Abstract
A 7-y-old sow with a large mass in the right upper thoracic limb was submitted for autopsy. Grossly, the mass encompassed the right humerus, elbow, and proximal radius and ulna. On cut surface, the mass was solid, lobulated, pale tan, and firm, with multifocal areas of necrosis and mineralization; it replaced the brachial musculature, invading and causing extensive humeral and ulnar osteolysis. The periosteum was roughened and irregular, and there was minor invasion into the elbow joint. Histologically, the mass was composed of densely cellular interweaving streams and bundles of pleomorphic spindle cells embedded in a scant fibrovascular stroma. There was moderate-to-strong, diffuse cytoplasmic or membranous immunoreactivity to claudin-1, laminin, and vimentin; weak-to-moderate, multifocal cytoplasmic and nuclear immunoreactivity to S100 and Sox-10, respectively, and weak cytoplasmic immunoreactivity for neuron-specific enolase. No neoplastic immunolabeling was detected with CD204, CD18, desmin, glial fibrillary acidic protein, ionized calcium-binding adapter molecule 1, melan A, neurofilament, nerve growth factor receptor, smooth muscle actin, or muscle pan-actin. A specific immunomarker for definitive diagnosis of a malignant nerve sheath tumor (MNST) and its differentiation from other nerve tumors or other spindle cell tumors is still lacking in veterinary medicine, and case-by-case or interspecies differences in immunohistochemistry (IHC) expression can occur even when applying a broad diagnostic IHC panel. However, the gross, histologic, and IHC features in our case were consistent with a MNST, an exceedingly rare neoplasm of pigs.
Keywords: Immunohistochemistry, malignant peripheral nerve sheath tumor, pigs
A 7-y-old mixed-breed sow was submitted for autopsy after a brief period of sternal recumbency. Physical examination revealed tachycardia, tachypnea, and a large, firm, well-vascularized mass involving the right upper thoracic limb. Radiographs revealed severe osteolysis and periosteal reaction in the distal humerus, humeral condyle, and proximal radius and ulna. On ultrasound, the mass was well vascularized and composed of heterogeneous soft tissue with multiple hyperechoic foci. The sow had been rescued ~2 wk prior from an unreported situation, and no additional history was known. Given declining quality of life and poor prognosis, euthanasia via pentobarbital overdose was elected.
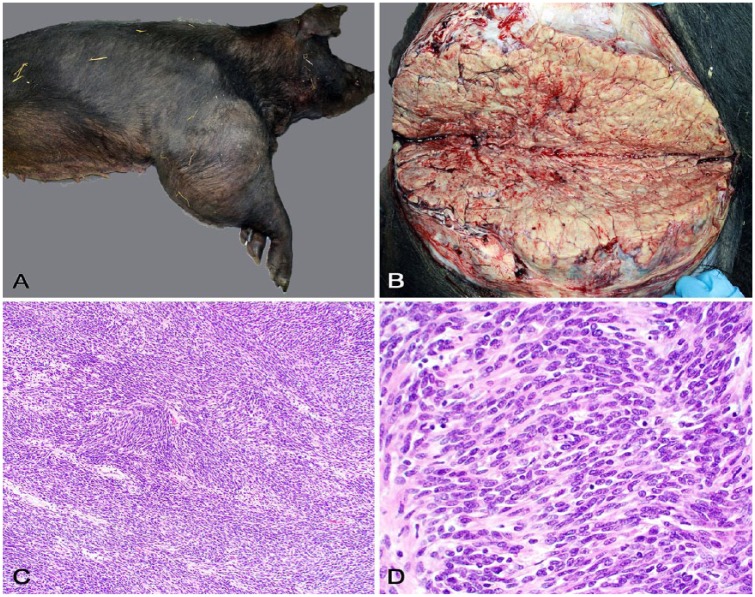
Gross anatomic changes (Fig. 1A) were restricted to the affected limb. A firm, 33-cm diameter mass encompassed the entire right humerus, elbow, and proximal radius and ulna. At its widest point, the mass circumference was ~105 cm. On cut surface (Fig. 1B), the mass was solid, lobulated, pale tan, and firm with friable or chalky debris in foci of necrosis and mineralization, respectively. Distal to the mass, the lower limb was severely edematous. The mass replaced the brachial musculature entirely, invading and causing extensive humeral and ulnar osteolysis. The periosteum was roughened and irregular with large defects in the cortical bone that exposed the bone marrow. There was minor invasion into the elbow joint. Severely engorged, varicose veins surrounded the mass and extended caudally along the right ventral thoracic body wall. No other significant changes were observed.
Figure 1.
Pathologic findings of a malignant nerve sheath tumor in a pig. A. A firm mass expands the right humerus, elbow joint, and proximal radius and ulna. B. On cut surface, the mass is lobulated, pale tan, and firm with friable or chalky debris in foci of necrosis and mineralization. C. The neoplasm is composed of closely packed, interweaving short streams and bundles of spindle cells in a fine fibrovascular stroma. H&E. D. Closer view of the neoplasm with interweaving streams and bundles of pleomorphic spindle cells. H&E.
Multiple tissue sections from the mass and other organs were immersed in 10% buffered formalin, routinely processed for histology, and stained with hematoxylin and eosin. Histologically (Fig. 1C), the mass was densely cellular and highly infiltrative, consisting of closely packed interweaving streams and bundles of spindle cells in a scant fibrovascular stroma (Fig. 1D). Neoplastic cells had moderate pleomorphism and indistinct cell borders, with scant, fibrillar, eosinophilic cytoplasm. Nuclei were round-to-elongate and had finely stippled chromatin and one evident nucleolus. There was moderate anisokaryosis and scattered multinucleate neoplastic cells. Mitotic count was 7 in ten 400× fields. There were foci of necrosis and mineralization throughout the tumor. Gomori reticulin stain revealed a fine network of basal lamina separating the neoplastic cells. No cross-striations within the cytoplasm of neoplastic cells were detected using phosphotungstic acid–hematoxylin stain. There was no gross or histologic evidence of metastasis in other examined tissues.
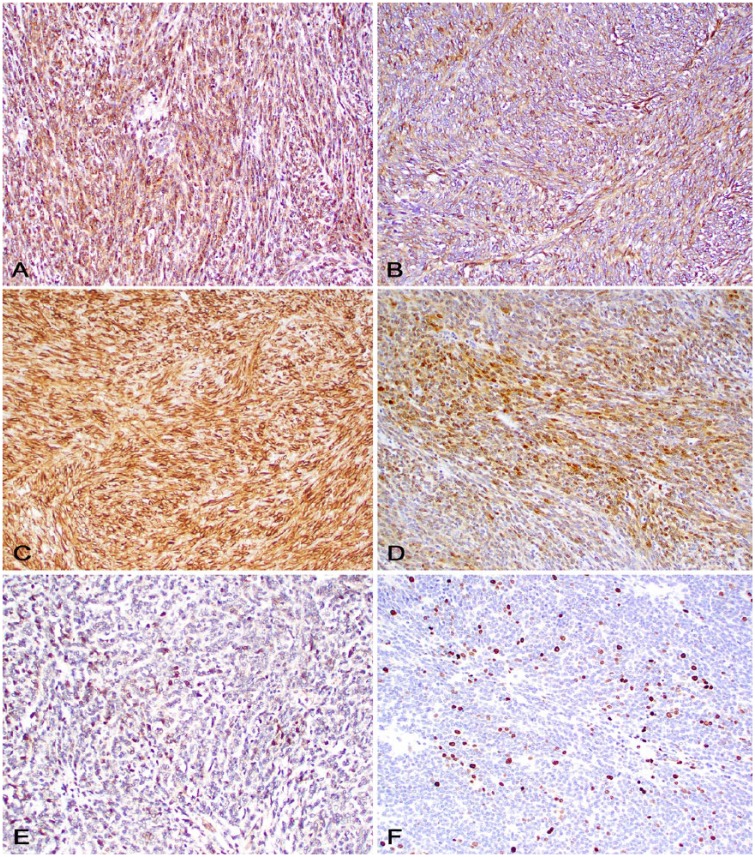
Sections of the neoplasm were subjected to immunohistochemistry (IHC) for diagnostic confirmation (Table 1, Fig. 2). There was moderate-to-strong, diffuse cytoplasmic or membranous immunoreactivity to claudin-1, laminin, and vimentin; weak-to-moderate, patchy cytoplasmic and nuclear immunoreactivity to S100 and Sox-10, respectively; and weak cytoplasmic immunoreactivity for neuron-specific enolase (NSE). Immunolabeling for CD204, CD18, and ionized calcium-binding adapter molecule 1 (Iba1) was observed in reactive macrophages throughout the neoplasm.
Table 1.
Immunohistochemistry and characterization of a malignant nerve sheath tumor in a pig.
| Antibody | Clonality | Concentration | Incubation (min) | Manufacturer | Neoplastic cells |
|---|---|---|---|---|---|
| Claudin-1 | Rabbit polyclonal | 1:50 | 60 | Abcam, Cambridge, UK | + (100%) |
| Laminin | Rabbit polyclonal | 1:200 | 60 | Dako, Santa Clara, CA | + (100%) |
| Vimentin | Mouse monoclonal | 1:3,000 | 60 | BioGenex, Fremont, CA | + (100%) |
| S100 | Mouse monoclonal | RTU | 30 | Cell Marque, Rocklin, CA | + (15%) |
| Sox-10 | Goat polyclonal | 1:100 | 60 | Santa Cruz, Dallas, TX | + (<5%) |
| Neuron-specific enolase | Mouse monoclonal | 1:2,000 | 60 | Invitrogen, Carlsbad, CA | + (<5%) |
| Ki67 | Rabbit monoclonal | RTU | 60 | Cell Marque, Rocklin, CA | + (15–20%) |
| Pan-cytokeratin (AE1/AE3) | Mouse monoclonal | RTU | 30 | Cell Marque, Rocklin, CA | – |
| CD204 | Mouse monoclonal | 1:500 | 60 | Cosmo Bio, Tokyo, Japan | – |
| CD18 | Mouse monoclonal | 1:10 | 60 | University of California, Davis, CA | – |
| Desmin | Mouse monoclonal | 1:10 | 90 | Biocare, Pacheco, CA | – |
| Factor VIII related antigen | Rabbit polyclonal | RTU | 60 | Cell Marque, Rocklin, CA | – |
| Glial fibrillary acidic protein | Mouse monoclonal | 1:4,000 | 60 | BioGenex, Fremont, CA | – |
| Ionized calcium-binding adapter molecule 1 | Rabbit polyclonal | 1:8,000 | 60 | Wako, Richmond, VA | – |
| Melan A | Mouse monoclonal | 1:30 | 90 | Dako, Santa Clara, CA | – |
| Neurofilament | Mouse monoclonal | 1:10,000 | 60 | BioGenex, Fremont, CA | – |
| Nerve growth factor receptor | Mouse monoclonal | 1:40 | 60 | Thermo Fisher Scientific, Waltham, MA | – |
| Smooth muscle actin | Mouse monoclonal | 1:1,500 | 60 | Dako, Santa Clara, CA | – |
| Muscle pan-actin | Mouse monoclonal | 1:100 | 30 | Dako, Santa Clara, CA | – |
RTU = ready to use.
Figure 2.
Immunohistochemistry of a malignant nerve sheath tumor in a pig. A. Neoplastic cells express strong cytoplasmic immunostaining for claudin-1. B. Neoplastic cells express moderate cytoplasmic immunostaining for laminin. C. Neoplastic cells express strong cytoplasmic immunostaining for vimentin. D. Subsets of neoplastic cells express strong cytoplasmic immunostaining for S100. E. Scattered neoplastic cells express strong nuclear immunostaining for Sox-10. F. Subsets of neoplastic cells express strong nuclear immunostaining for Ki67.
The tumor location, cellular morphology, high mitotic count, and degree of invasiveness in our case are consistent with a malignant nerve sheath tumor (MNST), and the diagnosis was supported by IHC. Nerve sheath tumors are widely referenced in the veterinary medical literature as peripheral nerve sheath tumors but the term “peripheral” is redundant, given that there are no such structures as central nerves.8 We will thus refer to these neoplasms as nerve sheath tumors (NSTs) or MNSTs in this article.
NSTs arise from Schwann cells (schwannomas), perineurial cells (perineuriomas), or a mixture of endoneurial and epineurial cells and stromal fibroblasts (neurofibromas and MNSTs).14 NSTs can occur nearly anywhere in the body, but have been most frequently reported involving the cranial nerves, spinal nerve roots, and subcutaneous tissue, typically on the head and limbs.3,12 In the veterinary medical literature, NSTs have been described most frequently in dogs, cats, cattle, and horses, with few reports in goats, sheep, camels, pigs, non-human primates, ferrets, rodents, birds, reptiles, and fish.2,3,13,18,23,26,29,34,36–38 NSTs are exceedingly rare in pigs, with only 2 reports of a cutaneous plexiform schwannoma and a pulmonary MNST described to date.31,37 Grossly, NSTs are well-circumscribed and pale yellow or white, whereas MNSTs are locally invasive and typically have areas of necrosis or hemorrhage, as observed in our case.4,12,24
Histologically, schwannomas are well circumscribed and composed of densely cellular interwoven bundles, streams, and concentric whorls of neoplastic cells (Antoni type A pattern) or less densely cellular areas with small nuclei in a loosely arranged fibrous stroma (Antoni type B pattern). Nuclear palisades, referred to as Verocay bodies, may be observed, but are not common in veterinary species.14 Perineuriomas consist of concentric whorls of neoplastic cells around myelinated axons, whereas neurofibromas consist of interwoven bundles, streams, and concentric whorls of neoplastic cells in a basophilic myxomatous matrix. Perineuriomas and neurofibromas have lower cellularity than schwannomas. Mitotic activity is rare in schwannomas, perineuriomas, and neurofibromas. Malignant NSTs are highly invasive and densely cellular tumors and consist of pleomorphic spindle cells arranged in interweaving streams and bundles. Mitotic activity is higher than other NSTs and areas of necrosis and hemorrhage are typically present.4,12,24 The morphologic features of the neoplasm in our report, particularly the local invasion into the adjacent soft tissues and bone, as well as its high cellularity, increased cell pleomorphism, and increased mitotic activity were consistent with a diagnosis of MNST.4,12,24
IHC is often required for diagnostic confirmation of NSTs and differentiation from other spindle cell tumors. Claudin-1, laminin, vimentin, S100, Sox-10, NSE, glial fibrillary acidic protein (GFAP), neurofilament (NF), nerve growth factor receptor, Leu-7, CNPase, collagen IV, and NF200 or periaxin have been listed as useful antibodies for diagnostic confirmation of NSTs.14 In our case, neoplastic cells had different degrees of immunoreactivity for claudin-1, laminin, vimentin, S100, Sox-10, and NSE.14 Claudin proteins have structural and functional involvement in tight junctions in non-transformed epithelial, endothelial, and perineurial cells.9,11 Claudin-1 IHC is useful in the diagnosis of canine and feline meningioma and may represent a potential immunomarker to distinguish schwannomas and other NSTs in dogs, which should be immunonegative and immunopositive, respectively, for this antibody.14,19,30
Laminin IHC is frequently utilized in the diagnosis of NSTs.14,17,27 Schwann and perineurial cells produce abundant basal lamina proteins that are reactive for laminin and type IV collagen IHCs. However, given its low specificity, laminin IHC should be interpreted in conjunction with other IHC markers or special stains. Gomori reticulin stain, which can be utilized as a less specific histochemical stain for type IV collagen, revealed an extensive network of reticular fibers in our case.14
Sox-10 and S100 IHCs are supportive of a diagnosis of neural crest tumors, including melanomas, gliomas, and NSTs.22 In dogs, Sox-10 IHC has been utilized to differentiate NSTs from perivascular wall tumors.14 Only a subset of neoplastic cells exhibited immunolabeling with Sox-10 and S100 in our case, and that may reflect downregulation of Schwann cell markers with progression toward malignancy, which is consistent with reports in the human medical literature.22,28 These immunomarkers are more strongly and diffusely expressed in human schwannoma and are considered specific, but not sensitive, immunomarkers in the diagnosis of MNSTs.20,28 Although the characteristic perineurioma morphology was lacking in our case, its IHC profile could reflect perineurial cell differentiation in a MNST.21,32 Previous reports of intraneurial perineuriomas in dogs found that perineural cells were negative for S100 and positive for laminin and claudin-1, similar to our case.5,15 In MNSTs with perineurial differentiation in humans, tumors were immunoreactive for vimentin, epithelial membrane antigen (EMA), type 4 collagen, claudin-1, and laminin, but not for S100, CD34, CD68, cytokeratin, desmin, smooth muscle actin (SMA), lysozyme, and factor XIIIa.21,32 Along with claudin-1, EMA IHC is routinely used in the diagnosis of perineuriomas and MNSTs with perineurial differentiation in humans, but this antibody is not available, to date, in veterinary medicine.16,21,30,32
NSE immunoreactivity has been reported in NSTs occurring in multiple species.12,24 Similar to our case, NSE expression was focal and weak in the cutaneous plexiform schwannoma and not assessed in the pulmonary MNST reported in pigs.31,37 Although GFAP immunoreactivity can occur in schwannomas, its expression is variable and often negative, making its usefulness in the diagnosis of NSTs limited.4,12,24,33
Differentiation of NSTs and other soft tissue sarcomas, such as skeletal or smooth muscle neoplasms and melanocytic neoplasms, can be achieved via IHC for desmin, SMA, and melan A, respectively.14 These IHCs were consistently negative in our case. The lack of immunolabeling for muscle pan-actin was used to differentiate our NST from a perivascular wall tumor, given that these typically express variable immunolabeling for claudin-1, vimentin, laminin, SMA, and pan-actin.1,19
The location of the neoplasm in our report also warrants its differentiation from histiocytic sarcomas and synovial cell sarcomas. Periarticular histiocytic sarcoma occurs mainly in dogs and less commonly in cats.6 Tumors are morphologically similar to histiocytic sarcomas occurring elsewhere and express leukocyte surface markers via IHC.6 We observed no immunolabeling for histiocytic markers (CD18, CD204, and Iba1) in our case, undermining a diagnosis of histiocytic sarcoma. Human synovial cell sarcoma arises from pluripotent mesenchymal stem cells throughout the body and not from synoviocytes, as previously thought.7 In veterinary medicine, the existence of synovial cell sarcoma has been questioned, and diagnostic confirmation based on cell morphology and IHC rather than tumor location has been advocated.6,10 However, the lack of immunomarkers for synoviocytes has made the characterization of possible primary synovial tumors difficult.7 Although CD18 has been utilized to label type A synoviocytes,7 the negative immunolabeling in our case must be interpreted with caution, because CD18 antibodies have not been standardized to be used on porcine tissues.
A specific IHC marker for the definitive diagnosis of NSTs has yet to be discovered.14,31 Given multiple subtypes and variable expression of many IHC markers, NSTs can be difficult to differentiate from other soft tissue sarcomas, requiring extensive investigation with IHC, which may be cost prohibitive in a diagnostic setting.24,35 When taken as a whole, our IHC results are more consistent with a NST than with other spindle cell sarcomas. Histologic features in our case would be consistent with a grade 1 tumor category according to the canine soft tissue sarcoma grading system.25
Acknowledgments
We thank Nicole Young and Lucy Dalton (Histology Laboratory, Department of Pathology, College of Veterinary Medicine, University of Georgia), Dr. Jose Ramos-Vara (Department of Comparative Pathobiology, College of Veterinary Medicine, Purdue University), Dr. Andrew D. Miller (Department of Biomedical Sciences, Section of Anatomic Pathology, College of Veterinary Medicine, Cornell University), Dr. Josepha DeLay (Animal Health Laboratory, University of Guelph), and Dr. Leonardo Susta (Department of Pathobiology, Ontario Veterinary College, University of Guelph) for support with immunohistochemistry.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Avallone G, et al. The spectrum of canine cutaneous perivascular wall tumors: morphologic, phenotypic and clinical characterization. Vet Pathol 2007;44:607–620. [DOI] [PubMed] [Google Scholar]
- 2. Barbolt TA, Egy MA. Intestinal schwannoma in a rhesus monkey. J Comp Pathol 1990;103:471–475. [DOI] [PubMed] [Google Scholar]
- 3. Brower A, et al. Unilateral limb enlargement in a dog with a malignant peripheral nerve sheath tumor. Vet Pathol 2005;42:353–356. [DOI] [PubMed] [Google Scholar]
- 4. Chijiwa K, et al. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet Pathol 2004;41:307–318. [DOI] [PubMed] [Google Scholar]
- 5. Cornelis I, et al. Claudin-1 and glucose transporter 1 immunolabelling in a canine intraneural perineurioma. J Comp Pathol 2012;147:186–190. [DOI] [PubMed] [Google Scholar]
- 6. Craig LE, et al. The diagnosis and prognosis of synovial tumors in dogs: 35 cases. Vet Pathol 2002;39:66–73. [DOI] [PubMed] [Google Scholar]
- 7. Craig LE, Thompson KG. Tumors of joints. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:337–355. [Google Scholar]
- 8. de Lahunta A. Feline nerve sheath tumors versus feline peripheral nerve sheath tumors. Vet Pathol 2010;47:758. [DOI] [PubMed] [Google Scholar]
- 9. Escudero-Esparza A, et al. The claudin family and its role in cancer and metastasis. Front Biosci (Landmark Ed) 2011;16:1069–1083. [DOI] [PubMed] [Google Scholar]
- 10. Fairley R. Synovial tumors in dogs. Vet Pathol 2002;39:413–414. [DOI] [PubMed] [Google Scholar]
- 11. Folpe AL, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol 2002;26:1620–1626. [DOI] [PubMed] [Google Scholar]
- 12. Gaitero L, et al. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: a histopathological and immunohistochemical study. J Comp Pathol 2008;139:16–23. [DOI] [PubMed] [Google Scholar]
- 13. Helfer DH, Stevens DR. Spinal neurofibroma in a sheep. Vet Pathol 1978;15:784–786. [DOI] [PubMed] [Google Scholar]
- 14. Higgins RJ, et al. Tumors of the nervous system. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley, 2017:834–891. [Google Scholar]
- 15. Higgins RJ, et al. Canine intraneural perineurioma. Vet Pathol 2006;43:50–54. [DOI] [PubMed] [Google Scholar]
- 16. Hirose T, et al. Perineurial malignant peripheral nerve sheath tumor (MPNST): a clinicopathologic, immunohistochemical, and ultrastructural study of seven cases. Am J Surg Pathol 1998;22:1368–1378. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman A, et al. Feline periocular peripheral nerve sheath tumor: a case series. Vet Ophthalmol 2005;8:153–158. [DOI] [PubMed] [Google Scholar]
- 18. Hohsteter M, et al. Intratesticular benign peripheral nerve sheath tumour in a ferret (Mustela putorius furo). J Small Anim Pract 2012;53:63–66. [DOI] [PubMed] [Google Scholar]
- 19. Jakab C, et al. Expression of claudin-1 in canine peripheral nerve sheath tumours and perivascular wall tumours. Immunohistochemical study. Histol Histopathol 2012;27:905–917. [DOI] [PubMed] [Google Scholar]
- 20. Kang Y, et al. Diagnostic utility of SOX10 to distinguish malignant peripheral nerve sheath tumor from synovial sarcoma, including intraneural synovial sarcoma. Mod Pathol 2014;27:55–61. [DOI] [PubMed] [Google Scholar]
- 21. Karaki S, et al. Low-grade malignant perineurioma of the paravertebral column, transforming into a high-grade malignancy. Pathol Int 1999;49:820–825. [DOI] [PubMed] [Google Scholar]
- 22. Karamchandani JR, et al. Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 2012;20:445–450. [DOI] [PubMed] [Google Scholar]
- 23. Khodakaram-Tafti A, Khordadmehr M. Multicentric fibromyxoid peripheral nerve sheath tumor (multicentric schwannoma) in a dromedary camel (Camelus dromedarius): morphopathological, immunohistochemical, and electron microscopic studies. Vet Pathol 2011;48:1180–1184. [DOI] [PubMed] [Google Scholar]
- 24. Ko S, et al. Cutaneous peripheral nerve sheath tumors in 15 dogs. Korean J Vet Res 2014;54:7–12. [Google Scholar]
- 25. Kuntz CA, et al. Prognostic factors for surgical treatment of soft-tissue sarcomas in dogs: 75 cases (1986–1996). J Am Vet Med Assoc 1997;211:1147–1151. [PubMed] [Google Scholar]
- 26. Lemberger KY, et al. Multicentric benign peripheral nerve sheath tumors in two related bearded dragons, Pogona vitticeps. Vet Pathol 2005;42:507–510. [DOI] [PubMed] [Google Scholar]
- 27. Macarenco RS, et al. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med 2007;131:625–636. [DOI] [PubMed] [Google Scholar]
- 28. Pekmezci M, et al. Morphologic and immunohistochemical features of malignant peripheral nerve sheath tumors and cellular schwannomas. Mod Pathol 2015;28:187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramirez GA, et al. Malignant peripheral nerve sheath tumour (malignant schwannoma) in the diaphragm of a goat. J Comp Pathol 2007;137:137–141. [DOI] [PubMed] [Google Scholar]
- 30. Ramos-Vara JA, et al. Immunohistochemical detection of CD34, E-cadherin, claudin-1, glucose transporter 1, laminin, and protein gene product 9.5 in 28 canine and 8 feline meningiomas. Vet Pathol 2010;47:725–737. [DOI] [PubMed] [Google Scholar]
- 31. Resende TP, et al. Malignant peripheral nerve sheath tumour in a sow. Acta Vet Scand 2015;57:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenberg AS, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma”. J Cutan Pathol 2002;29:362–367. [DOI] [PubMed] [Google Scholar]
- 33. Schulman FY, et al. Feline peripheral nerve sheath tumors: histologic, immunohistochemical, and clinicopathologic correlation (59 tumors in 53 cats). Vet Pathol 2009;46:1166–1180. [DOI] [PubMed] [Google Scholar]
- 34. Sirri R, et al. Ultrasonographic and pathologic study of schwannoma in a goldfish (Carassius auratus). Vet Clin Pathol 2015;44:586–591. [DOI] [PubMed] [Google Scholar]
- 35. Sirri R, et al. Reclassification of 21 presumptive canine peripheral nerve sheath tumors (PNST) using a literature-based immunohistochemical panel. Acta Veterinaria-Beograd 2016;66:455–465. [Google Scholar]
- 36. Snyder LA, et al. Malignant peripheral nerve sheath tumor in a hamster. J Am Assoc Lab Anim Sci 2007;46:55–57. [PubMed] [Google Scholar]
- 37. Tanimoto T, Ohtsuki Y. Cutaneous plexiform schwannoma in a pig. J Comp Pathol 1993;109:231–240. [DOI] [PubMed] [Google Scholar]
- 38. Wernick MB, et al. Peripheral nerve sheath tumor in a subadult golden eagle (Aquila chrysaetos). J Avian Med Surg 2014;28:57–63. [DOI] [PubMed] [Google Scholar]