Abstract
Blood samples were collected from late-gestation ewes to determine the agreement of a point-of-care (POC) Precision Xtra meter and a standard laboratory test for β-hydroxybutyrate (BHBA). Fresh whole blood samples were immediately tested with the POC instrument, and serum samples were analyzed with a standard commercial biochemical analyzer. Ewes were classified as having ketonemia if their BHBA concentrations were ≥800 µmol/L. Scatter plots, paired t-tests, Bland–Altman limits of agreement, and Gwet AC1 tests were used to compare results. The 2 tests had very good agreement. The values between instruments were not statistically different based on paired t-tests (p = 0.312). The intercept and slope of a linear mixed model, containing the standard test results as an outcome and the POC meter results as a predictor, were 0.02 (95% CI: 0.00, 0.04) and 0.98 (95% CI: 0.96, 1.01), respectively. When the samples were classified into ketonemic classes (non-ketonemic and ketonemic) based on BHBA concentrations obtained from each test, the Gwet AC1 statistic was 0.94 (95% CI: 0.91, 0.97; p < 0.001). The ketosis classification agreed in 95% of samples. Based on the Bland–Altman plot and limits of agreement, the optimal cutoff to diagnose ketonemia with the POC meter was 1,000 µmol/L, which is 200 µmol/L higher than the laboratory BHBA medical decision limit. The Precision Xtra meter provided excellent correlation and substantial agreement with the standard laboratory technique for measuring blood BHBA in late-gestation ewes.
Keywords: β-hydroxybutyrate, diagnostic agreement, ketonemia, late-gestation ewe, point-of-care testing, Precision Xtra meter
Introduction
Ketosis is a common metabolic disorder of late-gestation ewes. Their increased energy requirement caused by the rapid growth of fetuses in the last month of pregnancy contributes to an energy deficit.3 Provision of rations that are too low in energy or over- or under-conditioning of ewes in late gestation have been reported as common risk factors for this disorder.6 Ewes carrying multiple fetuses are more susceptible to ketosis than ewes carrying just one fetus.13,17 Stress and concurrent diseases are also predisposing factors for ketosis, likely because they contribute to appetite suppression.4 Placental transfer of ketones from ewes to fetal lambs was confirmed previously.12 Although the significance of this finding is not known, low birth weight, weak and nonviable lambs, and poor milk availability are reported consequences in lambs of maternal ketosis.4
Monitoring the concentration of β-hydroxybutyrate (BHBA) in blood or urine of late-gestation ewes is an effective means of predicting clinical ketosis during the peripartum period because it is the most stable of the physiologic ketone substances.8 However, the stability of BHBA in storage is of concern, given that BHBA concentrations in human sera stored at −20°C for 40 d were different than in freshly tested sera.7 Laboratory analysis of blood BHBA concentrations is considered a reliable test, but commercial testing is time-consuming (Oetze GR. Herd-level ketosis—diagnosis and risk factors. Pre-conference seminar 7C: Dairy herd problem investigation strategies: transition cow troubleshooting, 40th Ann Conf Am Assoc Bov Pract; Sept 2007; Vancouver, BC, Canada. Available from: https://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/ketosis.pdf) and expensive at the flock-level. An accurate, less expensive animal-side test for successful disease detection would be useful.3 Point-of-care (POC) tests used for the purposes of measuring blood BHBA concentrations have been evaluated in other domestic ruminants under field conditions, and have generally been found to be accurate.5,11,19 The Precision Xtra meter (Abbott Diabetes Care, Saint-Laurent, QC, Canada) is an example of an animal-side test used for monitoring blood concentrations of glucose and BHBA in diabetic human patients and also for measuring blood BHBA concentrations in cattle.
Two previous studies have examined the usefulness of a POC test from the same manufacturer as the Precision Xtra meter to measure whole blood concentrations of glucose and ketones of sheep.15,16 The concentrations obtained using the test were strongly correlated with the results from traditional laboratory analysis; however, the target group of those studies included both late-gestation and early-lactation ewes. Our objective was to evaluate the agreement between the Precision Xtra meter and a standard laboratory serum BHBA test for measuring blood BHBA concentrations in late-gestation ewes at risk of ketosis in commercial flocks.
Materials and methods
Our study was conducted between September 2014 and May 2015 in 48 breeding groups from 34 commercial sheep flocks, drawn from ~100 flocks that comprised the sheep industry of Prince Edward Island (PEI), Canada, at that time. The lambing groups were visited 1–3 wk before the expected first lambing date. The number of bred ewes comprising each lambing group ranged from 12 to 172. Prior consent for the study was obtained for all participating producers, and the study protocols were reviewed and approved by the University of Prince Edward Island (UPEI) Animal Care Committee.
In each group, 8 late-gestation ewes were selected for sample collection through convenience sampling. Blood samples were collected from the jugular vein into serum separator tubes containing a silicone plug and silica clot activator (Vacutainer, BD Diagnostics, Quebec, QC, Canada) without any collection site preparation. A drop of unclotted blood was immediately taken from the tube using the Precision Xtra test strip and tested at ambient barn temperature (–20 to 27°C; approximate ambient temperatures at the time of sampling were obtained from a data repository: http://www.timeanddate.com) for BHBA concentration using the POC meter. The current change created from conversion of BHBA to acetoacetate is electrochemically measured by the device. This device displays a one decimal point result for BHBA concentration in mmol/L, with a reported assay range of 0–6,000 µmol/L. Clotted blood samples were transported upright on ice to the laboratory, which took a maximum of 6 h after collection. Once the samples arrived at the laboratory, serum was separated from the clot by centrifuging at 595 × g for 10 min at room temperature. Serum samples were stored at −20°C until laboratory analysis could be performed. Samples were transported by a courier service in foam boxes with ice-packs to the Animal Health Laboratory, University of Guelph, Guelph, Ontario. The samples were thawed at room temperature, mixed by inversion, then checked for fibrinogen clots and/or gel and centrifuged before analysis. The degree of hemolysis of serum samples was classified as negative, mild, or moderate. Serum BHBA concentration was determined using a commercial biochemical analyzer (Cobas 6000 c501, Roche Diagnostics, Laval, QC, Canada), which is a photometric system–based machine using Ranbut enzymatic reagents (Randox Laboratory, ESBE Scientific, Markham, ON, Canada). Results were reported in μmol/L. The diagnosticians performing each test were blind to the results of the other test.
Statistical analyses were performed (Stata Statistical Software: Release 13, StataCorp, College Station, TX). Blood BHBA concentrations obtained from the laboratory analysis and the POC analyzer were first evaluated on their continuous scale and compared using descriptive statistics. The difference between the values from the 2 tests was calculated: the result from the POC meter (in mmol/L) minus the result from the laboratory test (in mmol/L), then converted to SI units (µmol/L). The percent difference was calculated by dividing the difference by the laboratory concentration and multiplying by 100. Pearson correlation coefficient between percent difference and approximate ambient temperature at the time of sampling was calculated to determine if ambient temperature had an effect on the percent difference. Limits of agreement (95%) were calculated and plotted, along with the differences and the means of each sample, to evaluate agreement of the 2 tests.2
A paired t-test was used to determine if a significant difference existed between the results of the 2 tests. A linear mixed model, with flock and lambing group as random effects to control for the effects of clustering of ewes within lambing groups and lambing groups within flocks, was also fitted to determine the intercept and slope (and 95% confidence intervals [CIs]) of the strength of association and significance of the relationship between the POC instrument results (predictor variable) and the laboratory test results (outcome variable).
To assist with the initial interpretation of results, ewes were classified as having a ketonemic state if their BHBA concentrations were ≥800 µmol/L1,15,16 for each test method. Inter-rater agreement for ketotic classification was performed using the Gwet AC1 statistic.9 For all statistical analyses, p ≤ 0.05 was considered statistically significant.
Results
A total of 34 flocks were enrolled in the study. Mean flock size was 93 (range: 13–250). Three flocks (8.8%) had only 1 breed: Hampshire, Suffolk, or Black Welsh Mountain. The other 31 flocks incorporated ≥2 of the following breeds: Suffolk, Dorset, Canadian Arcott, Rideau, Texel, Cheviot, Hampshire, Ile de France, Border Leicester, Corriedale, Finnsheep, Romanov, Shetland, and Southdown. All of the producers typically sold market lambs. Fleece, breeding stock, milk, and hide were also cited as other meaningful sources of income on 38%, 24%, 9%, and 3% of farms, respectively.
Blood samples were collected from 384 ewes. The mean serum storage time prior to commercial laboratory testing was 251 d (SD = 62 d), with 24 samples stored for >1 y. Sixteen samples from 2 lambing groups could not be analyzed with the Precision Xtra meter because of the low ambient temperature (≤0°C). Moreover, 1 sample clotted before the Precision Xtra test was performed, and there was insufficient serum for laboratory testing of 2 samples. In total, 366 samples were tested with both the Precision Xtra meter and the commercial laboratory assay.
Negative, mild, and moderate hemolysis was noted in 310 (81%), 69 (18%), and 3 (0.8%) serum samples, respectively. When the 65 hemolyzed samples were included in the paired t-test analysis, the 2 tests were significantly different (p = 0.02), with higher serum values with the standard laboratory test compared to the Precision Xtra meter results. A total of 301 paired values were available for further comparison after exclusion of the mildly and moderately hemolyzed serum samples.
Scatter plots (Fig. 1) demonstrate a high concordance between the 2 methodologies; all points are close to the reduced major axis and the line of perfect concordance, with these 2 lines overlapping when BHBA concentrations were identical in 131 (43%) samples, higher in serum results in 99 (33%) samples, and higher with the POC analyzer in 71 (24%) samples.
Figure 1.
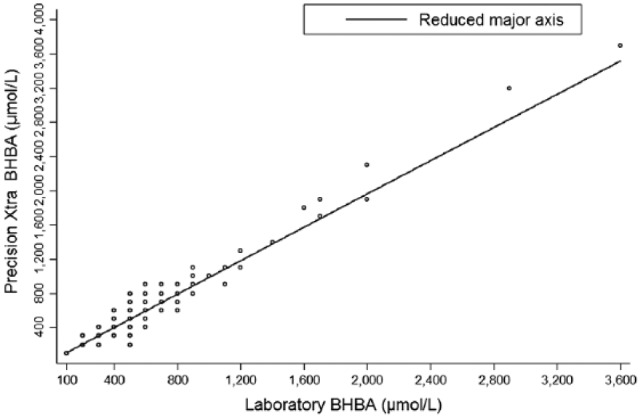
Scatter plots of blood β-hydroxybutyrate concentrations (µmol/L) in 301 non-hemolyzed blood samples of late-gestation ewes in Prince Edward Island, between September 2014 and August 2015, comparing the standard laboratory test and Precision Xtra meter.
Overall results were very similar (Table 1). Two samples had substantial differences (>300 µmol/L) between test results (one of which was hemolyzed) as shown by the minimum and maximum differences and percent differences. Twenty-eight (9%) samples had a difference of >100 µmol/L. The Pearson correlation coefficient between percent difference and approximate ambient temperature was low but significant (r = −0.12, p = 0.044).
Table 1.
β-hydroxybutyrate concentrations in 301 non-hemolyzed blood samples and 65 hemolyzed blood samples of late-gestation ewes in Prince Edward Island, between September 2014 and August 2015, measured in serum with a commercial analyzer and in whole blood with a point-of-care instrument (Precision Xtra meter).
| Commercial analyzer (µmol/L) | Precision Xtra meter (µmol/L) | Absolute difference* (µmol/L) | Percent difference† (%) | |
|---|---|---|---|---|
| Non-hemolyzed samples (n = 301) | ||||
| Mean (SD) | 560 (490) | 550 (490) | −10 (100) | −1.44 (19.6) |
| Median (interquartile range) | 500 (400–600) | 400 (300–600) | 0 (−10 to 0) | 0.0 (−20.0 to 0.0) |
| Range | 100–6,700 | 100–6,100 | −600 to 300 | −60.0 to 60.0 |
| Hemolyzed samples (n = 65) | ||||
| Mean (SD) | 520 (310) | 480 (290) | 400 (130) | −8.11 (19.0) |
| Median (interquartile range) | 400 (400–600) | 400 (300–600) | 0 (−100 to 0) | 0.0 (−25.0 to 0.0) |
| Range | 200–2,500 | 200–1,800 | −700 to 300 | −33.3 to 50.0 |
Absolute difference = Precision Xtra − laboratory test.
Percent difference = (Precision Xtra − laboratory test)/laboratory test × 100%.
Bias was not evident in a Bland–Altman plot (Fig. 2) in the 301 non-hemolyzed samples. The 95% limits of agreement were −200 to +200 µmol/L. There was one observation that clearly deviated from other observations; the laboratory serum concentration of this sample was substantially higher than the concentration obtained by the POC meter. However, this sample had a high BHBA concentration on both tests, and would still be categorized as ketotic using either method. The limits of agreement for the 65 hemolyzed serum samples was worse than for the 301 non-hemolyzed samples; the range of the limits of agreement was wider (–290 to +210 µmol/L vs. −200 to +200 µmol/L). The paired t-test for the non-hemolyzed samples was not significantly different (p = 0.312, n = 301). The intercept and slope of the linear mixed model were 0.02 (95% CI: 0.00, 0.04) and 0.98 (95% CI: 0.96, 1.01), respectively.
Figure 2.
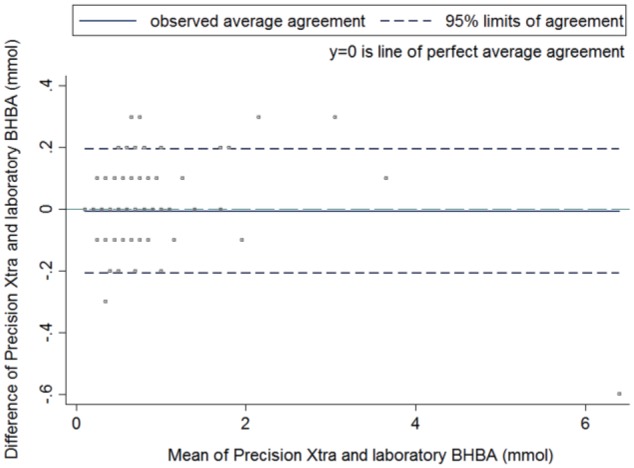
Bland–Altman plot of differences between β-hydroxybutyrate concentrations (µmol/L) from the Precision Xtra meter and standard laboratory test against their mean β-hydroxybutyrate concentrations in 301 non-hemolyzed blood samples of late-gestation ewes in Prince Edward Island, between September 2014 and August 2015. The middle solid line displays the mean; the 2 dashed lines display the 95% limits of agreement; the middle blue dashed line displays the perfect average agreement.
Based on results of the laboratory test, most ewes were classified as normal. Ketonemic ewes were identified in many of the lambing groups, with 14 (29%) lambing groups having at least 1 ketonemic ewe. The number of affected ewes in groups ranged from 0 to 5 of 8 (0–62%). The mean number of ketotic ewes in affected groups was 1.6.
The ketosis classification was the same in 95% of the samples; 5% of samples were categorized differently (Table 2). The Gwet AC1 statistic was 0.94 (95% CI: 0.91, 0.97; p < 0.001). This demonstrates good classification reliability between the 2 tests with our results, whereby >85% were normal on both tests.
Table 2.
Ketosis classification based on the standard laboratory test and Precision Xtra meter for β-hydroxybutyrate in 301 blood samples of late-gestation ewes in Prince Edward Island, between September 2014 and August 2015.
| Precision Xtra meter | Laboratory test |
Total | |
|---|---|---|---|
| Normal < 800 µmol/L | Ketosis ≥ 800 µmol/L | ||
| Normal < 800 µmol/L | 261 | 15 | 276 |
| Ketosis ≥ 800 µmol/L | 0 | 25 | 25 |
| Total | 261 | 40 | 301 |
Discussion
We did not include hemolyzed samples in all inferential statistics because the 2 tests were significantly different based on the paired t-test, as expected given that the laboratory test was a photometric test.
For successful treatment and prevention of ketosis, early and accurate detection of elevated ketones is required.3 Although the physiologic upper threshold for ketonemia in sheep has been suggested to be 800 µmol/L for laboratory testing,1,6 the Bland–Altman plot in our study had a limit of agreement of −200 to +200 µmol/L; therefore, the physiologic upper threshold in this POC meter should be 1,000 µmol/L. Results of 600–1,000 µmol/L should be considered inconclusive. The mean difference (–10 µmol/L) between the 2 results in our study was lower than that found in a previous study (–150 µmol/L), which reported using an animal-side test from the same manufacturer of the Precision Xtra.16 The highest between-method difference was >1,000 µmol/L in that study, whereas it was only 600 µmol/L in our study.
In a prior study, BHBA concentrations in serum samples stored at −20°C were stable for at least 40 d.7 In our study, all samples were stored for >40 d, and 24 samples were stored for >1 y. Based on the good agreement between the 2 tests in our study, the stability of serum BHBA in samples stored at −20°C might be >1 y. Further research is warranted to confirm this hypothesis.
There was a sample in our study in which a large between-method difference was noted (600 µmol/L, 9% of the laboratory test value); this sample had a high mean blood BHBA concentration (6,400 µmol/L). However, this sample was classified as ketosis (≥800 µmol/L) regardless of measurement techniques, and the difference would not have clinical impact.
A different POC instrument model by the same manufacturer as that used in our study underestimated results when it was used in dairy cows10 and in sheep elsewhere.16 However this was not apparent in our study; the intercept of the linear mixed model was not significantly different from 0 (p = 0.081). In addition, the slope of the model was not significantly different from 1.0. These results demonstrate the similarity of the continuous scale results from the 2 tests when using non-hemolyzed serum samples.
The 2 tests had good agreement in classifying ketosis based on the Gwet AC1 statistic, although there were some false positives. Of 15 false-positive samples reported by the POC meter, the greatest difference between the POC instrument and reference laboratory value was 300 µmol/L. This finding indicates that even when categorical results were not in agreement, the difference in concentration between the 2 tests was still small.
In a 2013 study, the performance of an animal-side test developed to measure milk BHBA concentration was not affected by temperature.18 However, the recommended temperature range for operating the Precision Xtra meter is 10–50°C, and using the device at very low temperatures resulted in an error reading in our study. In most cases, this problem was overcome by storing the device and test strips in coverall pockets. However, variation because of ambient temperature may have occurred; therefore, the difference between the field and laboratory test values (i.e., percent difference using the laboratory concentration as a denominator) compared to the recorded daytime temperature at the time of sampling were examined. We found that percent difference was significantly and negatively correlated to daytime temperature. Therefore, ambient temperature when conducting the meter test should be further investigated and potentially standardized.
Because quality assurance and quality control of the POC meter were not performed, results of our study should be interpreted with caution. For example, control solution testing was not performed throughout the study, and within-run precision and between-run precision were not determined for both tests used in the study. In addition, sample type varied, given that whole blood was used for the POC meter and serum was used for the standard laboratory test. In another study, a conversion factor was required when glucose concentration was measured in different types of samples containing different water contents (Gerber KL, Freeman KP. ASVCP guidelines: Quality assurance for portable blood glucose meter (glucometer) use in veterinary medicine. Vol. 1. Am Soc Vet Clin Pathol 2015. Available from: https://cdn.ymaws.com/www.asvcp.org/resource/resmgr/QALS/Other_Publications/ASVCP_Guidelines_for_Glucose.pdf).
In addition to good agreement with a standard laboratory test, there are a number of additional benefits of the POC meter. Only 1.5 µL of blood is required for testing. A drop of blood from the ear vein was sufficient for sample analysis using a similar device in an ovine study.16 The cost of the BHBA animal-side test is also less than the laboratory test,15 and the cost of collection and transport of the sample to a laboratory is eliminated. Considering that less equipment is required to collect a sample for the POC instrument, the total cost of this instrument is lower. A Precision Xtra meter and a ketone test strip were available online (http://www.google.ca) at ~US$38 and ~US$4, respectively, in 2017. Some distributors offered a free test meter for purchase of a specified number of the test strips. Additionally, waste produced by performing the field test is minimal. The device provides a result within 10 s. Although short turnaround time analyzers have been available at laboratories, time for sample transportation is unavoidable for a laboratory test. Perhaps most importantly, treatment and husbandry and/or management changes can be instituted immediately after measuring BHBA concentration, thereby reducing the risk of ewes developing (or worsening) clinical ketosis or pregnancy toxemia while waiting for a laboratory result.15
Animal-side tests have been used for measuring BHBA concentrations in the urine and milk of cows; however, the results have been less accurate when compared to blood analysis.10,14 Thus, evaluation of the POC meter in sheep urine or milk samples is worth studying.
Given the good agreement with a standard laboratory test and the practical advantages of the Precision Xtra meter, the animal-side test is reliable for use on the farm for real-time determination of BHBA concentrations in late-gestation ewes. Further research is warranted, such as field trials to determine the impact of management changes (e.g., nutrition) on morbidity and mortality of ewes and their fetuses pre- or postpartum when diagnosing ketosis with this animal-side test.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638718819688 for Evaluation of the Precision Xtra meter for monitoring blood β-hydroxybutyrate concentrations in late-gestation ewes by Niorn Ratanapob, John VanLeeuwen, Shawn McKenna, Maureen Wichtel, Juan C. Rodriguez-Lecompte, Paula Menzies and Jeffrey Wichtel in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank PEI sheep producers participating in this study, Jane Saunders-Jewell, our project technician, for her assistance with sample collection, and Theresa Andrews for her assistance with providing medical supplies.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was a component of the Increasing Perinatal Lamb Survival in Island Sheep Flocks project, which was funded by the Province of PEI Agriculture Research and Innovation Program (Growing Forward).
ORCID iDs: Niorn Ratanapob  https://orcid.org/0000-0002-0937-5971
https://orcid.org/0000-0002-0937-5971
Maureen Wichtel  https://orcid.org/0000-0002-6441-4400
https://orcid.org/0000-0002-6441-4400
References
- 1. Andrews A. Pregnancy toxemia in the ewe. In Pract 1997;19:306–312. [Google Scholar]
- 2. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;8:307–310. [PubMed] [Google Scholar]
- 3. Brozos C, et al. Treatment and control of peri-parturient metabolic diseases: pregnancy toxemia, hypocalcemia, hypomagnesemia. Vet Clin North Am Food Anim Pract 2011;27:105–113. [DOI] [PubMed] [Google Scholar]
- 4. Cal-Pereyra L, et al. Toxemia de la gestación en ovejas: Revisión [Ewe pregnancy toxemia. Review]. Revista Mexicana de Ciencias Pecuarias 2012;3:247–264. Spanish. [Google Scholar]
- 5. Dore V, et al. Evaluation of the accuracy of an electronic on-farm test to quantify blood β-hydroxybutyrate concentration in dairy goats. J Dairy Sci 2013;96:4505–4507. [DOI] [PubMed] [Google Scholar]
- 6. Edmondson MA, et al. Theriogenology of sheep and goats. In: Pugh DG, Baird AN, eds. Sheep and Goat Medicine. 2nd ed. Maryland Heights, MO: Elsevier, 2012:150–230. [Google Scholar]
- 7. Fritzsche I, et al. Stability of ketone bodies in serum in dependence on storage time and storage temperature. Clin Lab 2001;47:399–403. [PubMed] [Google Scholar]
- 8. Fthenakis GC, et al. Health management of ewes during pregnancy. Anim Reprod Sci 2012;130:198–212. [DOI] [PubMed] [Google Scholar]
- 9. Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol 2008;61:29–48. [DOI] [PubMed] [Google Scholar]
- 10. Iwersen M, et al. Evaluation of an electronic cowside test to detect subclinical ketosis in dairy cows. J Dairy Sci 2009;92:2618–2624. [DOI] [PubMed] [Google Scholar]
- 11. Kanz P, et al. Suitability of capillary blood obtained by a minimally invasive lancet technique to detect subclinical ketosis in dairy cows by using 3 different electronic hand-held devices. J Dairy Sci 2015;98:6108–6118. [DOI] [PubMed] [Google Scholar]
- 12. Miodovnik M, et al. Effect of maternal ketoacidemia on the pregnant ewe and the fetus. Am J Obstet Gynecol 1982;144:585–593. [DOI] [PubMed] [Google Scholar]
- 13. Moallem U, et al. Plasma concentrations of key metabolites and insulin in late-pregnant ewes carrying 1 to 5 fetuses. J Anim Sci 2012;90:318–324. [DOI] [PubMed] [Google Scholar]
- 14. Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet Clin North Am Food Anim Pract 2004;20:651–674. [DOI] [PubMed] [Google Scholar]
- 15. Panousis N, et al. Evaluation of precision Xceed meter for on-site monitoring of blood β-hydroxybutyric acid and glucose concentrations in dairy sheep. Res Vet Sci 2012;93:435–439. [DOI] [PubMed] [Google Scholar]
- 16. Pichler M, et al. Thresholds of whole blood β-hydroxybutyrate and glucose concentrations measured with an electronic hand-held device to identify ovine ketonemia. J Dairy Sci 2014;97:1388–1399. [DOI] [PubMed] [Google Scholar]
- 17. Ratanapob N, et al. The association of serum β-hydroxybutyrate concentration with fetal number and health indicators in late-gestation ewes in commercial meat flocks in Prince Edward Island. Prev Vet Med 2018;154:18–22. [DOI] [PubMed] [Google Scholar]
- 18. Shire J, et al. The effect of temperature on performance of milk ketone test strips. J Dairy Sci 2013;96:1677–1680. [DOI] [PubMed] [Google Scholar]
- 19. Tatone EH, et al. Evaluation of a handheld device for measurement of β-hydroxybutyrate concentration to identify prepartum dairy cattle at risk of developing postpartum hyperketonemia. J Am Vet Med Assoc 2015;246:1112–1117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638718819688 for Evaluation of the Precision Xtra meter for monitoring blood β-hydroxybutyrate concentrations in late-gestation ewes by Niorn Ratanapob, John VanLeeuwen, Shawn McKenna, Maureen Wichtel, Juan C. Rodriguez-Lecompte, Paula Menzies and Jeffrey Wichtel in Journal of Veterinary Diagnostic Investigation


