Abstract
The urine gamma-glutamyl transferase (GGT)-to-creatinine ratio has been used to monitor patients at risk of acute renal injury. We validated the spectrophotometric quantification of GGT in urine in a commercial biochemistry analyzer. The assay was precise, accurate, and linear. Intra-assay precision was 3.59% in 4 samples, with GGT concentrations of 47–195 U/L. Inter-assay precision in 3 samples with activities of 11–51 U/L was 7.74%. Accuracy was 97.3%, with an absolute bias of 2.7 U/L. Urine GGT was unaffected by hematuria, hemoglobinuria, or bacteriuria. Urine GGT was stable at 20°C and 4°C for up to 3 d. Storage by freezing at −20°C resulted in a significant reduction in enzyme activity. A pH outside the range of 6.5–8 resulted in reduced GGT activity. The biological variation of urine GGT-to-creatinine ratio provided an index of individuality of 1.6, indicating that a population-based reference interval (RI) can be used. The reference change value was calculated, and an increase in consecutive measurements >43% is required to be regarded as significant. The urine GGT-to-creatinine ratio RI obtained in a population of 41 healthy dogs was 8.5–28.5 U/g.
Keywords: Acute kidney injury, creatinine, dogs, gamma-glutamyl transferase, GGT:creatinine ratio, urine
Introduction
Serum urea and creatinine concentrations reflect the glomerular filtration rate, but they are poor indicators of renal tubular damage in dogs. Enzymes in urine, such as gamma-glutamyl transferase (GGT), N-acetyl-β-D-glucosaminidase, and alkaline phosphatase, have been shown to be useful markers of tubular damage.3
GGT is a peptidase enzyme found in many cell types, including the brush border of renal proximal tubular epithelial cells.3 Tubular injury from various causes can result in cell damage and release of GGT into the tubular lumen. Plasma GGT is not able to pass through the healthy glomerulus into the ultrafiltrate by virtue of its large molecular size, therefore increased concentrations of GGT in the urine reflect tubular injury.3 However, this is only true when the glomerular barrier is intact, because glomerular disease may result in serum proteins, including serum GGT, passing into the ultrafiltrate.
Changes in urine GGT concentration have been studied in both experimental and naturally occurring acute renal tubular injury in the dog,4,8,14 and may have a place in the veterinary hospital in identifying dogs with acute tubular injury, such as those undergoing treatment with nephrotoxic drugs (e.g., aminoglycoside antibiotics). A previous review of renal biomarkers did not identify published validation studies for a spectrophotometric GGT assay in canine urine,9 and little is known about the reference interval (RI) and biological variation of this analyte in the urine of healthy dogs.
We validated a spectrophotometric method for the measurement of GGT activity in canine urine, including evaluation of the effects of interfering substances and pre-analytical storage duration and conditions. GGT is found widely in nature, and versions of this enzyme are found in bacteria as well as mammalian cells. Given the frequent contamination of urine samples with bacteria, we also assessed the effect of in vitro bacterial proliferation on GGT concentration.
We evaluated the effect of urine pH on GGT enzyme activity. Urine pH has been reported to affect the catalytic activity of GGT10 and, in a study of dogs, a significant difference in urine GGT-to-urine creatinine (GGT:creatinine) ratio was seen in samples with urine pH <7 and those >7.2 By measuring urine GGT:creatinine ratio in a population of healthy dogs, we established a population RI, and calculated the index of individuality and the reference change value (RCV) to help guide clinicians in interpretation of this parameter.
Materials and methods
Samples
The canine urine samples used for the validation, interfering substances, and effect of pH study were residual material of diagnostic specimens from hospitalized patients submitted to our laboratory. The urine samples for the stability study were collected from catheterized dogs undergoing treatment for intervertebral disc disease in our veterinary hospital (Dick White Referrals, Six Mile Bottom, Cambridgeshire, UK). The dogs had indwelling catheters for bladder emptying. Urine was collected via the indwelling catheter during routine care immediately prior to the beginning of the stability study.
Consent for the use of residual diagnostic material for research purposes was given by the animal’s owner through the institution’s standard treatment and hospitalization consent form.
The gross appearance of urine samples was recorded, and a dipstick analysis (Multistix 10 SG, Siemens Healthineers, Erlangen, Germany) was performed prior to centrifugation of 5 mL of sample in a conical tube at 890 × g for 7 min (EB series centrifuge, Centurion Scientific, Chichester, UK). The supernatant was recovered for GGT activity quantification, leaving 0.5 mL of supernatant to resuspend the sediment pellet. Fifty μL of the resuspended unstained sediment was placed on a slide and covered by a 20-mm2 coverslip to allow microscopic examination. Exclusion criteria for the use of urine samples included the presence of hematuria, pyuria, and bacteriuria detected by urinalysis or sediment examination. Urine was centrifuged and analyzed immediately unless otherwise specified in the method.
Analysis
Biochemical analyses were performed on an automated spectrophotometric biochemistry analyzer (AU480, Beckman Coulter, Brea, CA). Urine GGT was analyzed using the same gamma-glutamyl-3-carboxy-4-nitroanalide method (Olympus GGT OSR 6020, Beckman Coulter) as supplied for quantification of GGT activity in serum. Urine creatinine was analyzed using the kinetic Jaffé method. An internal quality control (QC) program for the analyzer was performed daily; the laboratory participates in an external quality assurance scheme.
Urine pH was measured using urine dipsticks (Multistix 10 SG, Siemens) in order to replicate the most widely available method of urine pH measurement. Urine dipstick analysis was performed by experienced biomedical scientists strictly following manufacturer’s instructions in a well-lit and temperature-controlled laboratory. The pH was measured before each GGT testing period to ensure that no change had occurred in urine pH.
Analytical validation
Analytical validation of urine GGT activity included the determination of precision, linearity, and accuracy and was performed using the guidelines published by the American Society for Veterinary Clinical Pathology (ASVCP Quality Control Guidelines–Principles of Quality Assurance and Standards for Veterinary Clinical Pathology 2006 Revision: Finalized 2009, https://www.asvcp.org/page/QALS_Guidelines).
Intra-assay precision was assessed in 4 urine samples with mean GGT activities of 14 U/L, 47 U/L, 69 U/L, and 195 U/L. Each sample was analyzed 20 times within a single analyzer run. The inter-assay precision was assessed in 3 samples with mean GGT activities of 11 U/L, 18 U/L, and 51 U/L. Each sample was analyzed in triplicate on 7 independent analyzer runs over 3 d. All samples were stored at 4°C, but brought to room temperature immediately prior to analysis. The mean and standard deviation (SD) of the replicates were used to calculate the coefficients of variation (CV%).
Linearity of the method was assessed (4–189 U/L GGT activity) by serially diluting a urine sample with high GGT activity with a sample with low GGT activity at ratios of 1:4, 2:3, 3:2, and 4:1 parts. Diluting a sample of high GGT activity with a urine sample of low GGT activity avoids matrix effects, which may result from dilution with a non-urine diluent. The dilutions and original urine samples were then analyzed in triplicate. An estimated value for each dilution was plotted against the mean of the measured values. The coefficient of correlation was calculated.
The accuracy of the assay was assessed by recovery. Four urine samples with different GGT concentrations were used. A small volume (<10% v/v) of serum GGT QC material (Beckmann Coulter) was mixed with each sample. The original urine sample, the QC material, and the spiked sample were run in duplicate in the same run. The predicted GGT activity in each mixed sample was estimated, and the difference between the mean of the measured values and the predicted value was used to calculate percentage recovery (%) and absolute bias.
The limit of the blank (LoB) was obtained by measuring the GGT activity on 10 replicates of physiologic saline and applying the following mathematical formula:
Stability
Four fresh urine samples were divided into twenty-nine 1-mL aliquots and distributed between 4 temperature-controlled locations: 20°C, 4°C, −20°C, and −80°C. One aliquot from each sample was analyzed immediately, and one aliquot of each sample was analyzed on d 1, 2, 3, 5, and 7 for aliquots stored at 20°C, 4°C, −20°C, and −80°C, and on d 14, 21, 28, and 56 for aliquots stored at −20°C and −80°C. At each time, urine GGT activity was evaluated in duplicate with the mean of the measured values calculated and the pH of each sample measured using a urine dipstick. Refrigerated and frozen samples were brought to room temperature immediately prior to analysis.
Interfering substances
To assess the effect of free hemoglobin on the measurement of GGT activity, 1-mL aliquots from 3 urine samples (GGT activity of 10 U/L, 43 U/L, and 67 U/L) were spiked with 5 μL of a variably diluted hemoglobin solution in order to produce blood-contaminated urine samples with 1+, 2+, and 3+ blood and/or hemoglobin detection on a urine dipstick. To assess the effect of red blood cell contamination, 1-mL aliquots from the same urine samples were spiked with 5 μL of diluted canine EDTA blood (residual diagnostic sample: PCV 50 L/L and GGT activity 14 U/L) to produce blood-contaminated urine samples with 1+, 2+, and 3+ blood and/or hemoglobin detection on a urine dipstick. Five μL of the same undiluted blood sample was added to another 1-mL aliquot of urine so that it was visibly blood-tinged. A control aliquot of urine was spiked with the same volume of physiologic saline. GGT was measured in triplicate in each sample and in a 1-mL aliquot of saline spiked with 5 μL of undiluted hemoglobin solution.
The effect of bacteria on GGT activity was investigated by spiking 1-mL aliquots of 2 urine samples that had a GGT activity of 26 U/L and 32 U/L, respectively, with 100 μL of a 0.5 McFarland equivalent standard (~1.5 × 108 bacteria/L) suspension of Escherichia coli, a 0.5 McFarland equivalent standard suspension of Enterococcus sp., or 100 μL of sterile physiologic saline. GGT activity was measured in triplicate immediately following addition of the bacterial suspension or physiologic saline and after 48 h stored at 20°C. Urine culture was performed on the bacterial suspensions and on the spiked urine samples after 48 h, incubated at 37°C at our microbiology laboratory, to demonstrate that bacteria were still viable. The mean of the measured GGT activities for aliquots spiked with E. coli or Enterococcus sp. was compared to those of the urine spiked with physiologic saline.
Effect of pH
A small volume (50 μL, <10% v/v) of different concentrations of hydrochloric acid and sodium hydroxide were added to 1-mL aliquots of urine to produce samples of pH 5.0–8.5 as measured by urine dipsticks. Acidified and alkalinized samples were analyzed in triplicate, and mean GGT activities were compared to the mean GGT activity of an aliquot diluted with 50 μL of physiologic saline that was also measured in triplicate. Acidified, alkalinized, and saline-diluted sample were analyzed in single analyzer runs.
Reference interval, biological variation, and reference change value
Free-catch convenience urine samples were collected from 46 owned healthy adult (>1-y-old) dogs during the 3-mo data collection period. Dogs were owned by staff and associates of our institution. Owners completed a questionnaire consenting to the inclusion of their dog’s urine in the study and confirming that their dog was healthy and not receiving any veterinary treatment other than vaccines and anti-parasite preparations. Eight of these individuals were recruited into the biological variation study and had 7 subsequent free-catch urine samples taken over ~4–6 wk with 1–3 d between samples. Urine samples were stored at 4°C if analysis could not be performed immediately. All samples were analyzed within 48 h of collection and were brought to room temperature prior to analysis. Urine GGT activity and creatinine concentration were measured in triplicate. Urine was tested with a urine dipstick and the specific gravity measured with a refractometer (Uricon Refractometer, Atago Corp, Tokyo, Japan). Sediment was not evaluated. Samples with >1+ protein or bilirubin, hemoglobin and/or blood, or pH <6.5 or >8.0 were excluded from the RI study. The means of the measured GGT activity and creatinine concentration were calculated, and the urine GGT:creatinine ratio (U/g) was calculated using the previously published formula of mean GGT activity (U/L) divided by mean urine creatinine concentration converted to the units g/L.2,9 Analytical imprecision of the urine GGT:creatinine ratio was calculated by performing 20 replicates on pooled urine samples from patients in the hospital with an average GGT concentration of 27 U/L and an average creatinine concentration of 10,438 μmol/L (1.18 g/L).
Statistical analysis
Arithmetic means, SDs, CVs, linear regression, and paired t-tests were calculated (Excel 2011 for Mac, Microsoft, Seattle, WA). Testing for outliers, tests for normality, and restricted maximum likelihood (REML) were performed (SPSS Statistics v.22.0 for Mac, IBM, Armonk, NY).
Interference by a substance (hemoglobin, blood, or bacteria) or alteration in pH was deemed unacceptable if the variance of GGT activity following intervention was greater than the inter-assay CV.
Within the reference population, outlying values were identified using the Dixon method (p = 0.05) and culled from the population. The effect of sex on urine GGT:creatinine ratio within the population of healthy dogs was assessed using an unpaired t-test. A RI for this data set was then calculated using the Reference Interval Macro for Excel.6
Data were assessed for normality by visual inspection of the histograms and normal probability plots of the residuals. Once normality was confirmed, the analytical (CVA), within-subject (CVI), and between-subject (CVG) components of variation of GGT:creatinine ratio were calculated using the REML approach as advised by current recommendations for conducting a biological variation study.5 Index of individuality (II) was calculated by:
A unidirectional RCV for 95% confidence interval (CI) in percentage terms was calculated using the following formula:
For parametric and nonparametric analyses, a value ofp ⩽ 0.05 was considered significant.
Results
Analytical validation
The intra-assay analytical variation for urine GGT activity was 1.76–7.89%, and the inter-assay analytical variation was 4.75–11.23% (Table 1). The mean intra-assay and inter-assay imprecision for urine GGT activity were 3.59% and 7.74%, respectively. The assay was linear for the measurement of urine GGT activity in the range 4–189 U/L (R2 = 0.997). Accuracy by the recovery method was 97.3% with an absolute bias of 2.7 U/L. LoB was calculated to be 0 U/L.
Table 1.
Intra- and inter-assay analytical variation of gamma-glutamyl transferase (GGT) activity of 4 free-catch canine urine samples with different GGT concentrations.
| Intra-assay analytical variation |
Inter-assay analytical variation |
||
|---|---|---|---|
| GGT concentration (U/L) | Coefficient of variation (%) | GGT concentration (U/L) | Coefficient of variation (%) |
| 14 | 7.89 | 11 | 11.23 |
| 47 | 2.90 | 18 | 7.25 |
| 69 | 1.82 | 51 | 4.75 |
| 195 | 1.76 | ||
| Mean | 3.59 | Mean | 7.74 |
Stability
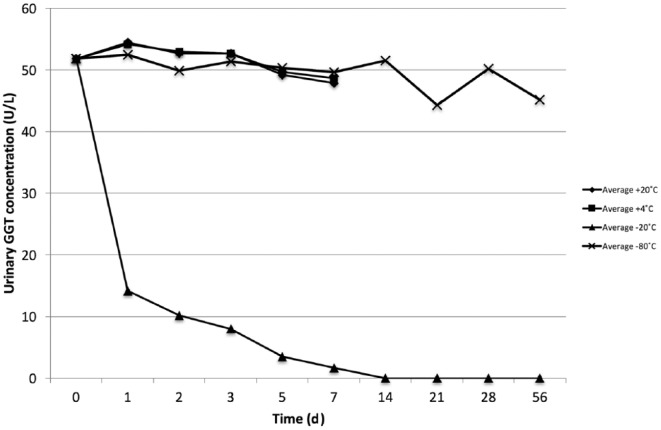
The average variance in GGT activity was within acceptable limits (less than the inter-assay CV of 7.74%) with up to 3 d storage at 20°C and 5 d at 4°C. Freezing at −20°C for a single day or longer markedly reduced GGT activity in all samples, with a mean reduction of 72%. For urine stored at −80°C, the mean variation in GGT activity was within the acceptable range for up to 14 d. However, there was unacceptable reduction in GGT activity in 1 sample at 14 d, with all samples showing unacceptable reduction in GGT activity by d 21 of storage (Fig. 1).
Figure 1.
Average urine gamma-glutamyl transferase (GGT) activity in 4 free-catch canine urine samples stored at different temperatures over 56 d.
Interfering substances and bacteria
GGT activity was not affected by hemoglobin or whole blood contamination up to 3+ as detected by urine dipstick or blood-tinged by macroscopic examination (i.e., less than the inter-assay CV of 7.74%). The difference in GGT activity between the urine samples spiked with 5 μL of hemoglobin solution or with 5 μL of whole blood was within the set acceptability limit (lower than the inter-assay CV) of the control urine samples spiked with 5 μL of physiologic saline.
Bacterial contamination with E. coli or Enterococcus sp. did not affect GGT activity. The difference between the GGT activity in the urine samples at the point of spiking and after 48 h incubation at 20°C was within set acceptability limits. Spiked urine samples were culture positive after 48 h, with pure growth of the inoculated bacteria.
Effect of pH
GGT activity was affected by urine pH. Urine GGT activity was preserved within the predefined acceptable limit when urine pH was within the range of 6.5–8.0. Outside this range, the GGT activity significantly reduced, with 80–100% loss of activity if pH was <5.0 or >8.5.
Reference interval, biological variation, and reference change value
Urine samples were submitted from 46 healthy owned dogs. The dogs included 29 males (8 intact, 21 neutered) and 17 females (2 intact, 15 neutered), 1–15 y of age. Breeds included: crossbreeds (16), Greyhound (6), Labrador Retriever (5), Staffordshire Bull Terrier (3), Cavalier King Charles Spaniel (2), English Springer Spaniel (2), Jack Russell Terrier (2), Lurcher (2), Bichon Frise (1), Bull Mastiff (1), Dachshund (1), English Bull Terrier (1), Husky (1), Pomeranian (1), Whippet (1), and Yorkshire Terrier (1). Results from 5 dogs were identified as significant outliers and were excluded from further analysis. These dogs were 4 intact males (2 Labradors, 1 Bull Mastiff, and 1 Pomeranian) and an intact female (Labrador). All urine samples had pH within the acceptable limit.
Significant differences between the urine GGT:creatinine ratio results of male compared to female dogs was not identified (T value = 1.66, p = 0.10). A lower limit of 8.5 U/g (90% CI: 8.4–9.2 U/g) and an upper limit of 28.5 U/g (90% CI: 27.0–28.6 U/g) were calculated using a robust nonparametric method.
The results obtained from the 8 subjects included in the biological variation study were assessed for outliers and normality. Examination of the histograms and normality Q-Q charts indicated that the results from each subject were normally distributed. CVI was 17.5% and CVG was 29.3%, as calculated from the output of restricted maximum likelihood analysis. CVA was 5.4%, as calculated from 20 replicates of pooled patient urine. The index of individuality was 1.6. Given that only increases in the GGT:creatinine ratio are considered clinically relevant, a single-sided RCV of 43% was calculated.
Discussion
Our validation study confirms that this serum assay of GGT activity is precise and accurate for canine urine, and shows analytical linearity of 4–189 U/L. A significant matrix effect was not identified when accuracy was determined by recovery. The limit of detection was not assessed as part of our validation study, because low values of urine GGT are not clinically significant. The LoB is 0 U/L. The assay is not affected by hemoglobinuria or hematuria, either grossly or as detected by a urine dipstick. The effect of pyuria was not studied, but in vitro bacterial proliferation did not have any impact on GGT activity.
The GGT activity in urine stored at +4°C and +20°C was stable in all samples up to 3 d, supporting the assertion from other studies7 that analysis of urine GGT should be conducted within this time frame. However, in 2 of the 4 urine samples, GGT activity was unaffected by up to 7 d with storage at +4°C and +20°C. The reason of this variation in stability is unknown, but it is not a result of pH change in these cases. The storage of urine at −20°C for any duration reduced GGT activity rapidly and markedly. Complete inhibition of GGT activity was seen by 14 d storage. At −80°C, enzyme activity was somewhat preserved, although urine GGT activity did start to decline following 14 d of storage. These findings are similar to those reported in human samples,11 and support the guidance that urine intended for GGT activity studies must not be frozen.
A study of GGT activity in human urine10 found that the enzyme was inactivated at pH <4.7. We demonstrated that canine urine GGT activity is also affected by pH, and that significant reductions in enzyme activity are observed with less marked changes in pH. The enzyme activity appeared to be stable within a range of pH 6.5–8.0, as determined by semi-quantitative colorimetric dipsticks, with a precipitous decline in activity outside this range. The semi-quantitative nature of urine dipsticks for the measurement of the pH is a limitation of our study, given its intrinsic inter-operator variation associated with subjective interpretation of the colorimetric change. However, the use of semi-quantitative urine dipsticks was intended to mirror the most available method of urine pH estimation in practice. The simplified model of the effect of urine pH used in our study does not take into account the duration of time to which the enzyme was exposed to unfavorable conditions, given that samples were run immediately following acidification and alkalinization. In vivo changes of the urine within the bladder were also not considered. Nonetheless, our results suggest that urine pH should be a consideration when interpreting urine GGT activity.
A study of urine enzyme activity in healthy dogs2 reported that dogs with a higher urine pH (⩾7.0) had significantly higher urine GGT activity than those with low urine pH (<7.0). However, 7 of the 20 dogs in the low urine pH (<7.0) group had a urine pH of 5.0. This is below the range of pH (6.5–8.0) in which urine GGT activity would be expected to be stable, as determined by our study. Thus, we speculate that the inclusion of dogs with urine pH of 5.0 (6.0 and below) might explain the significant difference in GGT activity between the 2 groups.
The urine GGT RI obtained by our study is similar to those reported by others.2,7 A significant difference between GGT:creatinine ratios between sexes was not observed, which is also in agreement with previous studies.2,16
We identified 5 dogs as outliers and excluded them from our study; 4 of the 5 dogs were intact males. Although the significance of this finding and its association with the neutered status cannot be proven, this might be a result of semen contamination of the urine as a consequence of retrograde ejaculation or residual seminal fluid within the urethra. Studies of enzyme activity in bovine, porcine, and equine semen12,13,17 demonstrated high concentrations of GGT in the seminal fluid and spermatozoa. It can be assumed that canine seminal fluid and spermatozoa also contain GGT and thus contamination of urine with seminal fluid or spermatozoa may affect the urine GGT:creatinine ratio. The number of intact male and female dogs available within our reference population group does not allow statistical comparison of the effect of male and female intact status on GGT:creatinine ratio. A study of semen quality in boars found significant differences in seminal GGT activity between different pig breeds. The possible effect of semen or seminal fluid contamination of urine in intact male dogs, including possible breed-related differences, may warrant further study.
Confirmation of the health status of the dogs that contributed to the reference population was limited to the assessment of urine dipstick, the declaration of the dog’s owner, and absence of current medication other than vaccines and anti-parasiticides. Evaluation of serum creatinine and urea or urine protein:creatinine ratio was not performed, given that the utility of these tests for the detection of tubular injury is suboptimal.9 Additionally, blood sampling of animals for research purposes is legally precluded without government license in the country where the study was performed. Confirming the absence of tubular disease using other markers of tubular injury, such as retinol-binding protein, neutrophil gelatinase–associated lipocalin, and cystatin C would be preferable to serum urea and creatinine, but these assays are not widely available.
Understanding of the biological variation of urine GGT:creatinine both within and between individuals is required for the appropriate interpretation of serial results. The index of individuality (1.6) supports the use of a population-based RI. Given that testing of GGT:creatinine ratio would normally be performed repeatedly to monitor for evidence of acute renal injury during a risk period, a change in GGT:creatinine ratio ⩾43% must be considered to be significant. Anecdotally, a doubling of the urine GGT:creatinine ratio from a baseline measurement has been regarded as an indicator of significant change.15 Our findings suggest that this threshold may be overly generous and that an increase of >43% from baseline would be greater than expected based on intra-individual biological variation and would suggest acute tubular injury. The reference population for our study comprised only healthy dogs, and the validity of extrapolating the biological variation characteristics to dogs with renal disease or comorbid disease is uncertain. Further study may be warranted into how renal disease and comorbid disease may affect within-subject biological variation of urine GGT:creatinine ratio.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev 2008;29(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 2. Brunker J, et al. Indices of urine N-acetyl-β-D-glucosaminidase and γ-glutamyl transpeptidase activities in clinically normal adult dogs. Am J Vet Res 2009;70:297–301. [DOI] [PubMed] [Google Scholar]
- 3. Clemo F. Urinary enzyme evaluation of nephrotoxicity in the dog. Toxicol Pathol 1998;26:29–32. [DOI] [PubMed] [Google Scholar]
- 4. De Schepper J, et al. Urinary gamma-glutamyl transferase and the degree of renal dysfunction in 75 bitches with pyometra. Res Vet Sci 1989;46:396–400. [PubMed] [Google Scholar]
- 5. Freeman KP, et al. Recommendations for designing and conducting veterinary clinical pathology biologic variation studies. Vet Clin Pathol 2017;46:211–220. [DOI] [PubMed] [Google Scholar]
- 6. Geffre A, et al. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Vet Clin Pathol 2011;40:107–112. [DOI] [PubMed] [Google Scholar]
- 7. Gossett KA, et al. Evaluation of gamma-glutamyl transpeptidase-to-creatinine ratio from spot samples of urine supernatant, as an indicator of urinary enzyme excretion in dogs. Am J Vet Res 1987;48:455–457. [PubMed] [Google Scholar]
- 8. Greco DS, et al. Urinary gamma-glutamyl transpeptidase activity in dogs with gentamicin-induced nephrotoxicity. Am J Vet Res 1985;46:2332–2335. [PubMed] [Google Scholar]
- 9. Hokamp J, Nabity M. Renal biomarkers in domestic species. Vet Clin Pathol 2016;45:28–56. [DOI] [PubMed] [Google Scholar]
- 10. Jung K, et al. Influence of pH on the activity of enzymes in urine at 37°C. Clin Chem 1982;28:1814. [PubMed] [Google Scholar]
- 11. Matteucci E, et al. Effects of storage time and temperature on urinary enzymes. Clin Chem 1991;37:1436–1441. [PubMed] [Google Scholar]
- 12. Pero ME, et al. Influence of γ-glutamyltransferase and alkaline phosphatase activity on in vitro fertilisation of bovine frozen/thawed semen. Ital J Anim Sci 2017;16:390–392. [Google Scholar]
- 13. Pesch S, et al. Determination of some enzymes and macro- and microelements in stallion seminal plasma and their correlations to semen quality. Theriogenology 2006;66:307–313. [DOI] [PubMed] [Google Scholar]
- 14. Rivers BJ, et al. Evaluation of urine gamma-glutamyl transpeptidase-to-creatinine ratio as a diagnostic tool in an experimental model of aminoglycoside-induced acute renal failure in the dog. J Am Anim Hosp Assoc 1996;32:323–336. [DOI] [PubMed] [Google Scholar]
- 15. Syme HE. Laboratory evaluation of renal disorders. In: Villiers E, Ristić J, eds. BSAVA Manual of Canine and Feline Clinical Pathology. 3rd ed. UK: British Small Animal Veterinary Association, 2016:219–236. [Google Scholar]
- 16. Uechi M, et al. Circadian variation of urinary enzymes in the dog. J Vet Med Sci 1994;5:849–854. [DOI] [PubMed] [Google Scholar]
- 17. Žaja IZ, et al. Influence of boar breeds or hybrid genetic composition on semen quality and seminal plasma biochemical variables. Anim Reprod Sci 2016;164:169–176. [DOI] [PubMed] [Google Scholar]