Abstract
We conducted a nested, case-control study of pre-weaned dairy calves (n = 477; 4 California dairy farms) to assess the association between bovine respiratory disease (BRD) and hematologic biomarkers, including plasma haptoglobin (Hp) and plasma bactericide (PB). At each location, heifer or bull dairy calves were observed 2–4 times per week until confirmed as BRD-positive using parallel interpretation of thoracic ultrasound examination and auscultation. In addition, control calves were enrolled after being confirmed as BRD-negative using ultrasound and auscultation. Complete blood counts (CBC), PB, and Hp concentrations were measured. Hp values were higher in calves with confirmed BRD than in controls (p < 0.01). The area under the curve (AUC) for the various biomarkers was obtained from the corresponding receiver operating characteristic curves. The AUC for Hp was 0.68, a value greater than those for PB or the remaining CBC parameters, indicating that Hp may be the most useful biomarker of BRD in pre-weaned dairy calves. The cutoff value for Hp was 0.195 g/L.
Keywords: Bovine respiratory disease, calves, haptoglobin, plasma bactericide
Introduction
Bovine respiratory disease (BRD) is a complex clinical syndrome that includes pneumonia, is one of the leading natural causes of death in U.S. beef and dairy cattle, and results in an annual loss of >1,000,000 animals and financial losses >$700 million.26 The disease etiology includes a multifactorial interaction of environment, including stressors, animal susceptibility, and pathogens. In the United States, BRD is responsible for 22% of all pre-weaning deaths and is the most common cause of death in post-weaning dairy heifers (https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_dr_PartI.pdf). Control and prevention of BRD is difficult given the disease’s multiple etiologies and a complex interaction among risk factors. Conventionally, BRD diagnosis is based on clinical signs, and varies among premises, calf caretakers, producers, and herd veterinarians leading to a proportion of false-negative and false-positive diagnoses. Such diagnostic inaccuracies lead to progression of disease, misuse of antimicrobials, production losses, and suboptimal animal welfare outcomes. As of 2018, 2 validated scoring systems exist for on-farm diagnosis of BRD in pre-weaning dairy calves, the California (CA) and the Wisconsin scoring systems.18 Both systems can be used to objectively identify BRD-positive calves for treatment with similar sensitivity and specificity. However, traditional BRD diagnostic methods and scoring systems are imperfect at predicting underlying respiratory system lesions. Furthermore, use of nasal swabs, either superficial nasal or deep nasopharyngeal, with aerobic bacterial and/or Mycoplasma cultures, had lower specificity than either scoring system.18
Acute-phase proteins contribute to restoring homeostasis and limiting microbial growth in animals subjected to infection, inflammation, or stress.8,21 Haptoglobin (Hp) is a major acute-phase protein in cattle with a significant positive association with BRD in calves.10,28 Hp is generated by hepatocytes13 in response to pro-inflammatory cytokines, particularly interleukin 6 (IL-6). Glucocorticoids may increase the responsiveness of hepatocytes to IL-6 stimulation.10 Under pro-oxidative and pro-inflammatory stress, Hp can be considered a scavenger protein through its binding capacity.22 Hp eliminates metabolites (e.g., free hemoglobin) released from cellular degradation, helping to prevent oxidative tissue damage because of its peroxidase activity. Hp also helps recycle heme residues for further metabolic processes,12 rather than be utilized by pathogens. In addition, elevated blood Hp concentration is used in detecting disease given its long-term response to infection.2 Previous studies considered Hp as a sign of inflammation and disease status in calves.14,25 Furthermore, elevated plasma Hp concentrations following parturition in multiparous Holstein cows were associated with leukocyte responses.23 More specifically, total neutrophil count was reduced, neutrophil surface expression of L-selectin was increased, and percentages of neutrophils needed to produce an oxidative burst were greatest in cows with greater Hp concentrations. Furthermore, Hp concentration can be significantly decreased in calves receiving antibiotic treatment.27 Acute-phase proteins also influence the effectiveness of humoral immunity19; therefore, we examined humoral bactericidal activity in calves as an additional measure of immune status. The objective of our study was to identify biomarkers to be used in BRD detection in pre-weaned calves, including Hp.
Materials and methods
Our study was approved by the University of California, Davis Institutional Animal Care and Use Committee (protocol 17496, approved March 21, 2013). Calves were enrolled as part of a nested case-control study designed to estimate the diagnostic accuracy of CA BRD scoring systems described elsewhere.18 Our study was conducted on 5 premises (A–E) located in the central San Joaquin Valley of California between April and September 2013. Of the 5 premises, 3 were dairy farms that raised their own calves, the fourth was a calf ranch that raised calves from 3 different source dairy farms, and the fifth was a calf ranch that raised calves from multiple dairy farms in California and Arizona. All hutch calves on the participating locations were eligible for enrollment in the study, unless they were vaccinated in the previous 14 d or treated with antibiotics in the previous 10 d. A total of 1,083 calves were eligible for enrollment and visually evaluated each morning at the start of each visit, which occurred 2–4 times a week. On any visit, calves with signs of BRD, including depression, dehydration or sunken eyes, cough, and abnormal respiration, were considered BRD-suspect calves and marked for further examination before being enrolled in the study as cases. Further examination started with the CA BRD scoring system, followed by auscultation (AU) and thoracic ultrasound (US) exam.18 Thoracic US and AU exam were conducted as described in the original nested case-control study report.18 Hence, a calf was confirmed as a BRD case if: 1) identified as suspect for BRD and had depression, dehydration or sunken eyes, cough, and abnormal respiration; and 2) confirmation of abnormal findings on either thoracic US or AU, or both. In addition, ~12 calves from the same herd were randomly selected at each visit and marked for further examination to confirm their BRD-negative status and enrolled as controls. A calf was confirmed as a control if it showed normal findings on both thoracic US and AU with no history of treatment for BRD based on the farm-specific BRD treatment protocols.
From each enrolled calf, 9 mL of blood were collected using vacutainers (6 mL in a heparin tube, and 3 mL in an EDTA-coated tube) via jugular venipuncture. A complete blood count (CBC) including an automated white blood cell (WBC) differential count was performed on EDTA samples (HEMAVET 950 Veterinary 5 part WBC hematology system set with blood cell parameters for the bovine species, Drew Scientific, Oxford, CT), and the neutrophil-to-lymphocyte (N:L) ratio was calculated from the CBC leukocyte differential count. The bactericidal activity of the plasma bactericide (Escherichia coli, % colony-forming units [CFU] killed) against a live culture of bacteria was measured using methods described previously.3 Briefly, blood was incubated with E. coli (E. coli 8739, Microbiologics, St. Cloud, MN) at a 1:20 ratio for 10 min, and then cultured on trypticase soy agar plates (Millipore Sigma, St. Louis, MO). The number of CFU was estimated 24 h after incubation, and the percent of CFU eliminated by plasma blood was calculated using controls (200 CFU in 50 µL of RPMI medium, Invitrogen, Carlsbad, CA).
Hp concentrations were measured following a colorimetric method based on peroxidase activity.7 Briefly, a plasma sample with a known high Hp concentration from a previous study30 was used for preparation of a standard curve by serial dilution. In a 16 × 100 mm borosilicate tube containing 10 µL of plasma, 7.5 mL of O-dianisidine solution (0.6 g/L) and 25 µL of a hemoglobin solution (0.3 g/L) were added and immediately incubated for 45 min in a bead bath at 37°C. After incubation, 100 µL of freshly prepared 156 mM hydrogen peroxide solution were added to each tube, finishing the assay with a 1-h final incubation at room temperature. After the final incubation, 200 µL of each tube were transferred into the corresponding well in a microplate and optical density immediately estimated at 450 nm using a microplate reader.
Statistical analysis
Mixed effects logistic regression
Univariate mixed-effects logistic regression models with dairy farm as a random intercept were specified for the association between each CBC parameter, N:L ratio, Hp and PB concentrations, and the outcome BRD. A calf’s BRD status was determined based on parallel interpretation of thoracic US and AU. The Box–Tidwell test was used to check variables for the linearity of log odds assumption.6 Variables that violated the assumption were categorized based on their frequency distribution to create approximately equal size categories. Subsequently, nonsignificant levels of categorical variables were collapsed for efficiency. Models for each hematologic parameter were then adjusted for calf age, sex, and breed and used to identify parameters for evaluation of their accuracy to identify BRD status. In addition, red cell distribution width, mean corpuscular volume (MCV), mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were contrasted between BRD cases and non-BRD controls.
A final model for the association between hematologic parameters and BRD was built using manual backward variable selection. A lower Akaike information criterion (AIC) estimate was used to select the final model from between models with absolute CBC counts and N:L ratio in the case of neutrophils and lymphocytes. Similar models with random intercept for dairy farm were used to predict the most important biomarker(s) as explained by calf age, breed, BRD, and significant interaction terms, if any. Significant differences were declared at p ⩽ 0.05. Mixed-effects models were specified using Stata v.13.1 (Stata Corp, College Station, TX).
BRD detection accuracy of hematologic parameters
All of the hematologic parameters measured were subjected to receiver operator characteristic (ROC) curve analyses,9 considering Hp as ROC contrast reference (SAS, SAS Institute, Cary, NC; SigmaPlot, Systat Software, San Jose, CA). Area under the curve (AUC) is a test accuracy measure that ranges from 0 to 1, with test values of 0.5 interpretable as of no diagnostic value and 1 as perfect accuracy. Cutoff values were determined as the point where the Youden index was maximum.11 Likelihood ratio positive (LR+) and likelihood ratio negative (LR–) were estimated for each parameter’s cutoff selected using the formulas:
Results
A total of 536 pre-weaned calves 15–137 d old were enrolled from study herds. For technical reasons, all but 40 samples from herd E were tested and hence a decision was made to exclude all of the samples from that herd to avoid potential selection bias. As a result, we used samples from 477 calves on premises A–D in our study.
Of the 477 calves at the study locations, 194 BRD cases were identified using thoracic US and/or AU during the study period. Subsequently, 283 randomly chosen calves served as a control group after being confirmed as BRD-negative using thoracic US and AU. Within BRD cases, 49 calves had normal AU but abnormal US, 43 calves had normal US but abnormal AU, 102 had both abnormal AU and abnormal US.
Basophil count violated the linearity of log odds and hence was categorized into 6 categories (0–0.01 as the Reference; 0.011–0.020; 0.021–0.030; 0.031–0.040; 0.041–0.050; and >0.050 × 109/L). The remaining hematologic parameters did not violate the linearity of log odds assumption and hence remained as continuous variables. Univariate models identified significant biomarkers including lymphocytes (absolute cell count, ×109/L; odds ratio, OR = 0.787, p = 0.003), eosinophil (absolute cell count, ×109/L; OR = 1.998, p = 0.031), basophils (absolute cell count, × 109/L: >0.050 × 109/L vs ⩽0.050 × 109/L; OR = 2.684, p = 0.002), neutrophils (absolute cell count, ×109/L; OR = 1.151, p = 0.008), Hp (g/L; OR = 1.002, p < 0.001), and MCV (p = 0.036). MCV was significantly associated with lower odds of BRD (OR = 0.887, p = 0.036). The remaining hematologic parameters and PB were not significant (p > 0.05). Estimates from uni-variate models for Hp, neutrophils, lymphocytes, neutrophil:lymphocyte ratio, and basophils remained significant after adjusting for calf age, breed, and sex (Table 1). The final multivariable mixed-effects logistic regression model for BRD in pre-weaned calves showed a positive association between Hp and BRD and a negative association between lymphocyte count and BRD (Table 2).
Table 1.
Results of univariate mixed-effects logistic regression models for the association between hematologic parameters and bovine respiratory disease in pre-weaned calves in 4 California herds using parallel interpretation of thoracic ultrasound and auscultation. Each model was adjusted for calf age, sex, and breed.
| Variable/model | Unit | Coefficient | SE | OR | OR (95% confidence limit) | p | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Haptoglobin | g/L | 2.17 | <0.49 | 8.78 | 3.33 | 23.13 | <0.01 |
| Neutrophils | ×109/L | 0.14 | 0.06 | 1.16 | 1.04 | 1.29 | 0.01 |
| Lymphocytes | ×109/L | −0.35 | 0.09 | 0.71 | 0.59 | 0.85 | <0.01 |
| Neutrophil:lymphocyte ratio | Counts | 0.69 | 0.20 | 1.99 | 1.35 | 2.94 | 0.01 |
| Basophils | ×109/L | ||||||
| 0–50 | Reference* | ||||||
| >50 | 0.95 | 0.34 | 2.57 | 1.31 | 5.04 | 0.01 | |
OR = odds ratio; SE = standard error.
The comparison group for the categorical variable basophil contrasts the basophil counts greater than the reference, which is <50 × 109/L.
Table 2.
Results of final mixed-effects logistic regression model for the association between hematologic parameters and bovine respiratory disease in pre-weaned calves in 4 California herds using parallel interpretation of thoracic ultrasound and auscultation.
| Variable | Coefficient | SE | OR | OR (95% confidence limit) |
p | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Haptoglobin (g/L) | 2.02 | 0.50 | 7.54 | 2.84 | 19.98 | <0.01 |
| Lymphocytes (×109/L) | −0.31 | 0.01 | 0.73 | 0.61 | 0.88 | <0.01 |
| Age (d) | 0.02 | 0.01 | 1.02 | 1.00 | 1.04 | 0.07 |
| Sex | ||||||
| Female | Reference* | |||||
| Male | 0.67 | 0.28 | 1.96 | 1.13 | 3.38 | 0.02 |
| Breed | ||||||
| Holstein | Reference | |||||
| Jersey | 0.59 | 0.32 | 1.80 | 0.97 | 3.35 | 0.06 |
OR = odds ratio; SE = standard error.
The comparison group for categorical variables. For calf sex, female was the comparison group; for breed, Holstein was the comparison group.
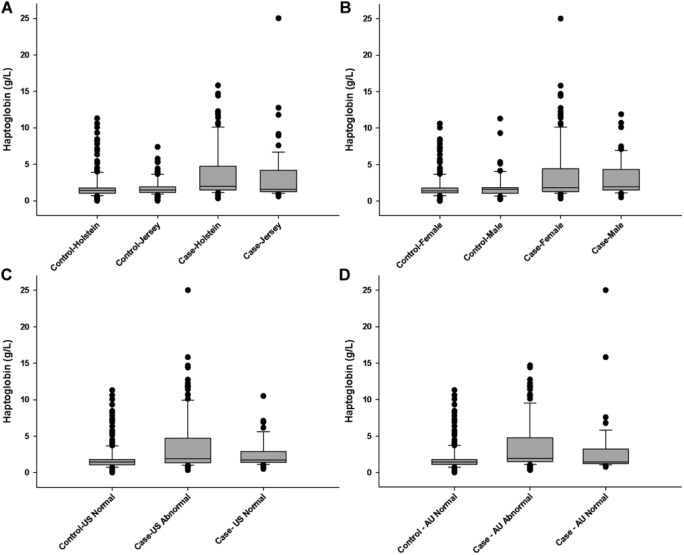
Hp had an AUC equal to 0.676 (Fig. 1). The remaining hematologic parameters had AUC values <0.60 and hence were not deemed sufficiently useful for BRD case identification (Table 3). The cutoff value of 0.195 g/L determined for Hp (Fig. 2) corresponds to a LR+ of 2.52 (95% confidence interval [CI]: 1.9, 3.4) and a LR– of 0.68 (95% CI: 0.6, 0.8). The LR+ for Hp can be interpreted as an increased Hp concentration beyond the cut-off is 2.5 times more likely in BRD cases than in non-BRD calves. There was greater variability in Hp concentration in BRD cases than in control calves (Fig. 3). In addition, Hp concentrations varied greatly by farm location (p = 0.006; Fig. 4).
Figure 1.
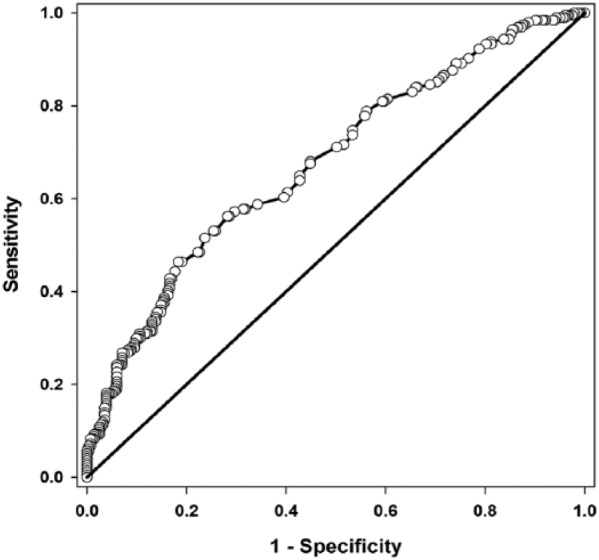
Receiver operating characteristics curve for haptoglobin concentration in blood for controls and cases of bovine respiratory disease in pre-weaned dairy calves. Area under the curve = 0.676; p < 0.01 for haptoglobin concentration.
Table 3.
Area under the curve (AUC), cutoff values, sensitivity and specificity percentages, likelihood ratios, and predictive values for complete blood count parameters, haptoglobin concentrations, and plasma bactericide for controls and cases of bovine respiratory disease in pre-weaned dairy calves diagnosed using parallel interpretation of thoracic ultrasound and auscultation. Only parameters with AUC ⩾0.60 were considered in the analyses.
| Hematologic parameter | Unit | AUC | Cutoff | Se (%) | Sp (%) | LR+ | LR– | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| Haptoglobin | g/L | 0.677 | 0.195 | 46.4 (39.2–53.7) | 81.6 (76.6–86.0) | 2.53 | 0.66 | 71.6 | 60.4 |
| Basophils | ×109/L | 0.599 | 0.03 | 46.9 (39.7–54.2) | 67.0 (64.3–75.3) | 1.56 | 0.76 | 61.0 | 56.9 |
| Neutrophil:lymphocyte ratio | Absolute counts | 0.583 | 1.19 | 38.7 (31.8–45.9) | 80.6 (75.5–85.0) | 1.99 | 0.76 | 66.6 | 56.8 |
| Neutrophil | ×109/L | 0.556 | 4.6 | 36.1 (29.3–43.3) | 79.9 (74.7–84.4) | 1.79 | 0.8 | 64.2 | 55.5 |
| MCHC | g/L | 0.553 | 314 | 42.3 (35.2–49.6) | 67.8 (62.1–73.3) | 1.31 | 0.85 | 56.8 | 54.0 |
| Eosinophil | ×109/L | 0.547 | 0.3 | 50.5 (43.3–57.8) | 60.8 (54.8–66.5) | 1.29 | 0.81 | 56.3 | 55.1 |
| Platelet | ×109/L | 0.541 | 430 | 52.1 (44.8–59.3) | 57.6 (51.6–63.4) | 1.23 | 0.83 | 55.1 | 54.6 |
| Monocyte | ×109/L | 0.540 | 0.6 | 43.3 (36.2–50.6) | 67.1 (61.3–72.6) | 1.32 | 0.85 | 56.9 | 54.2 |
| RBC | ×1012/L | 0.537 | 10.0 | 48.5 (41.2–55.7) | 60.1 (54.1–65.8) | 1.21 | 0.86 | 54.8 | 53.8 |
| E. coli | % CFU killed | 0.522 | 89.6 | 24.2 (18.7–30.9) | 85.2 (80.5–89.1) | 1.63 | 0.89 | 62.0 | 52.9 |
| Total leukocytes | ×109/L | 0.516 | 10.0 | 33.0 (26.4–40.1) | 76.3 (70.9–81.2) | 1.39 | 0.88 | 58.2 | 53.3 |
| Hemoglobin | g/L | 0.515 | 104 | 29.4 (23.1–36.3) | 76.0 (70.6–80.8) | 1.22 | 0.93 | 55.0 | 51.8 |
| Mean platelet volume | fL | 0.509 | 5.4 | 25.3 (19.3–32.0) | 82.7 (77.8–86.9) | 1.46 | 0.90 | 59.3 | 52.5 |
% (CFU) killed = percent E. coli colony forming units killed by plasma (plasma bactericide); CFU = colony-forming units; CI = confidence interval; LR = likelihood ratio; MCHC = mean corpuscular hemoglobin concentration; NPV = negative predictive value; PPV = positive predictive value; RBC = red blood cell; Se = sensitivity; Sp = specificity. Hematocrit, mean corpuscular hemoglobin, mean corpuscular volume, RBC distribution width, lymphocyte absolute number had AUC values <0.5. Numbers in parentheses are 95% confidence intervals.
Figure 2.
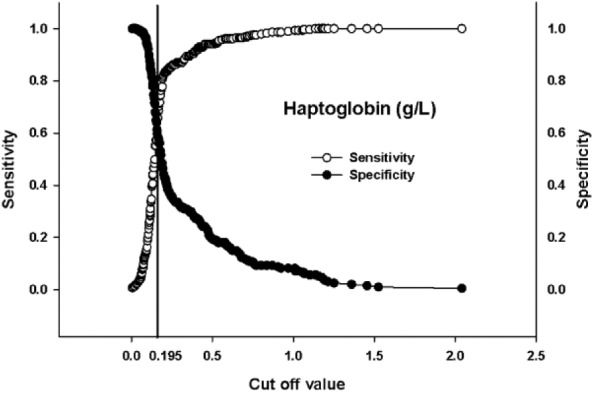
Sensitivity and specificity curves for haptoglobin concentration based on controls and cases of bovine respiratory disease in pre-weaned dairy calves diagnosed using parallel interpretation of thoracic ultrasound and auscultation. The cutoff value is represented by the vertical line at the intersection of the curves.
Figure 3.
Boxplot graphs depicting haptoglobin concentrations in plasma samples from control calves and bovine respiratory disease cases by A. breed, B. sex, C. ultrasound, and D. auscultation findings.
Figure 4.
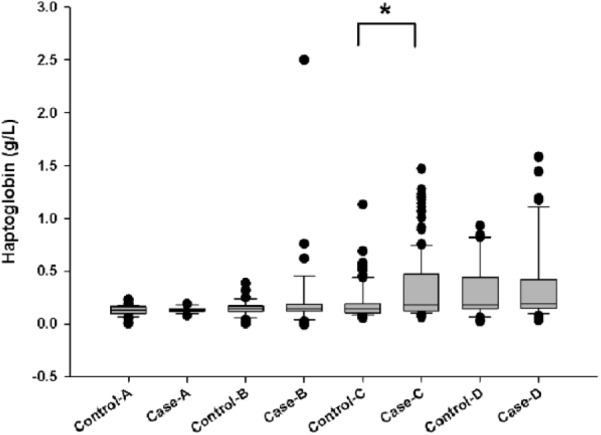
Boxplot graph depicting haptoglobin concentrations in control and BRD cases in calves by premises (A–D); asterisk denotes a significant difference between control and case calves at the same location (p ⩽ 0.05).
Discussion
Of the biochemical and hematologic biomarkers analyzed in our study, Hp had a high specificity of 82% and was the most promising biomarker of BRD in pre-weaned dairy calves. Using a cutoff of 0.195 g/L of Hp, the LR+ of 2.5 indicates that an increased Hp concentration is associated with BRD. Furthermore, elevated Hp and decreased lymphocyte count were significantly associated with BRD in calves after adjusting for calf age, sex, and breed. The importance of Hp as a biomarker is consistent with a previous study in which >75% of calves that required antimicrobial and anti-inflammatory treatment of bronchopneumonia were identified using Hp levels.16 Finally, associations between serum Hp concentration and subsequent clinical respiratory tract disease and pulmonary lesions at slaughter were observed in a population of feedlot cattle.29
Another CBC parameter of importance was basophil count, which had the second greatest AUC value. Basophils store histamine and are the least common granulocytes, representing ~0.01–0.3% of circulating leukocytes.4 In cattle, basophils release histamine as a consequence of a bacterial or viral infection resulting in airway inflammation.15,20 Receptors on the basophil cell surface bind with IgE to initiate cytokine release, which further sustains airway inflammation.1,25 Findings from previous studies agree with our finding that basophils are a potential biomarker for BRD in pre-weaned dairy calves. In a previous study, significant basophil differences were detected in beef calves grouped based on their pulmonary score used to diagnose BRD.17
An interesting finding was the association of lower MCV values in pre-weaned calves with higher odds of BRD. Further research is required to explore the lower MCV values in calves with BRD compared to their healthy counterparts. Multivariate analysis also showed that lymphocytes, not basophils, were significantly associated with BRD after adjusting for plasma Hp concentration. The negative association between lymphocyte count and BRD in pre-weaned calves may be explained by cases of BRD caused by viral infection, which typically result in lower lymphocyte counts.24 Alternatively, exposure to endogenous corticosteroids as a result of stress may result in a decrease in lymphocytes. However, lymphocyte count as a biomarker on its own had a lower AUC to correctly identify a calf’s BRD status.
Results from our study allow us to recommend using Hp concentrations as an additional tool for BRD detection, but it does not replace standard procedures for BRD detection because Hp can be released after other events (e.g., castration, vaccination). Additionally, we believe that pre-analysis blood extraction, manipulation, and processing (e.g., temperature variations or sun exposure) may also alter Hp concentration estimation, which could decrease its usefulness.5 Therefore, further research is needed to determine the cutoff values for Hp in response to other insults, and to determine if multiple insults can cause an additive Hp response.
Acknowledgments
We thank the study dairy farm owners, calf caretakers, and herd veterinarians for their participation in the study. We also thank Mr. Casey Glenn and Mr. Justin Tonooka for technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding for our study was provided by competitive grant 1753 “Risk assessment, welfare analysis, and extension education for dairy calf respiratory disease management in California,” to S Aly (PI), provided by the University of California Division of Agriculture and Natural Resources. Undergraduate (SC Trombetta) and post-doctoral scholar (SJ Moisá), and additional plasma analyses were partially funded through Kansas State University HA (Hatch Act of 1887) distributions representing the USDA-NIFA Multistate Project W-3173 (Impacts of Stress Factors on Performance, Health, and Well-Being of Farm Animals). Internship (EM Bortoluzzi) was funded through the Brazilian Scientific Mobility program—Science Without Borders program.
ORCID iD: Sharif S. Aly  https://orcid.org/0000-0003-0330-5013
https://orcid.org/0000-0003-0330-5013
References
- 1. Abbas JS, et al. Effector mechanism of immunoglobulin E-initiated immune reactions. In: Cellular and Molecular Immunology. 2nd ed. Philadelphia, PA: Saunders, 1997:278–292. [Google Scholar]
- 2. Angen O, et al. Respiratory disease in calves: microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol 2009;137:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballou MA. Immune responses of Holstein and Jersey calves during the preweaning and immediate postweaned periods when fed varying planes of milk replacer. J Dairy Sci 2012;95:7319–7330. [DOI] [PubMed] [Google Scholar]
- 4. Blann A. While blood cells in health and disease. In: Blann A, Ahmed N, eds. Blood Science: Principles and Pathology. Indianapolis, IN: Wiley-Blackwell, 2014:109–134. [Google Scholar]
- 5. Blumberg BS, et al. Alterations in haptoglobin levels. J Am Med Assoc 1963;184:1021–1023. [DOI] [PubMed] [Google Scholar]
- 6. Box GEPT, Tidwell PW. Transformation of the independent variables. Technometrics 2012;4:531–550. [Google Scholar]
- 7. Cooke RF, Arthington JD. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J Anim Physiol Anim Nutr (Berl) 2013;97:531–536. [DOI] [PubMed] [Google Scholar]
- 8. Eckersall PD, Bell R. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 9. Gardner IA, Greiner M. Receiver-operating characteristics curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol 2006;35:8–17. [DOI] [PubMed] [Google Scholar]
- 10. Godson DL, et al. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet Immunol Immunopathol 1996;51:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habibzadeh F, et al. On determining the most appropriate test cut-off value: the case of tests with continuous results. Biochem Med (Zagreb) 2016;26:297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanthorn CJ, et al. Serum concentrations of haptoglobin and haptoglobin-matrix metalloproteinase 9 (Hp-MMP 9) complexes of bovine calves in a bacterial respiratory challenge model. BMC Vet Res 2014;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higuchi H, et al. Dexamethasone-induced haptoglobin release by calf liver parenchymal cells. Am J Vet Res 1994;55:1080–1085. [PubMed] [Google Scholar]
- 14. Holland BP, et al. Effectiveness of sorting calves with high risk of developing bovine respiratory disease on the basis of serum haptoglobin concentration at the time of arrival at a feedlot. Am J Vet Res 2011;72:1349–1360. [DOI] [PubMed] [Google Scholar]
- 15. Holroyde MC, Eyre P. Immunological release of histamine from bovine leucocytes. Unusual adrenergic modulation. Immunology 1976;31:167–170. [PMC free article] [PubMed] [Google Scholar]
- 16. Humblet MF, et al. Acute phase proteins assessment for an early selection of treatments in growing calves suffering from bronchopneumonia under field conditions. Res Vet Sci 2004;77:41–47. [DOI] [PubMed] [Google Scholar]
- 17. Leach RJ, et al. The change in differing leukocyte populations during vaccination to bovine respiratory disease and their correlations with lung scores, health records, and average daily gain. J Anim Sci 2013;91:3564–3573. [DOI] [PubMed] [Google Scholar]
- 18. Love WJ, et al. Sensitivity and specificity of on-farm scoring systems and nasal culture to detect bovine respiratory disease complex in preweaned dairy calves. J Vet Diagn Invest 2016;28:119–128. [DOI] [PubMed] [Google Scholar]
- 19. Millet S, et al. Quantifying and comparing constitutive immunity across avian species. Dev Comp Immunol 2007;31:188–201. [DOI] [PubMed] [Google Scholar]
- 20. Motomura Y, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014;40:758–771. [DOI] [PubMed] [Google Scholar]
- 21. Murata H, et al. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J 2004;168:28–40. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen MJ, et al. Haptoglobin-related protein is a high-affinity hemoglobin-binding plasma protein. Blood 2006;108:2846–2849. [DOI] [PubMed] [Google Scholar]
- 23. Nightingale CR, et al. Elevated plasma haptoglobin concentrations following parturition are associated with elevated leukocyte responses and decreased subsequent reproductive efficiency in multiparous Holstein dairy cows. Vet Immunol Immunopathol 2015;164:16–23. [DOI] [PubMed] [Google Scholar]
- 24. Roland L, et al. Hematology as a diagnostic tool in bovine medicine. J Vet Diagn Invest 2014;26:592–598. [DOI] [PubMed] [Google Scholar]
- 25. Sihra BS, et al. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol 1997;99:699–706. [DOI] [PubMed] [Google Scholar]
- 26. Svensson C, et al. Evaluating the efficacy of serum haptoglobin concentration as an indicator of respiratory-tract disease in dairy calves. Vet J 2007;174:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wittum TE, et al. Haptoglobin response to clinical respiratory tract disease in feedlot cattle. Am J Vet Res 1996;5:646–649. [PubMed] [Google Scholar]
- 28. Wolfger B, et al. A systematic review of bovine respiratory disease diagnosis focused on diagnostic confirmation, early detection, and prediction of unfavorable outcomes in feedlot cattle. Vet Clin North Am Food Anim Pract 2015;31:351–365. [DOI] [PubMed] [Google Scholar]
- 29. Young CR, et al. Serum haptoglobin concentrations in a population of feedlot cattle. Am J Vet Res 1996;57:138–141. [PubMed] [Google Scholar]
- 30. Yuan K, et al. Effects of yeast product supplementation on immunity and uterine inflammation in transition dairy cows. J Dairy Sci 2014;98:3236–3246. [DOI] [PubMed] [Google Scholar]