant drugs; placenta praevia; cornual placenta; decreased or increased amniotic fluid volume; extended fetal legs; multiple pregnancy; prematurity; short umbilical cord; decreased fetal activity; impaired fetal growth; fetal anomaly; and fetal death.
Breech babies tend to be at higher risk of adverse outcomes, with increased neonatal morbidity and mortality (Conde‐Agudelo 2000), although it is unclear whether this is due to pre‐existing vulnerabilities (perhaps also the factors that caused the initial breech presentation), or the effects of delivery in this position. The interpretation of observational studies that compare outcomes after vaginal breech birth and cephalic birth is confounded by the fact that breech presentation per se appears to be a marker for poor perinatal outcome. For example, the incidence of childhood handicap among singleton breech babies, born at term, has been found to be high (19.4%) and similar for those delivered following trial of labour and those following an elective caesarean section (Danielian 1996). Thus, poor outcomes following vaginal breech birth may be the result of underlying conditions causing breech presentation rather than damage during delivery. However, the care during labour, the delivery methods used, and skill of the birth attendant may also influence outcome.
Description of the intervention
There is concern that vaginal delivery for babies in the breech position increases the risks of compression of the umbilical cord causing oxygen deprivation and distress, cord prolapse, head entrapment, rapid decompression of the head, spinal cord injuries, and other birth trauma. Delivering a breech baby by caesarean section avoids these potential complications and may result in fewer poor outcomes for infants. However, it carries risks for the mother during delivery, in the postoperative recovery, and in future pregnancies, e.g. repeat caesarean section, risk of ruptured scars, placental invasion of the uterus and hysterectomy (Lawson 2012). The routine use of caesarean section for breech presentation became widespread prior to evidence from randomised trials that the benefits of such a policy outweighed the risks. As caesarean section has increased for breech delivery, the skills for vaginal breech delivery have become scarcer, and more birth attendants have become inexperienced at vaginal breech delivery.
In a review of two randomised trials and seven cohort studies, the risk difference between trial of labour and planned caesarean section for any perinatal injury or death was 1.1% (Gifford 1995), findings similar to a previous review (Cheng 1993). An observational prospective study with an intent‐to‐treat analysis was conducted in France and Belgium in 174 maternity units where vaginal breech birth is commonly practised (Goffinet 2006). The study included 8105 pregnant women delivering singleton term breech babies. Multivariate analysis was used to control for confounding variables. The composite outcome fetal and neonatal mortality and severe neonatal morbidity was low in both groups. In the planned vaginal delivery group it was 1.60%; (95% confidence interval (CI) 1.14 to 2.17). This was not significantly different from that in the planned caesarean delivery group (unadjusted odds ratio = 1.10, 95% CI 0.75 to 1.6; adjusted odds ratio = 1.40, 95% CI 0.89 to 2.23). The authors concluded that "where planned vaginal delivery is a common practice and when strict criteria are met before and during labour, planned vaginal delivery of singleton fetuses in breech presentation at term remains a safe option". A large observational study in Canada found increased perinatal mortality and morbidity following vaginal birth or caesarean section during labour than following carsarean section without labour (Lyons 2015). However, cohort studies are fundamentally flawed by the fact that factors which influence the choice of method of delivery may have more to do with the outcome for the baby than the method of delivery.
Why it is important to do this review
Information from randomised trials is required to determine whether benefits (if any) of routine caesarean section for the infant are sufficient to justify subjecting mothers to the increased current and future risks of caesarean section. Attention should be paid to the selection criteria for allowing a trial of labour and the skill and experience of the clinician at delivery.
Objectives
To assess, from the best available evidence, the effects of a policy of routine versus selective caesarean delivery for term singleton breech presentation on perinatal or neonatal death (excluding fatal anomalies) or serious neonatal morbidity, perinatal, neonatal, or infant death (excluding fatal anomalies) or disability in childhood, and maternal death or maternal morbidity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials, quasi‐randomised or cluster‐randomised trials comparing planned caesarean section for singleton breech presentation at term with planned vaginal birth, subject to a management protocol.
Types of participants
Women with breech presentation considered suitable for vaginal delivery. Subgroup analysis was performed for countries with low (20 or less per 1000) and high (more than 20 per 1000) national perinatal mortality rates, as defined in the Term Breech Trial (Hannah 2000). This analysis was not specified in the original review protocol.
Types of interventions
Planned caesarean section compared with planned vaginal birth subject to the requirements of the clinical trial protocol.
Types of outcome measures
The list of outcome measures was developed in 2000 as a generic list for reviews of planned caesarean section for various indications. The list was revised in 2003 and 2004 to include additional measures of neonatal and maternal morbidity (marked * and ** respectively).
Primary outcomes
Perinatal or neonatal death (excluding fatal anomalies) or serious neonatal morbidity (e.g. seizures, birth asphyxia as defined by trial authors, neonatal encephalopathy, birth trauma);
perinatal, neonatal or infant death (excluding fatal anomalies) or disability in childhood;
maternal death or serious maternal morbidity (e.g. admission to intensive care unit, septicaemia, organ failure).
Secondary outcomes
Short‐term perinatal/neonatal outcomes
Perinatal/neonatal death (excluding fatal anomalies);
serious neonatal morbidity (e.g. seizures, birth asphyxia as defined by trial authors, neonatal encephalopathy, birth trauma);
Apgar score less than seven at five minutes;
*Apgar score less than four at five minutes;
cord blood pH less then 7.2;
*cord blood pH less than 7.0;
*base deficit at least 15;
neonatal intensive care unit admission;
neonatal encephalopathy, as defined by trial authors;
*birth trauma, as defined by trial authors;
brachial plexus injury.
Long‐term infant outcomes (at two years)
Death (excluding fatal anomalies);
disability in childhood, as defined by trial authors;
**medical problems.
Short‐term maternal outcomes
Caesarean section;
regional analgesia;
general anaesthesia;
instrumental vaginal delivery;
death;
serious maternal morbidity (e.g. intensive care unit admission, septicaemia, organ failure);
postpartum haemorrhage (as defined by the trial authors);
postpartum anaemia, as defined by trial authors;
blood transfusion;
wound infection;
woman not satisfied with care.
Longer‐term maternal outcomes (at three months)
Breastfeeding failure, as defined by trial authors;
perineal pain;
abdominal pain;
backache or back pain;
any pain;
dyspareunia, as defined by trial authors;
uterovaginal prolapse;
urinary incontinence;
flatus incontinence;
faecal incontinence;
postnatal depression, as defined by trial authors;
postnatal self‐esteem, as defined by trial authors;
postnatal anxiety, as defined by trial authors;
relationship with baby, as defined by trial authors;
relationship with partner, as defined by trial authors.
Long‐term maternal outcomes (at two years)
Breastfeeding failure, as defined by trial authors;
perineal pain;
abdominal pain;
backache or back pain;
any pain;
dyspareunia, as defined by trial authors;
uterovaginal prolapse;
urinary incontinence;
flatus incontinence;
faecal incontinence;
infertility;
subsequent pregnancy;
miscarriage or termination of a subsequent pregnancy;
caesarean section in a subsequent pregnancy;
uterine rupture in a subsequent pregnancy;
dysmenorrhoea;
menorrhagia;
postnatal depression, as defined by trial authors
postnatal self‐esteem, as defined by trial authors;
postnatal anxiety, as defined by trial authors;
relationship with child, as defined by trial authors;
relationship with partner, as defined by trial authors.
Health services
Caregiver not satisfied;
cost.
Outcomes were included if clinically meaningful; reasonable measures had been taken to minimise observer bias; missing data were insufficient to materially influence conclusions; data were available for analysis according to original allocation, irrespective of protocol violations; data were available in a format suitable for analysis.
Only outcomes for which data were available have been included in the analysis tables.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 March 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review,seeHofmeyr 2003 .
No new trials were identified by the updated search. However, the following methods were used to update the 'Risk of bias' assessment for the trials included in previous versions of the review.
Selection of studies
Two review authors (GJ Hofmeyr (GJH)) and (T Lawrie (TL)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion but would have involved another author (M Hannah (MH)) if necessary.
Data extraction and management
We designed a form to extract data. For eligible studies, GJH and TL extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, would have consulted MH. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. Information provided was clear and it was not necessary to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
GJH and TL independently assessed the risk of bias for eligible studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have resolved any disagreement by discussion or by involving MH. Additional 'Risk of bias' assessment for blinding of outcome assessment was carried out by GJH for the updated review.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessing the quality of the evidence using GRADE
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes.
Perinatal/neonatal death or severe neonatal morbidity.
Birth trauma as defined by trial authors.
Death or neurodevelopmental delay at two years.
Caesarean section.
Short‐term maternal morbidity.
Any pain after three months.
GRADE profiler (Grade 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. In future updates, if appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
There were no cluster‐randomised trials for consideration in the review update. However, we would include cluster‐randomised trials in the analyses along with individually‐randomised trials as per the methods described in the Handbook [Section 16.3.4 and 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011). If we used ICCs from other sources, we would report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We would consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We would also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
Had there been 10 or more studies in the meta‐analysis, we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where random‐effects analyses have been used, the results are presented as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analysis.
Participants from countries with low perinatal mortality rates (20/1000 or less).
Participants from countries with high perinatal mortality rates (more than 20/1000).
We restricted subgroup analysis to the review's primary outcomes.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We were unable to perform sensitivity analyses. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
Our search identified 29 reports corresponding to five studies (some of the included studies were described in multiple publications). Three studies were assessed as being eligible for inclusion in the review (Collea 1980; Gimovsky 1983; Hannah 2000) and two studies were excluded (Confino 1985; Stiglbauer 1989).
Included studies
Three trials contributed data to the review. The trials included a total of 2396 women whose babies were in breech presentation. Two trials were conducted in the USA (Collea 1980; Gimovsky 1983), and one was a large international multi‐centre trial conducted in 121 centres in 26 countries (Hannah 2000).
Interventions
In all included studies, women were randomly allocated to a policy of elective caesarean section or a protocol allowing vaginal delivery within prescribed limitations (e.g. where there was no evidence of pelvic disproportion). The choice of analgesia and anaesthesia was left for the woman and her care providers in all trials. Assisted breech delivery was performed in Gimovsky 1983, with a midline episiotomy or episioproctotomy, and elective use of Piper forceps. In Hannah 2000, the protocol specified that there should be no intervention until there was spontaneous exit of the infant to the umbilicus and minimum intervention thereafter with no traction on the body, and controlled delivery of the aftercoming head usually either with the use of forceps or the Mauriceau‐Emellie‐Veit manoeuvre (chosen by the clinician). The vaginal breech delivery methods used were not described in Collea 1980. Vaginal breech deliveries were carried out by a resident physician (Gimovsky 1983), the senior obstetric resident or an assistant resident under supervision (Collea 1980), and a clinician who the head of department had confirmed as experienced in vaginal breech delivery (Hannah 2000).
Intravenous oxytocin was permitted in all three trials when augmentation of labour was deemed necessary. However, the definition of adequate labour progress varied between trials. In Hannah 2000, adequate progress in the first stage was "a rate of cervical dilation of at least 0.5 cm per hour after the onset of active labour", and in the second stage was "descent of the breech to the pelvic floor within 2 hours of full dilatation, with delivery being imminent within 1 hour of beginning active pushing". Collea 1980 and Gimovsky 1983 viewed minimal acceptable active phase progress as 1.2 cm/hour for nulliparous women and 1.5 cm/hour for multiparous women. Oxytocin augmentation was indicated in Collea 1980 if the active‐phase dilatation was protracted according to these criteria or there was a prolonged latent phase of more than 10 hours in primigravidas and more than six hours in multigravidas. The difference in these definitions means that delay would be identified at very different points in labour, introducing different thresholds for interventions such as augmentation with oxytocin, or delivery by caesarean section.
Participants
All studies included women with a singleton fetus in breech presentation. In two studies from the same unit, women with frank (Collea 1980) or non‐frank (Gimovsky 1983) breech presentation were included, and Hannah 2000 included babies in both the frank or complete breech presentation. In all three studies, women were delivered in hospital. Women were over 37 weeks gestational age (Hannah 2000) and over 36 weeks (Collea 1980; Gimovsky 1983). In all three studies, women could be randomised while in labour, although Collea 1980 and Gimovsky 1983 specified that cervical dilatation must be ≤ 7 cm for women to be eligible. Estimated fetal weight for randomisation needed to be 2500 g to 3800 g (Collea 1980), 2000 g to 4000 g (Gimovsky 1983) or less than 4000 g (Hannah 2000), with no evidence of fetopelvic disproportion, assessed by x‐ray pelvimetry (Collea 1980; Gimovsky 1983) or ultrasound (Hannah 2000). Women were excluded from participation in all studies if there was a contraindication to either labour or vaginal delivery or obstetric indication for caesarean section, hyperextension of the fetal head, or fetal anomalies.
Excluded studies
We excluded two studies because they were not randomised trials. Confino 1985 and Stiglbauer 1989 compared the outcomes for clinics with different protocols for the management of breech birth, but participants were not randomly allocated to the intervention they received.
Risk of bias in included studies
See table of Characteristics of included studies, Figure 1 and Figure 2.
1.
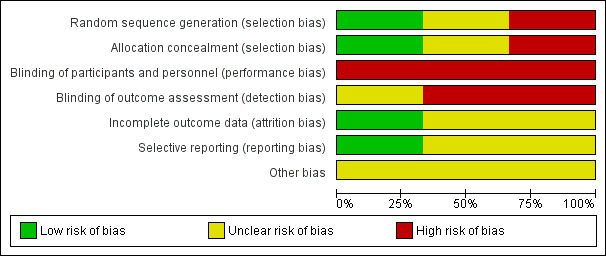
'Risk of bias. graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

.Risk of bias. summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of randomisation was not specified by Collea 1980 and Gimovsky 1983, other than that women were allocated 'by random selection'. In Collea 1980, a large discrepancy in numbers between groups (93 versus 115 total, and 37 versus 57 multiparous women) is not accounted for. Hannah 2000 used a central computerised randomisation system, accessed by telephone, and stratified by parity (0 and ≥ 1).
Blinding
Blinding of women and clinicians was not possible due to the nature of the intervention. No attempts at partial blinding were described by Collea 1980 and Gimovsky 1983. Outcome assessment was blinded for a few outcomes in Hannah 2000 (i.e. diagnosis of severe morbidity was made by the steering committee, masked to the group allocation (Hannah 2000 p1377) and diagnosis of neonatal outcomes, such as lethal congenital abnormality and Down's syndrome, were also masked to group allocation (Whyte 2004 p865)).
Incomplete outcome data
Some of the reported analyses were by actual method of delivery rather than intention‐to‐treat (Collea 1980; Gimovsky 1983), however the data presentation allows analysis according to primary allocation as presented in this review. A large discrepancy in numbers between groups (93 versus 115 total, and 37 versus 57 multiparous women) is not accounted for in Collea 1980. In Gimovsky 1983, three women appear to have been excluded shortly after randomisation and not included in the analyses: two progressed so rapidly to emergency caesarean section that x‐rays could not be obtained, and the third had inadequate pelvic dimensions so elected for caesarean section.
In Hannah 2000, outcome data were collected at three months and two years after birth from women who had delivered at centres able to ensure greater than 80% follow‐up.
Selective reporting
There was insufficient evidence to assess whether all prespecified outcomes were reported in Collea 1980 and Gimovsky 1983. Hannah 2000 reported all prespecified outcomes.
Other potential sources of bias
Several publications have commented on the limitations of the Term Breech Trial (Hannah 2000). Lawson 2012 highlighted limitations and protocol violations in the trial that may have biased the results towards favouring caesarean section. Some selection criteria in the trial protocol were violated, including the recruitment of babies who may already have been dead, twin pregnancies, not having an experienced clinician at vaginal breech deliveries, and including babies with footling or "uncertain" breech presentation. Lawson 2012 reports that the protocol had been compromised in 58 out of 646 women who had vaginal deliveries. The data monitoring committee stopped Hannah 2000 before the sample size of 2800 was reached because pre‐defined criteria of benefit to the caesarean section group were met. However, deaths unrelated to mode of delivery may have contributed to the early trial cessation. The participating countries were classified as having low (20 per 1000 or less) or high (greater than 20 per 1000) national perinatal mortality rates. The different definitions of adequate labour progress might have affected the results of the studies (see Included studies).
Effects of interventions
See: Table 1
Three trials with 2396 participants were included in the review. No new studies were identified for this update, however the background, methods, 'Risk of bias' assessments, and conclusions have been updated, and a GRADE 'Summary of findings' assessment has been added (Table 1).
Primary outcomes
Perinatal or neonatal death (excluding fatal anomalies) or severe neonatal morbidity was reduced with a policy of planned caesarean section in settings with a low national perinatal mortality rate (risk ratio (RR) 0.07, 95% confidence interval (CI) 0.02 to 0.29, one study, 1025 women, evidence graded moderate quality, Analysis 1.1), but not in settings with a high national perinatal mortality rate (RR 0.66, 95% CI 0.35 to 1.24, one study, 1053 women, evidence graded low quality). The difference between subgroups was significant (Test for subgroup differences: Chi² = 8.01, df = 1 (P = 0.005), I² = 87.5%, Analysis 1.1). Due to this significant heterogeneity, a random‐effects analysis was performed. The average overall effect was not statistically significant (RR 0.23, 95% CI 0.02 to 2.44, one study, 2078 infants, Analysis 1.1) (seeDiscussion).
1.1. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 1 Perinatal/neonatal death or severe neonatal morbidity.
A two‐year follow‐up was conducted at the Term Breech Trial centres which felt they would be able to achieve follow‐up rates of about 80%. The primary outcome death or neurodevelopmental delay at age two years was similar between the two groups (RR 1.09, 95% CI 0.52 to 2.30, one study, 920 children, evidence graded low quality,Analysis 1.2).
1.2. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 2 Death or neurodevelopmental delay at age 2 years.
Secondary outcomes
Short‐term perinatal/neonatal outcomes
Perinatal or neonatal death (excluding fatal anomalies) was reduced overall (RR 0.29, 95% CI 0.10 to 0.86, three studies, 2388 infants, Analysis 1.3) with a policy of planned caesarean section. The reduction in risk was similar for countries with low and high national perinatal mortality rates, although the numbers in these subgroups were too small for valid statistical evaluation. There were also significant reductions in neonatal morbidity overall and in specific measures of neonatal morbidity. Five‐minute Apgar scores below four were reduced in one study (RR 0.11, 95% CI 0.01 to 0.87, 2062 infants, Analysis 1.5). Apgar scores below seven were reduced significantly with planned caesarean section in the large trial of Hannah 2000, but not overall (random‐effects, average RR 0.43, 95%CI 0.12, to 1,47, three studies, 2375 infants, Heterogeneity: Chi² = 3.89, df = 2 (P = 0.14); I² = 49%, Analysis 1.4). Cord blood pH less than 7.0 was reduced with planned caesarean section (RR 0.15, 95% CI 0.03 to 0.67, one study, 1013 infants, Analysis 1.6) and cord blood base excess at least 15 (RR 0.30, 95% CI 0.10 to 0.92, one study, 899 infants, Analysis 1.7). The numbers studied were too small to satisfactorily address reductions in birth trauma and brachial plexus injury with planned caesarean section. Both outcomes did not reach statistical significance (birth trauma: RR 0.42, 95% CI 0.16 to 1.10, one study, 2062 infants (20 events), evidence graded low quality, Analysis 1.8; brachial plexus injury: RR 0.35, 95% CI 0.08 to 1.47, three studies, 2375 infants (nine events), Analysis 1.9).
1.3. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 3 Perinatal/neonatal mortality (excluding fatal malformations).
1.5. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 5 5 minute Apgar < 4.
1.4. Analysis.
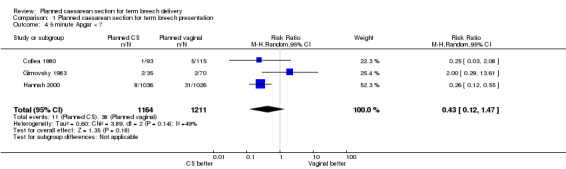
Comparison 1 Planned caesarean section for term breech presentation, Outcome 4 5 minute Apgar < 7.
1.6. Analysis.
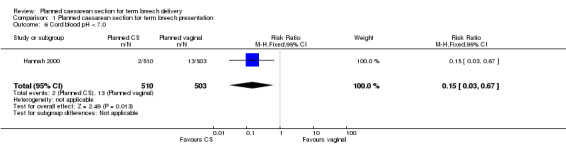
Comparison 1 Planned caesarean section for term breech presentation, Outcome 6 Cord blood pH < 7.0.
1.7. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 7 Cord blood base deficit =/> 15.
1.8. Analysis.
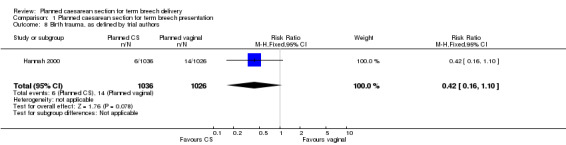
Comparison 1 Planned caesarean section for term breech presentation, Outcome 8 Birth trauma, as defined by trial authors.
1.9. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 9 Brachial plexus injury.
Long‐term infant outcomes (two years)
More infants who had been allocated to planned caesarean delivery had medical problems at two years (RR 1.41, 95% CI 1.05 to 1.89, one study, 843 children, Analysis 1.10). There were no statistically significant differences in neurodevelopmental delay at two years (RR 1.74, 95% CI 0.69 to 4.37, one study, 920 children, Analysis 1.11).
1.10. Analysis.
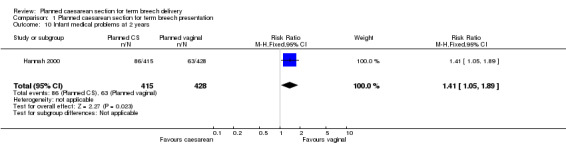
Comparison 1 Planned caesarean section for term breech presentation, Outcome 10 Infant medical problems at 2 years.
1.11. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 11 Neurodevelopmental delay at age 2 years.
Short‐term maternal outcomes
Caesarean delivery occurred in 1060/1169 (91%) of those women allocated to planned caesarean section, and 550/1227 (45%) of those allocated to a vaginal delivery protocol, although the high heterogeneity shows that the proportion of women receiving the randomly assigned treatment varied significantly between studies (random‐effects, average RR 1.88, 95% CI 1.60 to 2.20, three studies, 2396 women, evidence graded low quality, Heterogeneity: Chi² = 6.11, df = 2 (P = 0.05); I² = 67%, Analysis 1.12). Planned caesarean section compared with planned vaginal birth was associated with a small increase in short‐term maternal morbidity, which was consistent between trials, and overall statistically significant (RR 1.29, 95% CI 1.03 to 1.61, three studies, 2396 women, evidence graded low quality, Analysis 1.13). There was no statistically significant difference in the levels of women's satisfaction (not satisfied: RR 1.00, 95% CI 0.64 to 1.56, one study, 1596 women, Analysis 1.14).
1.12. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 12 Caesarean section.
1.13. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 13 Short‐term maternal morbidity.
1.14. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 14 Woman not satisfied.
Longer‐term maternal outcomes (three months)
Follow‐up for women at centres participating in the three‐month follow‐up of the Term Breech Trial (Hannah 2000) was greater than 82%. At three months after delivery, women allocated to the planned caesarean section group reported less urinary incontinence (RR 0.62, 95% CI 0.41 to 0.93, one study, 1595 women, Analysis 1.22); more abdominal pain (RR 1.89, 95% CI 1.29 to 2.79, one study, 1593 women, Analysis 1.18); and less perineal pain (RR 0.32, 95% CI 0.18 to 0.58, one study, 1593 women, Analysis 1.17). There was no statistically significant difference in any pain at three months (RR 1.09, 95% CI 0.93 to 1.29, one study, 1593 women, evidence graded low quality,Analysis 1.20), There were also no statistically significant differences in other outcomes at three months (postnatal depression (RR 0.93, 95% C 0.70 to 1.24, one study, 1586 women, Analysis 1.15), not breastfeeding (RR 1.04, 95% CI 0.90 to 1.21, one study, 1557 women, Analysis 1.16), backache (RR 0.93, 95% CI 0.71 to 1.22, one study, 1593 women, Analysis 1.19), dyspareunia (RR 0.91, 95% CI 0.72 to 1.14, one study, 1329 women, Analysis 1.21), flatus incontinence (RR 1.10, 95% CI 0.79 to 1.53, one study, 1222 women, Analysis 1.23), and faecal incontinence (RR 0.54, 95% RR 0.18 to 1.62, one study, 1226 women, Analysis 1.24)).
1.22. Analysis.
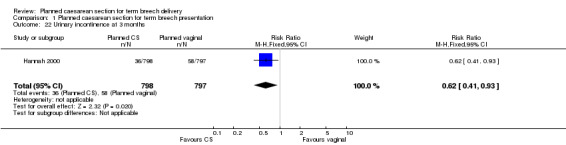
Comparison 1 Planned caesarean section for term breech presentation, Outcome 22 Urinary incontinence at 3 months.
1.18. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 18 Abdominal pain at 3 months.
1.17. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 17 Perineal pain at 3 months.
1.20. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 20 Any pain after at 3 months.
1.15. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 15 Postnatal depression at 3 months, as defined by trial authors.
1.16. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 16 Not breastfeeding at 3 months.
1.19. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 19 Backache after at 3 months.
1.21. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 21 Dyspareunia at 3 months.
1.23. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 23 Flatus incontinence at 3 months.
1.24. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 24 Faecal incontinence at 3 months.
Long‐term maternal outcomes (two years)
The two‐year follow‐up of women enrolled in the Term Breech Trial measured a wide range of outcomes relating to the women's health and wellbeing. The study was underpowered to detect modest differences in most of these outcomes. There was an increase in constipation in the planned caesarean section group (RR 1.34, 95% CI 1.06 to 1.70, one study, 917 women, Analysis 1.33). No differences were detected in the following outcomes: incontinence of urine (RR 0.82, 95% CI 0.63 to 1.06, one study, 917 women, Analysis 1.30), flatus incontinence (RR 1.14, 95% CI 0.81 to 1.61, one study, 917 women, Analysis 1.31) or faecal incontinence (RR 1.11, 95% CI 0.47 to 2.58, one study, 917 women, Analysis 1.32), haemorrhoids (RR 1.10, 95% CI 0.85 to 1.43, one study, 917 women, Analysis 1.34), pain (headache: RR 1.05, 95% CI 0.88 to 1.25, one study, 917 women, Analysis 1.25; perineal pain: RR 0.65, 95% CI 0.36 to 1.15, one study, 917 women, Analysis 1.26; back pain: RR 1.03, 95% CI 0.88 to 1.20, one study, 917 women, Analysis 1.27), menstruation (painful menstrual periods: RR 0.90, 95% CI 0.71 to 1.15, one study, 917 women, Analysis 1.37; heavy menstrual periods: RR 1.09, 95% CI 0.78 to 1.52, one study, 917 women, Analysis 1.38), sexual function (sexual problems: RR 0.95, 95% CI 0.62 to 1.48, one study, 917 women, Analysis 1.28; painful intercourse: RR 1.48, 95% CI 0.53 to 4.12, one study, 830 women, Analysis 1.29; unhappy with sexual relations: RR 0.87, 95% CI 0.51 to 1.50, one study, 702 women, Analysis 1.42), depression (RR 0.89, 95% CI 0.62 to 1.29, one study, 917 women, Analysis 1.39), relationship with baby (difficulty caring for child: RR 0.96, 95% CI 0.72 to 1.29, one study, 873 women, Analysis 1.40) and partner (relationship with partner unhappy: RR 1.02, 95% CI 0.63 to 1.66, one study, 856 women, Analysis 1.41), and subsequent pregnancies (subsequent birth or pregnant at 2 years: RR 0.93, 95% CI 0.71 to 1.24, one study, 917 women, Analysis 1.35; subsequent caesarean section at two years: RR 1.24, 95% CI 0.60 to 2.55, one study, 917 women, Analysis 1.36).
1.33. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 33 Constipation at 2 years.
1.30. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 30 Urinary incontinence at 2 years.
1.31. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 31 Flatus incontinence at 2 years.
1.32. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 32 Faecal incontinence at 2 years.
1.34. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 34 Haemorrhoids at 2 years.
1.25. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 25 Headache at 2 years.
1.26. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 26 Perineal pain at 2 years.
1.27. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 27 Back pain at 2 years.
1.37. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 37 Painful menstrual periods at 2 years.
1.38. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 38 Heavy menstrual periods at 2 years.
1.28. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 28 Sexual problems at 2 years.
1.29. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 29 Painful intercourse at 2 years.
1.42. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 42 Unhappy with sexual relations at 2 years.
1.39. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 39 Depression at 2 years.
1.40. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 40 Difficulty caring for child at 2 years.
1.41. Analysis.
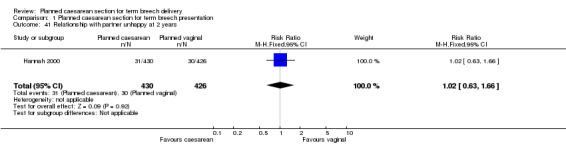
Comparison 1 Planned caesarean section for term breech presentation, Outcome 41 Relationship with partner unhappy at 2 years.
1.35. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 35 Subsequent birth or pregnant at 2 years.
1.36. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 36 Subsequent caesarean section at 2 years.
Health service outcomes
As part of the Term Breech Trial, economic aspects were evaluated for countries with low perinatal mortality rates only: the protocol of planned caesarean section was associated with lower healthcare costs (Palencia 2006; mean difference of ‐$877, in 2002 Canadian dollars, one study, 1027 women, Analysis 1.43).
1.43. Analysis.

Comparison 1 Planned caesarean section for term breech presentation, Outcome 43 Estimated cost of intervention (in Canadian dollars).
The following prespecified outcomes were not reported by the trials included in this review.
Primary outcomes
maternal death or serious maternal morbidity (e.g. admission to intensive care unit, septicaemia, organ failure).
Secondary outcomes
Short‐term perinatal/neonatal outcomes
Serious neonatal morbidity (e.g. seizures, birth asphyxia as defined by trial authors, neonatal encephalopathy, birth trauma);
cord blood pH less then 7.2;
neonatal intensive care unit admission;
neonatal encephalopathy, as defined by trial authors.
Short‐term maternal outcomes
Regional analgesia;
general anaesthesia;
instrumental vaginal delivery;
death;
serious maternal morbidity (e.g. intensive care unit admission, septicaemia, organ failure);
postpartum haemorrhage (as defined by the trial authors);
postpartum anaemia, as defined by trial authors;
blood transfusion;
wound infection.
Longer‐term maternal outcomes (at three months)
Uterovaginal prolapse;
postnatal self‐esteem, as defined by trial authors;
postnatal anxiety, as defined by trial authors;
relationship with baby, as defined by trial authors;
relationship with partner, as defined by trial authors.
Long‐term maternal outcomes (at two years)
Breastfeeding failure, as defined by trial authors;
abdominal pain;
any pain;
uterovaginal prolapse;
infertility;
miscarriage or termination of a subsequent pregnancy;
uterine rupture in a subsequent pregnancy;
postnatal self‐esteem, as defined by trial authors;
postnatal anxiety, as defined by trial authors.
Health services
Caregiver not satisfied.
Sensitivity analysis
Exclusion of the two less methodologically sound trials (Collea 1980; Gimovsky 1983) does not change the conclusions of the review, except that the excess of maternal morbidity in the planned caesarean section group is no longer statistically significant.
Discussion
Summary of main results
The three trials reviewed studied different populations of breech presentation: frank (Collea 1980), complete or footling (Gimovsky 1983), and frank or complete (Hannah 2000)). In the first two trials x‐ray pelvimetry and continuous electronic fetal monitoring in labour were used for all women; in the Term Breech Trial (Hannah 2000), these tests were used selectively. However, the estimates of effects are compatible between the trials. Due to the relative sizes of the trials, the findings of this review reflect mainly the findings of the Term Breech Trial. The interventions being compared in this review are planned caesarean section versus planned vaginal birth according to a clinical protocol. The comparison is thus not only of the intended method of delivery, but includes possible effects of shorter pregnancies and fewer labours in the planned caesarean section group. This reflects the reality of implementing either policy in practice.
Overall, planned caesarean section compared with planned vaginal birth reduced perinatal or neonatal death or serious neonatal morbidity, at the expense of somewhat increased maternal morbidity soon after birth and three months postnatally. At two years, more infants who had been allocated to planned caesarean delivery had medical problems, however there were no differences in the combined outcome 'death or neurodevelopmental delay' and maternal outcomes were also similar. Of 18 infants in the Term Breech Trial with short‐term severe morbidity, one died following surgery for subglottic stenosis thought to be congenital in origin, and the remaining 17 had no evidence of neurodevelopmental delay at age two years. There is thus no evidence of long‐term disability following the diagnosis of severe perinatal morbidity in this trial.
To determine whether the reduced mortality/neonatal morbidity in the planned caesarean section group might be specific to certain subgroups of women, the Term Breech Trial authors undertook numerous subgroup analyses. The reduction was greater in countries with low national perinatal mortality rates. The lack of similar reductions in high perinatal mortality rate countries appears anomalous. One possible explanation is that in these countries women are frequently discharged home shortly after vaginal birth. Documentation of neonatal complications following vaginal birth may have been less complete than for babies born by caesarean section, who spend a longer time under observation in hospital.
The subgroup analyses found similar reductions in risk of the main outcome (perinatal or neonatal death [excluding fatal anomalies] or serious neonatal morbidity) with planned caesarean section, compared to planned vaginal birth for all other subgroups defined by the baseline variables.
To determine whether the poorer short‐term outcome in the planned vaginal birth group might be due to differences in practice in individual cases, the Term Breech Trial authors also undertook sensitivity analyses after excluding women having a vaginal breech delivery after augmentation or induction of labour with oxytocin or prostaglandins, if:
labour was prolonged;
there was a footling breech or breech of uncertain type at delivery;
epidural analgesia was not used; and
there was no experienced clinician at the birth. Experienced clinician was defined in three different ways: according to the study protocol, as one who considered him or herself skilled and experienced in vaginal breech delivery, confirmed by the individual's head of department, as a licensed obstetrician, as a clinician with over 10 years of vaginal breech delivery experience and as a clinician with over 20 years of vaginal breech delivery experience.
The main outcome (perinatal or neonatal death (excluding fatal anomalies) or serious neonatal morbidity) remained significantly less frequent in the planned caesarean section group after excluding these cases (Hannah 2000).
Perinatal or neonatal death (excluding fatal anomalies) was also reduced overall with planned caesarean section compared to planned vaginal birth. This reduction was similar for countries with low and high national perinatal mortality rates.
Short‐term maternal morbidity was modestly increased with a policy of planned caesarean section. At three months after the birth, urinary incontinence was reduced by planned caesarean section. Although there was no difference in pain at three months after the birth in the Term Breech Trial (Hannah 2000), abdominal pain was more common following planned caesarean section while perineal pain was more common following planned vaginal birth. There were no statistically significant differences between groups for back pain, faecal or flatus incontinence, postnatal depression, maternal dissatisfaction with the experience, breastfeeding, relationship with the baby, relationship with the woman's partner, or dyspareunia. At two years, the only difference found was increased constipation in the planned caesarean section group. The added morbidity related to having a scarred uterus in subsequent pregnancies, and the ability to perform everyday activities were not assessed in these trials (seeImplications for research).
In a secondary analysis of the data from the Term Breech Trial (not according to group allocation), adverse perinatal outcome was lowest with prelabour caesarean section and increased with caesarean section in early labour, in active labour, and vaginal birth. For women having labour, adverse perinatal outcome was also associated with labour augmentation, birthweight less than 2.8 kg, longer time between pushing and delivery and no experienced clinician at delivery (Su 2003).
In another secondary multiple regression analysis of Term Breech Trial data (not according to group allocation) (Su 2007), the authors conclude that the maternal risk of caesarean section may be similar to vaginal birth if the caesarean section is undertaken before labour. In this report, maternal morbidity was more than two‐fold higher than vaginal birth in women who had a caesarean section in early labour and more than three‐fold higher in women who had a caesarean section in active labour. Intrapartum factors associated with maternal morbidity were the duration of the passive phase of the second stage of labour (P = 0.007), duration of the active phase of the second stage of labour (P = 0.002), and episiotomy (P = 0.02).
Overall completeness and applicability of evidence
As the Term Breech Trial was conducted in a wide range of clinical settings, the results of the Term Breech Trial, and thus this review, may be generalised to a similarly wide range of clinical settings. However, the results of this review cannot be generalised to settings where women labour and birth at home, or where caesarean section is not readily available, or to methods of breech delivery which differ materially from the clinical delivery protocols used in the trials reviewed. Also, as is the case with all randomised controlled trials, uncertainty remains as to whether results may be generalised to those who would not have agreed to randomisation because of strong views as to their preferred method of delivery. The results should also not be generalised to the preterm breech presentation or to twin pregnancies in which the first fetus is presenting cephalic and the second twin is presenting breech.
Quality of the evidence
All of the trials included in this review had design limitations, and the GRADE level of evidence was mostly low. No studies attempted to blind the intervention, and the process of random allocation was suboptimal in two studies. Two of the three trials had serious design limitations, however these studies contributed to fewer outcomes than the large multi‐centre trial with lower risk of bias.
Potential biases in the review process
The assessment of risk of bias involves subjective judgements. This potential limitation is minimised by following the procedures in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with review authors independently assessing studies and resolving any disagreement through discussion, and if required involving a third assessor in the decision.
Mary Hannah is principal investigator and Justus Hofmeyr a collaborator of the Term Breech Trial (Hannah 2000), which is included in this review. Mary Hannah was not involved in data extraction or 'Risk of bias' assessments.
Agreements and disagreements with other studies or reviews
The conclusions of this meta‐analysis of randomised controlled trials differ from those of the large observational prospective study of 8105 pregnant women delivering singleton term breech babies, conducted in France and Belgium in 174 maternity units where vaginal breech birth is commonly practised (Goffinet 2006). This trial found no significant difference in fetal and neonatal mortality and severe neonatal morbidity between women intending to delivery vaginally and those planning caesarean delivery, with low adverse outcomes in both groups, in a setting where vaginal breech delivery was regularly and routinely practiced.
Authors' conclusions
Implications for practice.
The reviewed trials indicate that a policy of planned caesarean section compared with planned vaginal birth according to a clinical protocol, for singleton term breech presentation, was associated with a decrease in perinatal or neonatal death and/or neonatal morbidity but no difference in the composite outcome death or neurodevelopmental delay at age two years. The latter finding was limited to a subset for whom long term follow up was possible. The numbers were small and the results do not exclude the possibility of important differences in either direction. As the long‐term outcome following perinatal morbidity appeared good, the most relevant outcome is the reduction in perinatal/neonatal death. This was 3/1166 (0.26%) in the planned caesarean section group versus 14/1222 (1.15%) in the planned vaginal birth group. At these rates (accepting that estimates based on small numbers are subject to wide variability), one death would be prevented for every 112 caesarean sections planned and one death would be prevented for every 53 additional caesarean sections performed.
For the mother, planned caesarean section was associated with a modest increase in short‐term maternal morbidity, possibly a decrease in urinary incontinence at three months but not two years, and an increase in constipation at two years after the birth. Other outcomes at two years were similar between the two groups. The effects of caesarean section on longer‐term outcomes, such as risks related to the scarred uterus, have not yet been addressed.
To reduce the problems associated with breech delivery, an active policy of external cephalic version at term may be considered (seeHofmeyr 1996; Hofmeyr 2004; Hutton 2006). Secondly, caesarean breech deliveries may be delayed to allow time for spontaneous version to take place. In the Term Breech Trial (Hannah 2000), cephalic birth occurred in 19/1041 of the planned caesarean section group, compared with 39/1042 of the planned vaginal birth group (P < 0.02).
The data from this review should be applied with due consideration to specific healthcare environments and the circumstances of individual women. A policy of planned caesarean section may not be affordable or feasible in resource‐poor settings. The long‐term risks of caesarean section may be increased for women who may not access health services in subsequent pregnancies.
Individual women should be informed of the risks of vaginal breech delivery, the present and future risks of caesarean section, and our lack of accurate knowledge in the latter field, so that as informed a choice as possible can be made in each case. A very large prospective study in France and Belgium provides reassuring evidence that a high level of safety for planned term vaginal breech birth can be achieved (Goffinet 2006).
A policy of planned caesarean section will reduce the overall incidence of cephalic birth and will not totally eliminate problems of vaginal breech birth (Hofmeyr 2001). In the group allocated to planned caesarean section in the Term Breech Trial (Hannah 2000), 100/1041 (9.6%) gave birth vaginally, most because the birth took place before caesarean section could be arranged; 22 (2.1%) experienced difficult deliveries; and six (0.6%) experienced birth trauma.
With a policy of routine caesarean section for breech presentation at term, in time, the clinical skills of vaginal breech delivery will be eroded, placing women who deliver vaginally at increased risk.
Implications for research.
Childbirth is a profound and unique human experience. Little is known about the evolutionary importance of the birth process to women's personal development, emotional wellbeing and adaptation to parenthood, and to subsequent child development, particularly for women who attach importance to giving birth normally. Future trials comparing planned caesarean section with planned vaginal birth should take care to ensure that the protocol for planned vaginal birth is designed to optimise the outcome for both mothers and infants. Further information on long‐term benefits and risks of caesarean section for the woman will be useful for clinical decision‐making. Research is needed to further investigate the finding of increased infant health problems following caesarean section in this review.
Given that by choice or by default, vaginal breech births will continue to take place, attention should be paid to techniques of vaginal delivery which might improve outcomes for the baby. For example, ready availability of symphysiotomy in the event of difficulty with delivery of the head (Hofmeyr 2010; Wykes 2003) might reduce adverse outcomes and give reassurance to women keen to give birth vaginally.
Feedback
Wayne, 21 October 2014
Summary
In this review the review authors state that "At two years, there were no differences in the combined outcome 'death or neurodevelopmental delay'." I think this means that for all participants in all included studies combined, there were no differences between the two groups in this outcome at two years after birth. However, the authors' conclusions then state: "Planned caesarean section compared with planned vaginal birth reduced perinatal or neonatal death or serious neonatal morbidity" but does not make any mention of the outcome at two years. Isn't the overall outcome at two years of age more important than the immediate perinatal or neonatal outcome? A baby's death at one day of life is no more significant than a baby's death at one year of life. Am I missing something? Does the outcome at two years after birth exclude those who died in the perinatal or neonatal period? I feel that in order to aid decision making, the longer‐term outcome is the one that should influence the conclusions of this review, not the short‐term outcome.
Comment sent by Carolyn Wayne, October 2014
Reply
Thank you for this important feedback. We placed more emphasis on the short‐term outcomes because they were available for the whole group, and included a very large reduction in perinatal death. The outcome "Death or neurodevelopmental delay" at 2 years was available for a sub‐set of less than 50% of the total sample size, and the numbers were rather small with wide confidence intervals. While this finding was reassuring in that it suggested that in the sub‐group who were followed up the perinatal morbidity did not translate to long‐term problems, it does not over‐ride the benefit of planned CS on the more serious outcome perinatal death. In response to your feedback, we have clarified the conclusions in the abstract and main text.
Contributors
G Justus Hofmeyr
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2019 | Amended | EditedJustus Hofmeyr's Declarations of interest. |
History
Protocol first published: Issue 2, 1995 Review first published: Issue 2, 1995
| Date | Event | Description |
|---|---|---|
| 30 November 2015 | Amended | An amended version of the Plain language summary has been added. |
| 30 June 2015 | Feedback has been incorporated | Authors have replied to Feedback 1. |
| 13 April 2015 | Feedback has been incorporated | Feedback 1 submitted from Carolyn Wayne. |
| 31 March 2015 | New search has been performed | Search updated, no new studies identified. Methods updated and a 'Summary of findings' table has been incorporated. |
| 31 March 2015 | New citation required but conclusions have not changed | Review updated. |
| 31 March 2011 | New search has been performed | Three new reports related to the Term Breech Trial (Hannah 2000) incorporated in the updated review; only one contributed additional data. Conclusions remain unchanged. |
| 2 July 2010 | Amended | Contact details updated. |
| 1 October 2009 | Amended | Search updated. Four reports added to Studies awaiting classification (Hodnett 2005; Palencia 2005; Palencia 2006; Su 2007). Stiglbauer 1989 now excluded. |
| 4 November 2008 | Amended | Converted to new review format. |
| 30 November 2004 | New search has been performed | The two‐year follow‐up data for the Term Breech Trial (Hannah 2000) have been included in this review. The conclusions have not changed. |
| 31 January 2003 | New search has been performed | The three‐month follow‐up data for the Term Breech Trial (Hannah 2000) have been included in this review. |
Acknowledgements
Denise Atherton for administrative assistance; Lynn Hampson for the literature search; Helen West for assistance with the 2015 revision.
This project was supported by the National Institute for Health Research, via Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Planned caesarean section for term breech presentation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal/neonatal death or severe neonatal morbidity | 1 | 2078 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.02, 2.44] |
| 1.1 Low national perinatal mortality rate | 1 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.02, 0.29] |
| 1.2 High national perinatal mortality rate | 1 | 1053 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.24] |
| 2 Death or neurodevelopmental delay at age 2 years | 1 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.30] |
| 3 Perinatal/neonatal mortality (excluding fatal malformations) | 3 | 2388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.86] |
| 3.1 Low national perinatal mortality rate | 3 | 1335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.00] |
| 3.2 High national perinatal mortality rate | 1 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.08, 1.09] |
| 4 5 minute Apgar < 7 | 3 | 2375 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.12, 1.47] |
| 5 5 minute Apgar < 4 | 1 | 2062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.87] |
| 6 Cord blood pH < 7.0 | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.67] |
| 7 Cord blood base deficit =/> 15 | 1 | 899 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.10, 0.92] |
| 8 Birth trauma, as defined by trial authors | 1 | 2062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.10] |
| 9 Brachial plexus injury | 3 | 2375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.08, 1.47] |
| 10 Infant medical problems at 2 years | 1 | 843 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.05, 1.89] |
| 11 Neurodevelopmental delay at age 2 years | 1 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.69, 4.37] |
| 12 Caesarean section | 3 | 2396 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.60, 2.20] |
| 13 Short‐term maternal morbidity | 3 | 2396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.03, 1.61] |
| 14 Woman not satisfied | 1 | 1596 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.64, 1.56] |
| 15 Postnatal depression at 3 months, as defined by trial authors | 1 | 1586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.24] |
| 16 Not breastfeeding at 3 months | 1 | 1557 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.21] |
| 17 Perineal pain at 3 months | 1 | 1593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.58] |
| 18 Abdominal pain at 3 months | 1 | 1593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.29, 2.79] |
| 19 Backache after at 3 months | 1 | 1593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.22] |
| 20 Any pain after at 3 months | 1 | 1593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.29] |
| 21 Dyspareunia at 3 months | 1 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.14] |
| 22 Urinary incontinence at 3 months | 1 | 1595 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.93] |
| 23 Flatus incontinence at 3 months | 1 | 1222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.79, 1.53] |
| 24 Faecal incontinence at 3 months | 1 | 1226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.18, 1.62] |
| 25 Headache at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.25] |
| 26 Perineal pain at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.36, 1.15] |
| 27 Back pain at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 28 Sexual problems at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.62, 1.48] |
| 29 Painful intercourse at 2 years | 1 | 830 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.53, 4.12] |
| 30 Urinary incontinence at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.63, 1.06] |
| 31 Flatus incontinence at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.81, 1.61] |
| 32 Faecal incontinence at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.58] |
| 33 Constipation at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.06, 1.70] |
| 34 Haemorrhoids at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.85, 1.43] |
| 35 Subsequent birth or pregnant at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.24] |
| 36 Subsequent caesarean section at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.60, 2.55] |
| 37 Painful menstrual periods at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.15] |
| 38 Heavy menstrual periods at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.78, 1.52] |
| 39 Depression at 2 years | 1 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.29] |
| 40 Difficulty caring for child at 2 years | 1 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.29] |
| 41 Relationship with partner unhappy at 2 years | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.63, 1.66] |
| 42 Unhappy with sexual relations at 2 years | 1 | 702 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.51, 1.50] |
| 43 Estimated cost of intervention (in Canadian dollars) | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | ‐877.0 [‐894.89, ‐859.11] |
| 43.1 Low national perinatal mortality rate | 1 | 1027 | Mean Difference (IV, Fixed, 95% CI) | ‐877.0 [‐894.89, ‐859.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Collea 1980.
| Methods | Allocation by "random selection". Method not specified. | |
| Participants | Inclusion criteria: singleton frank breech presentation; 36 weeks or more gestation; estimated fetal weight between 2500 and 3800 g; cervical dilation 7 cm or less. Exclusion criteria: hyperextension of the fetal head or evidence of fetal skeletal anomalies on abdominal x‐ray; elderly primigravidae; obstetric indication for caesarean section; class B‐F diabetes mellitus; floating station; involuntary infertility; pelvic contracture by previous x‐ray pelvimetry; history of previous difficult or traumatic delivery. 208 women randomised to vaginal delivery group (115 women) and caesarean section group (93 women). | |
| Interventions | Planned delivery by caesarean section compared with a policy of vaginal breech delivery; x‐ray pelvimetry was performed and if 1 or more pelvic inlet or mid‐cavity measurements were reduced, caesarean section performed; oxytocin induction was permitted only for premature rupture of membranes with the fetus engaged in the maternal pelvis; oxytocin augmentation of labour was used for prolonged latent phase and protracted active phase dilation; fetal heart rate and uterine contractions were monitored throughout labour. Delivery by or supervised by a senior obstetric resident. | |
| Outcomes | Actual use of caesarean section; brachial plexus injury; Apgar score < 7 at 5 minutes; short‐term neonatal morbidity; perinatal mortality; maternal morbidity. | |
| Notes | Los Angeles, California, USA. Data presented for 4 groups according to protocol selection and actual method of delivery. For this review, analysed according to protocol selection only (i.e. according to 'intention‐to‐treat'). A large discrepancy in numbers between groups (93 versus 115, and 37 versus 57 multiparous women) is not accounted for. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation by "random selection". Method not specified. Reason for large discrepancy in group sizes not given. High risk of selection bias. |
| Allocation concealment (selection bias) | High risk | Inadequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to the nature of the intervention. No attempt at partial blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete data. Difference in group sizes not explained. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence to assess whether all prespecified outcomes were reported. |
| Other bias | Unclear risk | Insuficient detail in reporting to be sure whether additional risk exists. |
Gimovsky 1983.
| Methods | 2‐arm trial. "Randomisation" in a ratio of 1 caesarean section to 2 trials of labour, to allow for exclusions from trial of labour. Method of randomisation not specified. | |
| Participants | Inclusion criteria: singleton pregnancy; non‐frank breech presentation on abdominal x‐ray; in labour; estimated gestational age 36‐42 weeks; estimated fetal weight 2000 to 4000 g; cervix < 7 cm dilated; non‐extended normal appearing fetal skull on x‐ray; no contraindication to labour. Of 105 enrolled, 35 allocated to caesarean section and 70 to trial of labour. Exclusion criteria: severe pregnancy‐induced hypertension; more than 1 prior caesarean section; previous stillbirth; history of infertility; class B diabetes mellitus; impaired intrauterine growth; abnormal antepartum fetal heart rate testing; abnormal amniotic fluid volume; multiple gestation. 105 women were randomised to trial of labour (70 women) or elective caesarean section (35 women). | |
| Interventions | Planned elective caesarean section compared with planned trial of labour: x‐ray pelvimetry performed and trial of labour allowed if measurements were at least 11 cm at anteroposterior diameter of the inlet, 12 cm at widest transverse diameter of the inlet and 10 cm between ischial spines at the midpelvis; continuous electronic fetal monitoring; oxytocin infusion on an optional basis for poor progress of labour; intravenous analgesia and assisted breech delivery with application of Piper forceps to aftercoming head. Delivery supervised by chief resident and/or obstetric staff. | |
| Outcomes | Actual use of caesarean section; brachial plexus injury; Apgar score < 7 at 5 minutes; perinatal mortality; maternal morbidity. | |
| Notes | Los Angeles. California, USA. Results reported in the study in 4 groups according to allocated and actual method of delivery. For this review analysed according to allocated method of delivery ('intention‐to‐treat') only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, stated only as "randomisation" done. High risk of selection bias. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to the nature of the intervention. No attempt at partial blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 women appear to have been excluded shortly after randomisation: 2 progressed so rapidly to emergency caesarean section that x‐rays could not be obtained, and the third had inadequate pelvic dimensions so elected caesarean section. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient evidence to assess whether all prespecified outcomes were reported. |
| Other bias | Unclear risk | Report not detailed enough to be sure of other bias |
Hannah 2000.
| Methods | Centrally controlled computerised randomisation, stratified by parity (0 or > 0) and block sizes of 2. | |
| Participants | Inclusion criteria: singleton live fetus; frank or complete breech presentation; 37 or more weeks' gestation. Exclusion criteria: fetopelvic disproportion; fetus judged to be 'large', or estimated 4000 g or more; hyperextension of fetal head; fetal anomaly or mechanical problem likely to affect delivery; contraindication to labour or vaginal delivery; known lethal fetal anomaly. 2088 women were randomly assigned to planned vaginal delivery (1045 women) or planned caesarean section (1043 women) | |
| Interventions | Planned caesarean section: if not in labour, scheduled for 38 or more weeks' gestation if known, or following maturity testing or onset of labour. If no longer breech presentation, method of delivery reviewed. Planned vaginal birth: await spontaneous labour; induction or augmentation allowed if indicated; caesarean section if indication arose, including fetal heart rate abnormality or inadequate labour progress; assisted breech delivery by an experienced clinician; total breech extraction avoided. | |
| Outcomes | Primary: perinatal or neonatal mortality up to 28 days of age (excluding lethal congenital abnormalities) or specified serious neonatal morbidity. Secondary: maternal mortality or specified serious maternal morbidity. 3‐month follow‐up: breastfeeding; infant health; ease of caring for infant; ease of adjusting to being a mother; sexual relations; relationship with partner; pain; urinary, flatus and faecal incontinence; depression; views regarding childbirth experience and participation in study, 2‐year follow‐up in selected centres: perinatal/infant death or neurodevelopmental delay at age 2 years; maternal health at 2 years; economic aspects (costs) of interventions. | |
| Notes | Multicentre trial. Countries classified as having low (20/1000 or less) or high perinatal mortality rates. Follow‐up at 3 months excluding centres unable to accomplish 80% follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally controlled computerised randomisation. |
| Allocation concealment (selection bias) | Low risk | Adequate, random allocation accessed by means of a touch‐tone telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible due to the nature of the intervention. No attempt at partial blinding described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not possible for most outcomes. Group allocation was masked for the assessment of a few outcomes (e.g. diagnosis of severe morbidity was made by the steering committee masked to the group allocation (Hannah 2000 p1377) and diagnosis of neonatal outcomes such as lethal congenital abnormality and Down syndrome were also masked to group allocation (Whyte 2004 p865)). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No unbalanced loss to follow‐up ‐ only 2 + 3 lost to follow‐up from 2088 women. |
| Selective reporting (reporting bias) | Low risk | All outcomes measured appear to have been reported |
| Other bias | Unclear risk | Baseline characteristics similar. Analysis by intention‐to‐treat. Study was stopped early because of significant differences in perinatal or neonatal mortality at less than 28 days of age (excluding lethal congenial anomalies). Some protocol violations may have biased the results towards favouring caesarean section (e.g. including the recruitment of babies who may already have been dead, twin pregnancies, not having an experienced clinician at vaginal breech deliveries, and including babies with footling or "uncertain" breech presentation). 58 out of 646 women who had vaginal deliveries violated the protocol (Lawson 2012). |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Confino 1985 | Excluded because not a randomised trial. Breech delivery outcomes were compared retrospectively for alternate‐day obstetric units. Unit 'B' used a conservative approach towards vaginal breech delivery and performed more caesarean sections (105/277, 38% versus 69/266, 26%). Unit 'A' made more use of x‐ray pelvimetry, early rupture of membranes and oxytocin augmentation of labour. There were no statistically significant differences in duration of labour, Apgar scores or neonatal morbidity. There were 2 (0.7%) neonatal deaths in unit 'B' and 7 (2.6%) in unit 'A'. |
| Stiglbauer 1989 | Not a randomised trial, but a comparison of the results of 2 clinics with differing protocols for management of breech birth. |
Differences between protocol and review
Methods updated to current Pregnancy and Childbirth Group standard text. A summary of findings table has been incorporated for the 2015 update.
The list of outcome measures was developed in 2000 as a generic list for reviews of planned caesarean section for various indications. The list was revised in 2003 and 2004 to include additional measures of neonatal and maternal morbidity (marked * and ** respectively) within "Types of outcomes".
Subgroup analysis was performed for countries with low (20 or less per 1000) and high (more than 20 per 1000) national perinatal mortality rates, as defined in the Term Breech Trial (Hannah 2000). This analysis was not specified in the original review protocol.
Contributions of authors
GJ Hofmeyr and ME Hannah prepared the first version of the review together. Justus Hofmeyr prepared the 2011 update with assistance from Tess Lawrie, and Mary Hannah approved it. Tess Lawrie assisted with study selection, data extraction, updating the 'Risk of bias' tables and editing the review. GJ Hofmeyr is responsible for maintaining the review and prepared the 2015 update.
Sources of support
Internal sources
University of the Witwatersrand, South Africa.
University of Toronto Maternal, Infant and Reproductive Health Research Unit, Canada.
(HW) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
South African Medical Research Council, South Africa.
The Nuffield Trust, UK.
HRP ‐ UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
(GJH) Rockefeller Foundation Residency, Oct 2004, USA.
(HW) National Institute for Health Research (NIHR), UKNIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Mary Hannah is principal investigator and Justus Hofmeyr a collaborator of the Term Breech Trial (Hannah 2000), which is included in this review. Tess Lawrie undertook assessment and data extraction for this trial. Justus Hofmeyr receives royalties from UpToDate for chapters related to breech pregnancy, delivery of a baby in breech presentation and external cephalic version. UpToDate is an electronic publication by Wolters Kluwer to disseminate evidence‐based medicine (such as Cochrane reviews).
Edited (no change to conclusions)
References
References to studies included in this review
Collea 1980 {published data only}
- Collea JV, Chein C, Quilligan EJ. A randomized management of term frank breech presentation: a study of 208 cases. American Journal of Obstetrics and Gynecology 1980;137:235‐44. [DOI] [PubMed] [Google Scholar]
- Collea JV, Rabin SC, Weghorst GR, Quilligan EJ. The randomized management of term frank breech presentation: vaginal delivery vs caesarean section. American Journal of Obstetrics and Gynecology 1978;131:186‐95. [DOI] [PubMed] [Google Scholar]
Gimovsky 1983 {published data only}
- Gimovsky ML, Wallace RL, Schifrin BS, Paul RH. Randomized management of the nonfrank breech presentation at term: a preliminary report. American Journal of Obstetrics and Gynecology 1983;146:34‐40. [DOI] [PubMed] [Google Scholar]
Hannah 2000 {published data only}
- Hannah M, Amankwah K, Chalmers B, Cheng M, Foster G, Guselle P, et al. Term Breech Trial (TBT): a RCT of planned caesarean section vs planned vaginal birth for breech at term. 12th EAGO Conference; 1997 June 25‐28; Dublin, Ireland. 1997.
- Hannah M, Amankwah K, Chalmers B, Cheng M, Foster G, Guselle P, et al. Term Breech Trial (TBT): a RCT of planned caesarean section vs planned vaginal birth for breech at term. Society of Obstetricians and Gynaecologists of Canada; 1997; Halifax, Nova Scotia, Canada. 1997.
- Hannah M, Hannah W for the TBT Group. Term Breech Trial (TBT): a RCT of planned caesarean section vs planned vaginal birth for breech at term. XV FIGO World Congress; 1997 August 3‐8; Copenhagen, Denmark. 1997.
- Hannah M, Hannah W for the Term Breech Trial Group. Term Breech Trial: a RCT of planned CS vs planned vaginal birth for breech at term. XVI FIGO World Congress of Obstetrics & Gynecology (Book 1); 2000 Sept 3‐8; Washington DC, USA. 2000:51.
- Hannah M, Hannah W, Hodnett E, Chalmers B, Kung R, Willan A, et al. Outcomes at three months postpartum for women enrolled in the multicentre international term breech trial of planned caesarean section and planned vaginal birth for breech presentation at term [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S114. [Google Scholar]
- Hannah M, Whyte H, Hannah W, Term Breech Trial Collaborative Group. Maternal outcome at 2 years postpartum in the Term Breech Trial. American Journal of Obstetrics and Gynecology 2003;189(6):S136. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Hannah W, Amankwah K, Cheng M, Chalmers B, Foster G, et al. Term Breech Trial (TBT): a RCT of planned caesarean section vs planned vaginal birth for breech at term. Birth Conference; 1998 June 5‐7; Boston, MA. 1998.
- Hannah ME, Hannah W, Amankwah K, Cheng M, Chalmers B, Foster G, et al. Term Breech Trial (TBT): a RCT of planned caesarean section vs planned vaginal birth for breech at term. Society of Obstetricians and Gynaecologists of Canada; 1998 June 25‐28; Victoria, BC, Canada. 1998.
- Hannah ME, Hannah WJ. Term Breech Trial (TBT): a randomised controlled trial (RCT) of planned caesarean section vs planned vaginal birth for breech at term. Society of Obstetricians and Gynaecologists of Canada; 2000 June 24‐29; Montreal, Canada. 2000.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR, Term Breech Trial Collaborative Group. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet 2000;356:1375‐83. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Hannah WJ, Hodnett ED, Chalmers B, Kung R, Willan A, et al. Outcomes at 3 months after planned cesarean vs planned vaginal delivery for breech presentation at term: the international randomized term breech trial. JAMA 2002;287(14):1822‐31. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Moutquin JM, Hannah W, the TBT Group. Term Breech Trial (TBT): a randomised controlled trial (RCT) of planned Caesarean section vs planned vaginal birth for breech at term. First Congress on Obstetrics, Gynecology & Infertility; 1999; Prague, Czech Republic. 1999.
- Hannah ME, Whyte H, Hannah WJ, Hewson S, Amankwah K, Cheng M, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191:917‐27. [DOI] [PubMed] [Google Scholar]
- Hodnett ED, Hannah ME, Hewson S, Whyte H, Amankwah K, Cheng M, et al. Mothers' views of their childbirth experiences 2 years after planned caesarean versus planned vaginal birth for breech presentation at term, in the international randomized term breech trial. Journal of Obstetrics & Gynaecology Canada 2005;27(3):224‐31. [DOI] [PubMed] [Google Scholar]
- McCleod L, Su M, Ross S, Hannah WJ, Willan A, Hutton E, et al. Predictors of maternal mortality or serious maternal morbidity in the term breech trial [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S100. [Google Scholar]
- Palencia R, Gafni A, Hannah ME, Ross S, Willan AR, Hewson S, et al. The costs of planned cesarean versus planned vaginal birth in the term breech trial. CMAJ Canadian Medical Association Journal 2006;174(8):1109‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia R, Gafni A, Ross S, Willan A, Hewson S, McKay D, et al. The costs of planned cesarean versus planned vaginal birth in the term breech trial [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S124. [Google Scholar]
- Su M, Hannah WJ, Willan A, Ross S, Hannah ME, Term Breech Trial collaborative group. Planned caesarean section decreases the risk of adverse perinatal outcome due to both labour and delivery complications in the Term Breech Trial. BJOG: an international journal of obstetrics and gynaecology 2004;111:1065‐74. [DOI] [PubMed] [Google Scholar]
- Su M, McCleod L, Ross S, Willan A, Hannah WJ, Hutton E, et al. Factors associated with adverse perinatal outcome in the term breech trial [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S69. [DOI] [PubMed] [Google Scholar]
- Su M, McLeod L, Ross S, Willan A, Hannah WJ, Hutton E, et al. Factors associated with adverse perinatal outcome in the Term Breech Trial. American Journal of Obstetrics and Gynecology 2003;189:740‐5. [DOI] [PubMed] [Google Scholar]
- Su M, McLeod L, Ross S, Willan A, Hannah WJ, Hutton EK, et al. Factors associated with maternal morbidity in the term breech trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2007;29(4):324‐30. [DOI] [PubMed] [Google Scholar]
- Walkinshaw S, Hannah M, Hannah W. Term breech trial (TBT): a randomised controlled trial of planned caesarean section against planned vaginal birth for breech presentation at term. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):65. [Google Scholar]
- Whyte H, Hannah M, Saigal S, Term Breech Trial Collaborative Group. Outcomes of children at 2 years of age in the Term Breech Trial. American Journal of Obstetrics and Gynecology 2003;189:S57. [DOI] [PubMed] [Google Scholar]
- Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191:864‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Confino 1985 {published data only}
- Confino E, Ismajovich B, Sherzer A, Peyser RM, David MP. Vaginal versus caesarean section oriented approaches in the management of breech delivery. International Journal of Gynaecology & Obstetrics 1985;23:1‐16. [DOI] [PubMed] [Google Scholar]
Stiglbauer 1989 {published data only}
- Stiglbauer M, Sevelda P, Vavra N, Weninger M, Sterniste W, Wagenbichler P. Cesarean section versus vaginal delivery of breech presentation in primiparous patients [Sectio versus vaginale Entbindung der Beckenendlage bei Primiparae]. Gynakologische Rundschau 1989;29 Suppl 2:319‐20. [PubMed] [Google Scholar]
Additional references
Cheng 1993
- Cheng M, Hannah ME. Breech delivery at term ‐ a critical review of the literature. Obstetrics & Gynecology 1993;82:605‐18. [PubMed] [Google Scholar]
Conde‐Agudelo 2000
- Conde‐Agudelo A, Belizan JM, Diaz‐Rossello JL. Epidemiology of fetal death in Latin America. Acta Obstetricia et Gynecologica Scandinavica 2000;79:371‐8. [PubMed] [Google Scholar]
Danielian 1996
- Danielian PJ, Wang J, Hall MH. Long term outcome by method of delivery of fetuses in breech presentation at term: population based follow up. BMJ 1996;312:1451‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gifford 1995
- Gifford DS, Morton SC, Kahn K. A meta‐analysis of infant outcomes after breech delivery. Obstetrics & Gynecology 1995;85:1047‐54. [DOI] [PubMed] [Google Scholar]
Goffinet 2006
- Goffinet F, Carayol M, Foidart JM, Alexander S, Uzan S, Subtil D, et al. Is planned vaginal delivery for breech presentation at term still an option? Results of an observational prospective survey in France and Belgium. American Journal of Obstetrics and Gynecology 2006;194(4):1002‐11. [DOI] [PubMed] [Google Scholar]
Grade 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodnett 2005
- Hodnett ED, Hannah ME, Hewson S, Whyte H, Amankwah K, Cheng M, et al. Mothers' views of their childbirth experiences 2 years after planned caesarean versus planned vaginal birth for breech presentation at term, in the international randomized term breech trial. Journal of Obstetrics & Gynaecology Canada 2005;27(3):224‐31. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1996
- Hofmeyr GJ, Kulier R. External cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000083] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2001
- Hofmeyr GJ. Commentary on "caesarean delivery of breech babies is beneficial for infant health but has no impact on maternal health". Evidence‐Based Healthcare 2001;5:75‐6. [Google Scholar]
Hofmeyr 2004
- Hofmeyr GJ, Gyte GML. Interventions to help external cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000184.pub2] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Shweni PM. Symphysiotomy for feto‐pelvic disproportion. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD005299.pub2] [DOI] [PubMed] [Google Scholar]
Hutton 2006
- Hutton EK, Hofmeyr GJ. External cephalic version for breech presentation before term. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000084.pub2] [DOI] [PubMed] [Google Scholar]
Lawson 2012
- Lawson GW. The Term Breech Trial ten years on: Primum non nocere?. Birth 2012;39(1):3‐9. [DOI] [PubMed] [Google Scholar]
Lyons 2015
- Lyons J, Pressey T, Bartholomew S, Liu S, Liston RM, Joseph KS, for the Canadian Perinatal Surveillance System (Public Health Agency of Canada. Delivery of Breech Presentation at Term Gestation in Canada, 2003‐2011. Obstet Gynecol. 2015;125(5):1153‐1161. [DOI] [PubMed] [Google Scholar]
Palencia 2005
- Palencia R, Gafni A, Ross S, Willan A, Hewson S, McKay D, et al. The costs of planned cesarean versus planned vaginal birth in the term breech trial. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S124. [Google Scholar]
Palencia 2006
- Palencia R, Gafni A, Hannah ME, Ross S, Willan AR, Hewson S, et al. The costs of planned cesarean versus planned vaginal birth in the term breech trial. CMAJ Canadian Medical Association Journal 2006;174(8):1109‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Su 2003
- Su M, McLeod L, Ross S, Willan A, Hannah WJ, Hutton E, et al. Factors associated with adverse perinatal outcome in the Term Breech Trial. American Journal of Obstetrics and Gynecology 2003;189:740‐5. [DOI] [PubMed] [Google Scholar]
Su 2007
- Su M, McLeod L, Ross S, Willan A, Hannah WJ, Hutton EK, et al. Factors associated with maternal morbidity in the term breech trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2007;29(4):324‐30. [DOI] [PubMed] [Google Scholar]
Whyte 2004
- Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. American Journal of Obstetrics and Gynecology 2004;191:864‐71. [DOI] [PubMed] [Google Scholar]
Wykes 2003
- Wykes CB, Johnston TA, Paterson‐Brown S, Johanson RB. Symphysiotomy: a life‐saving procedure. BJOG: an international journal of obstetrics and gynaecology 2003;110:219‐21. [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyer 2001b
- Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Hannah ME. Planned caesarean section for term breech delivery. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000166] [DOI] [PubMed] [Google Scholar]


