Abstract
Ameloblastic fibroma (AF) and ameloblastic fibro-odontoma (AFO) are mixed odontogenic tumors (odontogenic tumors with induction) that are reported only rarely in dogs. These tumors are histologically complex and, to a degree, recapitulate the early stages of tooth development, comprising 2 types of tissue: neoplastic odontogenic epithelium, and induced ectomesenchyme (dental pulp). AFOs are distinguished from AFs by the additional presence of hard dental matrices such as dentin. Herein, we describe the key diagnostic features of AF and AFO in 4 young dogs.
Keywords: Ameloblastic fibroma, ameloblastic fibro-odontoma, dogs, induction, odontogenic
Ameloblastic fibroma (AF) and ameloblastic fibro-odontoma (AFO) are mixed odontogenic tumors that arise from germinal tissues of developing teeth and feature both epithelial and mesenchymal components. AFO differs from AF by the additional presence of dental matrix (dentin with or without enamel). These tumors have been diagnosed in humans11 and, more rarely, dogs,3,4,9,10 cats,3 cattle,6 and horses.7 In humans, AF and AFO are most often diagnosed in children to young adults (average age for AF 14.6–15.5 y, 98% of AFO below age 20) and are primarily located in the posterior mandible.5 In cattle, AF tends to arise from the rostral mandibular incisors in young animals (newborn to 1.5 y) of either sex.6 AFO has been reported in both the mandible and maxilla in dogs up to 1 y of age.4,13,14 AF has only been reported in one 4-y-old dog, to our knowledge.10
Although AF and AFO are slowly progressive lesions, they can be locally destructive, potentially destabilizing the adjacent jawbone and resulting in pathologic fractures. In humans and animals, the tumors may appear radiographically as uni- to multilobular radiolucent masses with evidence of osteolysis and/or mineralization. When these lesions are associated with unerupted teeth, such radiographic features may be similar to the appearance of a dentigerous cyst. Complete surgical excision may be possible given the generally well-demarcated nature of these lesions. There have been a few case reports in cattle and dogs in which such lesions have demonstrated infiltrative or metastatic behavior.3,6,17 Note that one author (C Bell) is skeptical of the diagnosis of metastatic AFO in a dog.17
AF and AFO share many histologic features with other mixed odontogenic tumors such as odontomas (complex or compound) and odontoameloblastomas, creating a potentially challenging situation for the examining pathologist. We describe herein the clinical and histologic features of AF (case 1) and AFO (case 2–4) in young dogs.
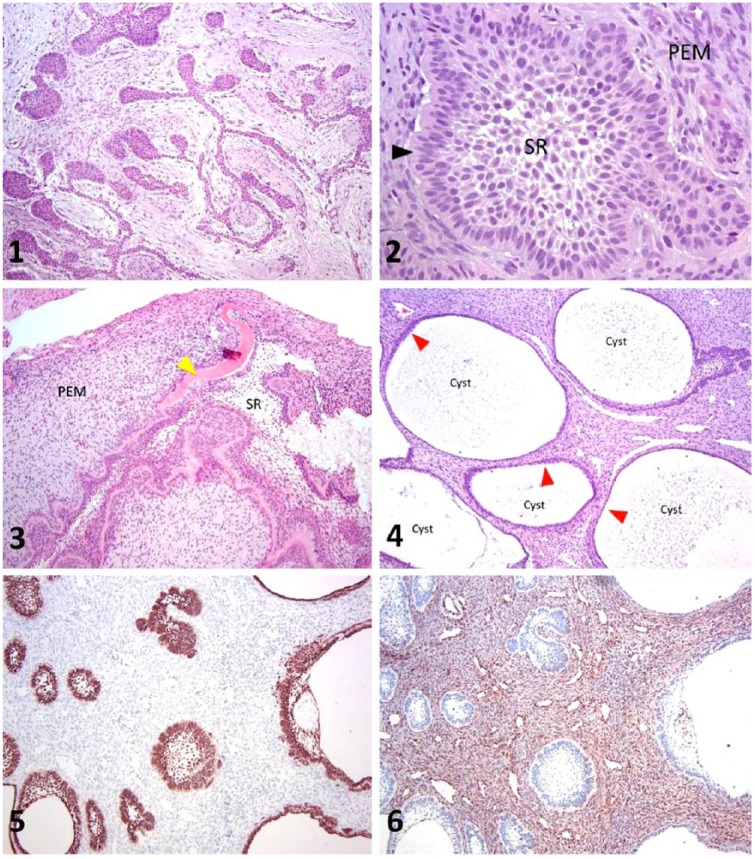
Case 1, a 4-mo-old intact female Labrador Retriever, was presented to Columbia River Veterinary Specialists (Vancouver, WA) because of persistent facial swelling. Intraoral radiographs revealed an expansive left intranasal mass with soft tissue opacity crossing into the right nasal cavity with bilateral disruption of the nasal turbinates. Additionally, the malformed crown of an unerupted left maxillary canine tooth (tooth 204) was noted caudal to the mass in the radiographs. The maldeveloped tooth bud and surrounding cystic tissue were surgically excised and submitted to the Center for Comparative Oral and Maxillofacial Pathology (CCOMP) at University of Wisconsin–Madison (UW-Madison, Madison, WI) for histologic examination. Follow-up information indicated that the patient recovered well from surgery and has yet to show any signs of tumor recurrence 3 y post-operation. Representative samples of the mass were fixed in 10% formalin and routinely demineralized, processed into paraffin blocks, sectioned at 5 µm, and stained with hematoxylin and eosin. Histologic sections revealed a proliferative lesion comprised of both odontogenic epithelium and ectomesenchymal tissue. The odontogenic epithelial component was arranged in thin plexiform ribbons with globular protuberances and irregular follicle-like aggregates (Fig. 1). The epithelial cells were generally columnar with anti-basilar nuclei. The epithelial structures were closely associated with, and embedded within, abundant, proliferative, loosely arranged ectomesenchymal stroma. This ectomesenchymal stroma was comprised of variably dense collections of angular-to-stellate cells amid basophilic mucinous ground substance reminiscent of pulp ectomesenchyme. Mineralized dental matrices (dentin and/or enamel) were not identified in any examined section. There were 0–1 mitotic figures per 10 high power fields (HPF), and mild anisocytosis and anisokaryosis. Collectively, these histopathologic and clinical features were consistent with a diagnosis of AF.
Figures 1–6.
Ameloblastic fibroma and ameloblastic fibro-odontoma in dogs. Figure 1. Histologic section of the lesion from case 1 (ameloblastic fibroma) has odontogenic epithelium arranged in branching cords with globular protuberances embedded within basophilic ectomesenchymal stroma. H&E. 100×. Figure 2. Histologic section of the lesion from case 1 (ameloblastic fibroma) demonstrates odontogenic epithelium embedded in pulp ectomesenchyme (PEM). Features of odontogenic epithelium shown here include palisading epithelium with anti-basilar nuclei (arrowhead) and centrally located cells with features of stellate reticulum (SR). H&E. 400×. Figures 3–4. Histologic sections from case 2 (ameloblastic fibro-odontoma). Figure 3 shows eosinophilic dental matrix (arrowhead) arranged in a ribbon-like pattern, separating the odontogenic epithelium from the basophilic pulp ectomesenchyme (PEM). Centrally, the odontogenic epithelium has features consistent with stellate reticulum (SR). H&E. 100×. Figure 4 depicts multiple cystic structures lined with attenuated, stratified squamous epithelium (arrowheads). H&E. 100×. Figures 5–6. Immunohistochemistry from case 2. In Figure 5, pan-cytokeratin identifies the epithelium. In Figure 6, vimentin identifies the ectomesenchymal tissue. 100×.
Cases 2–4 were diagnosed as AFO. Case 2 was a 4-mo-old spayed female Labrador Retriever who was presented to the Veterinary Medical Teaching Hospital Dentistry and Oral Surgery Service (VMTH DOSS) at UC Davis (Davis, CA) with swelling around an embedded left maxillary canine tooth. Case 3 was an 8-mo-old spayed female Beagle cross who was presented with swelling in the area of a missing left maxillary molar at Tennessee Veterinary Dentistry and Oral Surgery (Brentwood, TN). Case 4, a 9-mo-old intact female mixed-breed dog, was referred to the Virginia-Maryland Veterinary Teaching Hospital (VM-VTH, Blacksburg, VA) with gingival swelling in the region of a previously extracted right maxillary premolar (tooth 107). Radiographs in all 3 cases demonstrated cystic lesions in the area of the tissue swellings. All 3 patients underwent surgery to remove the lesions; the excised tissues were subsequently submitted to the associated university diagnostic laboratories (tissue from case 3 was submitted to CCOMP at UW-Madison) and processed as described previously. In case 2, immunohistochemistry (IHC) was additionally performed with murine monoclonal antibodies against pan-cytokeratin and vimentin according to manufacturer protocols (pan-cytokeratin Lu-5, Biocare Medical, Concord, CA; vimentin clone Vim 3B4, Dako, Glostrup, Denmark).
Microscopically, all 3 cases had tissues with poorly demarcated borders. Neoplastic odontogenic epithelium was arranged in cystic structures, plexiform ribbons, and follicles separated by abundant loosely arranged ectomesenchymal stroma. The neoplastic epithelium demonstrated multiple odontogenic features including peripheral palisading with anti-basilar nuclei and prominent basilar cytoplasmic clearing (Fig. 2). Centrally, the neoplastic epithelium was intermittently comprised of polygonal-to-stellate epithelial cells with prominent and elongated intercellular bridges (stellate reticulum). In addition, the epithelial islands were occasionally associated with ribbon-like deposits of homogeneously hyalinized eosinophilic matrical material histologically consistent with atubular dentin (Fig. 3). The abundant ectomesenchymal stroma consisted of loosely arranged, plump fusiform-to-angular polygonal cells embedded in basophilic ground substance reminiscent of dental pulp. There was mild anisocytosis and anisokaryosis with rare mitotic figures (0–1 mitotic figures per 10 HPF). Cystic structures were lined by attenuated stratified squamous epithelial cells generally lacking the cardinal features of odontogenic epithelium (Fig. 4). In case 2, IHC assays for pan-cytokeratin and vimentin sharply delineated the epithelial and ectomesenchymal components, respectively (Figs. 5, 6). In aggregate, these features were considered to be diagnostic for AFO.
Odontogenic tumors are categorized based upon the presence or absence of specific tissue types involved in the process of tooth embryogenesis.1 Normal tooth embryogenesis is initiated by the proliferation of the dental lamina, extending as a cup-shaped bud from the gingival mucosa into the subjacent connective tissue. This epithelial bud sequentially folds into a “cap” and then into a “bell”-shaped structure lined by palisading ameloblasts (enamel organ).8 During this developmental process, the ameloblasts release growth factors and other signaling molecules that induce the subjacent neural crest–derived ectomesenchymal cells to proliferate around each epithelial bud, resulting in the formation of the dental papilla, and eventually, the dental pulp. This process is called odontogenic induction. During induction, the pulp ectomesenchyme immediately subjacent to the enamel organ differentiates into aggregating odontoblasts that secrete dentinal matrix. In a reciprocal fashion, the presence of the dentinal matrix stimulates the ameloblasts to secrete enamel matrix. Both dentin and enamel subsequently mineralize to become hard tissues of the developing tooth. This cross-talk between epithelium and ectomesenchymal layers is referred to as “reciprocal induction,” and allows for the formation of the tooth crown and root.12
To date, mixed odontogenic tumors in domestic animals include AF, AFO, odontoameloblastoma (OA), and odontoma. Odontomas can either be classified as complex (disorganized deposits of dentin and/or enamel) or compound (organized denticles or odontoids). Mixed odontogenic tumors are tripartite lesions comprised of 1) odontogenic epithelium, 2) ectomesenchyme reminiscent of dental pulp, and 3) mineralized dental matrices (AF lacks dental matrices). The neoplastic odontogenic epithelium is often arranged in follicular or thin ribbon-like patterns, sometimes with globular buds resembling the structure of the early enamel organ (dental lamina). The epithelium may also demonstrate one or more of the following cardinal odontogenic features: peripheral palisading, apically placed nuclei, basilar cytoplasmic clearing, and/or centrally located epithelial cells resembling stellate reticulum. These so-called “cardinal” odontogenic features may or may not be present.
The pulp ectomesenchyme is typically characterized by variably dense, stellate-to-fusiform cells embedded in mucinous ground substance containing collagen and proteoglycan molecules, contributing to the pulp-like basophilic staining and edematous histologic appearance. In some lesions, it is possible to confuse the histologic features of stellate reticulum for pulp ectomesenchyme. IHC assays for vimentin (pulp ectomesenchyme) and pan-cytokeratin (stellate reticulum) can be helpful in this regard.
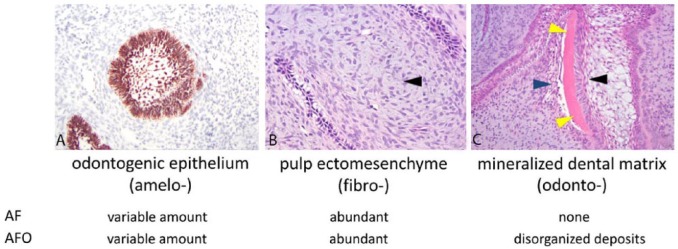
The presence or absence of mineralized dental matrix (dentin and/or enamel) is a key diagnostic feature of mixed odontogenic tumors. In AFO, it is important to distinguish between dental matrix and metaplastic bone or cementum. In a normal tooth, dentin stains as an eosinophilic matrix similar to that of bone, but uniquely includes tiny parallel tubes referred to as dentinal tubules. In reparative and neoplastic processes, odontoblasts may become engulfed in newly deposited dentin, a matrical mimic of bone referred to as osteodentin.16 Lesion-associated dentin often lacks dentinal tubules (atubular dentin). It is useful to note that although osteodentin and atubular dentin are typically arranged in thin, undulating ribbon or ring-like patterns, metaplastic bone and cementum may appear as anastomosing trabeculae and irregularly sized sheets instead.12 Through careful observation, loosely arranged aggregates of odontoblasts may be identified on one surface of the dental matrix, resulting in a “sandwich” of palisading ameloblasts, dentinal matrix, and aggregating odontoblasts (Fig. 7). Because enamel consists mostly of minerals, it is generally eliminated from the section by demineralization procedures. Exceptions to this include incomplete demineralization or the presence of immature enamel, which has a higher proportion of organic material, thus leaving behind a lightly basophilic to gray-staining matrix.16 The principal diagnostic differences between AF and AFO are summarized in Figure 7.
Figure 7.
Summary of the major features and differences between ameloblastic fibroma (AF) and ameloblastic fibro-odontomas (AFO). A. Pan-cytokeratin immunohistochemistry showing a follicle of odontogenic epithelium. B. Tissue section showing abundant pulp ectomesenchyme (arrowhead), which is characterized by variably dense sheets of angular to spindle-shaped ectomesenchymal cells embedded within basophilic matrix. H&E. C. Tissue section demonstrating a linear deposit of mineralized dental matrix (yellow arrowheads). Note that the dental matrix is sandwiched between palisading ameloblasts (black arrowhead) and odontoblasts (blue arrowhead). H&E.
Because the tripartite features of AFO are also those of OA and odontomas, these mixed odontogenic tumors often pose a diagnostic conundrum for the examining pathologist. In addition to the types of tissue present within the examined lesion, the proportions of different odontogenic tissues are of importance in histologically differentiating AFO, OA, and odontomas. AFO has the highest proportion of pulp ectomesenchyme strands of dental matrix and fewer islands of odontogenic epithelium. In contrast, OA has the greatest proportion of neoplastic odontogenic epithelium; this proliferative and often dysplastic epithelium generally accounts for a majority of the mass effect.12 In general, the odontogenic epithelium is relatively sparse in odontomas, and the tissue components (odontogenic epithelium, pulp ectomesenchyme, and dental matrix) are more organized than in either AFO or OA.
Investigators have argued whether AF, AFO, and odontomas collectively represent different stages of a single disease process or if they are unique entities.5 Some consider AF, AFO, and odontomas a single continuous process, with AF representing the most primitive lesion (lacking production of mineralized dental matrix) and compound odontomas representing the most well-differentiated lesion of this group (hamartoma).15 Others believe that additional information regarding the biologic behavior of these 3 lesions is needed before determining their relation with each other. For example, the locally infiltrative behavior that has been reported in dogs with AF and AFO contrasts with the generally benign and expansile behavior of odontomas.3 Thus, it is our opinion that the mixed odontogenic tumors should not be considered to be different stages of a single disease process, but rather as distinct, yet related entities with potential fluidity between lesions from progressive mutations and dysregulation.
The precise pathogenesis of AF and AFO has not been well scrutinized in either human or animals given the scarcity of diagnosed cases. One hypothesis is that neoplastic odontogenic epithelium releases signaling molecules that induce expansive, non-neoplastic proliferation of the underlying ectomesenchymal tissue (dental pulp).14 Further differentiation of odontoblasts and/or stimulation of ameloblasts result in deposition of layered dental matrix (AFO). Others believe that these lesions represent a hamartomatous process rather than a neoplastic one because they recapitulate the complex signaling and differentiation mechanisms found during normal tooth embryogenesis. A hamartomatous growth, derived from the enamel organ, dental pulp, and connective tissues of the follicle, may form when continued tooth development is no longer guided by appropriate molecular signals or spatial anatomic relationships. Therefore, physical disruption (trauma) of a developing tooth may be an important predisposing factor for AF and AFO. Such hypotheses have yet to be rigorously tested. A 2012 review highlighted efforts in identifying molecular mechanisms in human ameloblastic fibroma and related lesions.2
AF and AFO are odontogenic lesions that are rarely identified in companion animals. The lesions have histologic features that overlap with other neoplasms such as OA and odontomas. The pathologist is tasked with definitively identifying the histologic presence or absence of 1) neoplastic odontogenic epithelium, 2) induced pulp ectomesenchyme, and 3) dentinal matrix. The relative proportion of these different components helps to guide the diagnostician to the most appropriate diagnosis. Other clinical features must be considered alongside histology prior to making a diagnosis, including gross features of the lesion, imaging results, and biologic behavior of the tumor. Our 4 cases add to the relatively sparse literature on odontogenic tumors in dogs.
Acknowledgments
We thank Drs. Kevin Stepaniuk (Columbia River Veterinary Specialists) and Keith Stein (Tennessee Veterinary Dentistry and Oral Surgery) for submitting cases 1 and 3, respectively, and Dr. Jason Soukup (UW-Madison) for assisting in the initial diagnosis of cases 1 and 3. We also thank Dr. Yoav Bar-Am (UC Davis) for work up and submission of case 2, as well as Dr. Kurt Zimmerman (Virginia-Maryland College of Veterinary Medicine) for review and histopathology in case 4. We are grateful for the technical support and expertise of the histotechnology staff at UC Davis, UW-Madison, and Virginia-Maryland College of Veterinary Medicine, respectively.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bell CM, Soukup JW. Nomenclature and classification of odontogenic tumors—part II: clarification of specific nomenclature. J Vet Dent 2014;31:234–243. [DOI] [PubMed] [Google Scholar]
- 2. Bernardes VF, et al. Molecular investigation of ameloblastic fibroma: how far have we gone? Head Neck Oncol 2012;4:45–48. [Google Scholar]
- 3. Boehm B, et al. Odontogene Tumoren bei Hund und Katze [Odontogenic tumours in the dog and cat]. Tierarztl Prax Ausg K Kleintiere Heimtiere 2011;39:305–312. German. [PubMed] [Google Scholar]
- 4. Boyd RC. Ameloblastic fibro-odontoma in a German shepherd dog. J Vet Dent 2002;19:148–150. [DOI] [PubMed] [Google Scholar]
- 5. Cohen DM, Bhattacharyya I. Ameloblastic fibroma, ameloblastic fibro-odontoma, and odontoma. Oral Maxillofacial Surg Clin North Am 2004;16:375–384. [DOI] [PubMed] [Google Scholar]
- 6. Gardner DG. Ameloblastic fibromas and related tumors in cattle. J Oral Pathol Med 1996;25:119–124. [DOI] [PubMed] [Google Scholar]
- 7. Knowles S, et al. Ameloblastic fibro-odontoma associated with a retained molar in an Oldenburg mare. J Vet Diagn Invest 2010;22:987–990. [DOI] [PubMed] [Google Scholar]
- 8. Koussoulakou DS, et al. A curriculum vitae of teeth: evolution, generation, regeneration. Int J Biol Sci 2009;5:226–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kramek BA, et al. Diagnosis and removal of a dentigerous cyst complicated by ameloblastic fibro-odontoma in a dog. J Vet Dent 1996;13:9–11. [Google Scholar]
- 10. Miles CR, et al. Maxillary ameloblastic fibroma in a dog. Vet Pathol 2011;48:823–826. [DOI] [PubMed] [Google Scholar]
- 11. Muller S, Vered M. Ameloblastic fibroma. In: El-Naggar AK, et al., eds. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: IARC, 2017:222–223. [Google Scholar]
- 12. Murphy B, et al. Mandibular odontoameloblastoma in a rat and a horse. J Vet Diagn Invest 2017;29:536–540. [DOI] [PubMed] [Google Scholar]
- 13. Nold JB, et al. Ameloblastic odontoma in a dog. J Am Vet Med Assoc 1984;185:996–998. [PubMed] [Google Scholar]
- 14. Poulet FM, et al. A survey of epithelial odontogenic tumors and cysts in dogs and cats. Vet Pathol 1992;29:369–380. [DOI] [PubMed] [Google Scholar]
- 15. Silva GCC, et al. Ameloblastic fibro-odontoma. Oral Oncol 2005;42:217–220. [Google Scholar]
- 16. Slootweg PJ. Histology of the teeth and surrounding structures. In: Slootweg PJ, ed. Dental Pathology. Heidelberger, Berlin: Springer-Verlag; 2013;9–18. [Google Scholar]
- 17. Ueki H, et al. Malignant ameloblastic fibro-odontoma in a dog. Vet Pathol 2004;41:183–185. [DOI] [PubMed] [Google Scholar]