Abstract
Eleven adult African pygmy hedgehogs (Atelerix albiventris) were added to a group of 35 animals, and within 10 d, respiratory distress affected 8 of 35 resident animals in the group, but none of the introduced animals. Three animals died following onset of clinical signs. Tissues from one animal were collected and submitted for histopathology, which revealed acute necrotizing bronchopneumonia and tracheitis with intraepithelial intranuclear inclusion bodies. Electron microscopy identified 75–90 nm diameter encapsulated icosahedral virions. Degenerate nested PCR analysis identified adenovirus within the affected lung tissue. Deep sequencing showed 100% homology to skunk adenovirus 1 (SkAdV-1). Adenoviruses are usually species-adapted and -specific, but our case supports the single previous report of non-skunk infection with SkAdV-1, indicating that this virus can infect other species, and further shows that it can cause fatal disease.
Keywords: Adenoviridae; African pygmy hedgehogs; Atelerix albiventris; bronchopneumonia, lung; PCR; skunk adenovirus 1; transmission electron microscopy
Adenoviruses are 70–90 nm, icosahedral, nonenveloped viruses, with a linear double-stranded DNA genome.7,14 Adenoviral infections are ubiquitous among vertebrates, largely subclinical in their hosts, and generally considered species-specific.7 Adenovirus-associated disease can occur; however, morbidity in the host species is associated with underlying immunosuppressive disease, such as in dogs with concurrent canine adenovirus 1 and canine distemper or parvovirus infection, or in Arabian foals with severe combined immune deficiency.3,6 In 2015, a single icteric skunk in Canada infected by the novel skunk adenovirus 1 (SkAdV-1; family Adenoviridae, genus Mastadenovirus, species Skunk mastadenovirus A) had necrotizing hepatitis, and in 2016 a single African pygmy hedgehog (APH; Atelerix albiventris) in Japan that died as a result of heart failure was found to have subclinical tracheitis caused by the same adenovirus.10,11 We report herein clinical and fatal disease caused by SkAdV-1 infection in a hedgehog.
Eleven apparently healthy APHs from 2 separate out-of-state facilities (3 from one facility and 8 from the second) were concurrently added to an array of 35 breeding animals for eventual sale, with no preceding medical examination or quarantine. Ten days after addition of the new animals, respiratory illness, characterized by oculonasal discharge, was noted in 8 of the 35 resident animals. Five to 10 d after the onset of clinical signs, 3 animals died, and tissues from one were submitted to the New Hampshire Veterinary Diagnostic Laboratory (Durham, NH) fixed in 10% neutral-buffered formalin. There were no gross anatomic lesions noted during autopsy of this animal. By 4 weeks after respiratory disease was noted, 15 resident animals had clinical signs. Enrofloxacin was prescribed for these animals at 10 mg/kg for 7 d, with no additional deaths occurring. The surviving animals recovered to apparent normal health. Evaluation of the sources of the introduced animals revealed that one source housed APHs in the same facility as skunks and other exotic mammals.
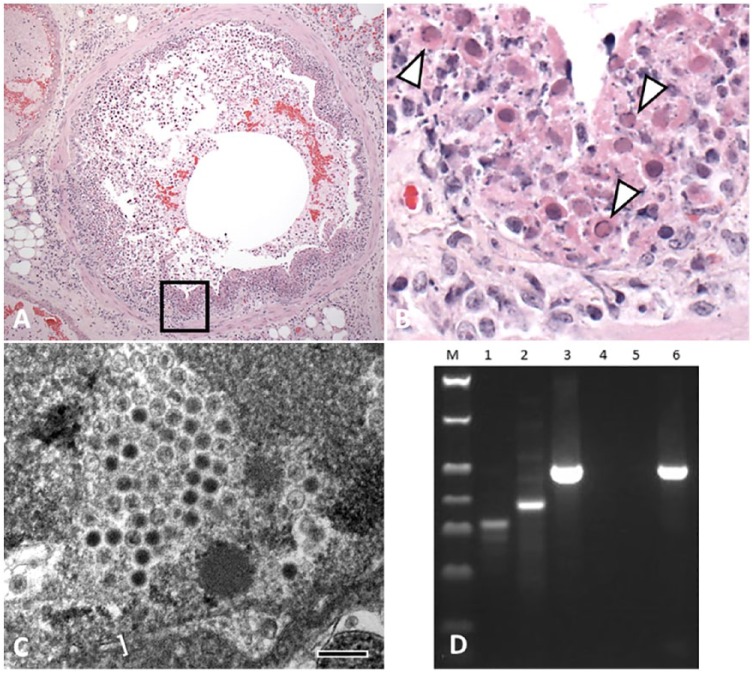
Tissues were processed routinely, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Trachea, lung, heart, kidney, spleen, liver, urinary bladder, esophagus, uterus, and pancreas were examined. There was multifocal, ulcerative, segmental-to-circumferential tracheal and bronchial epithelial necrosis (Fig. 1A). The airway lumina contained sloughed epithelial cells that were swollen and fragmented, with moth-eaten and pale, or deeply eosinophilic, cytoplasm, and karyorrhectic-to-karyolytic nuclei (Fig. 1A, 1B). Necrotic cells were mixed with amorphous granular eosinophilic necrotic debris and a few erythrocytes. Intact cells were attenuated, laterally elongated, and lacked cilia. Chromatin of numerous sloughed cells was compressed against the nuclear envelope by homogeneous, slightly refractile, pale basophilic-to-eosinophilic inclusion bodies (Fig. 1B). Rare degenerate epithelial cells had Cowdry type A inclusions, with a central eosinophilic inclusion surrounded by a nonstaining halo and chromatin compressed against the nuclear envelope. The adventitia of the airways and the alveolar interstitium contained a few lymphocytes, macrophages, and fibrin. There was mild, multifocal, alveolar interstitial edema and a few megakaryocytes scattered within the alveolar septa; ~30–40% of the examined lung area contained lesions. Coronary vessels were dilated. There was rare, multifocal, mild individual cardiomyocyte degeneration and loss, with fibrosis, which was considered incidental to the death of the animal. No additional lesions were noted. Aerobic culture of lung swabs from the autopsy grew moderate numbers of Morganella morganii and Enterococcus sp.
Figure 1.
Fatal bronchopneumonia caused by skunk adenovirus 1 in an African pygmy hedgehog (Atelerix albiventris). A. A bronchus with nearly complete acute epithelial necrosis, with luminal sloughed and necrotic cellular debris admixed with acute hemorrhage. There is a mild nonsuppurative infiltrate in the adjacent interstitium. H&E. 100×. B. Higher magnification of the boxed area in the bronchus in panel A, highlighting a segment of acute epithelial necrosis, with amorphous necrotic debris and karyorrhectic nuclear debris. There are multiple variably necrotic epithelial cells containing eosinophilic intranuclear inclusions with peripheralized chromatin (arrowheads). H&E. 200×. C. Transmission electron micrograph showing virions and degenerate nucleus. The viral particles are closely apposed and icosahedral, with variably electron-dense genetic material, often with a clear space and a peripheral icosahedral capsid. A portion of what appears to be the nuclear envelope with distended intermembrane space is visible along the bottom of the image (bracket). 71,000×. Bar = 100 nm. D. PCR detection of adenovirus in hedgehog second-round PCR. Lane M: size marker (Bionexus, Oakland, CA); lanes 1–3: positive control raccoon DNA; lanes 4–6: hedgehog DNA; lanes 1, 4: fully nested (polFinner/polRinner); lanes 2, 5: semi-nested (polFouter/polRinner); lanes 3, 6: semi-nested (polFinner/polRouter). See Supplementary data for methods. There is a strong, clear band with the positive control in lane 3 at ~500 bp length and, using the same semi-nested primer pairings, lane 6 has a similarly strong, clear band at ~500 bp.
Formalin-fixed lung was submitted for transmission electron microscopy (TEM). The tissue was washed in sodium cacodylate buffer, fixed in modified Karnovsky fixative, processed (Lynx tissue processor, Electron Microscopy Science, Hatfield, PA), embedded in epoxy resin, cut into thin sections, stained, and examined (Morgagni TEM, FEI, Hillsboro, OR) as described previously.4 Icosahedral viral particles with capsids were noted in necrotic nuclear and cellular debris (Fig. 1C). With their capsids, the viral particles measured 75–90 nm. The virions had variably electron-dense cores of genetic material, often surrounded by a halo of electron-lucent material and a peripheral capsid. Viral particles were occasionally aggregated in a relatively regular (paracrystalline) packing.
Based on histology, herpesvirus or adenovirus was suspected. DNA was extracted from dewaxed and washed formalin-fixed, paraffin-embedded lung tissue (ZR genomic DNA tissue miniprep kit, Zymo Research, Irvine, CA), according to the manufacturer’s instructions. A pan-herpesvirus nested PCR assay was performed on lung tissue using degenerate pan-herpesvirus primers as described previously (Supplementary data)8,15; no amplicon was detected by this method. Nested PCR amplification of a 500-bp fragment of the adenoviral DNA polymerase gene (as described previously, Supplementary data) resulted in generation of an amplicon (Fig. 1D) that was then sequenced and had 100% homology to SkAdv-1.10,11,16
SkAdV-1 is most closely related, phylogenetically, to canine adenovirus 1.10 The hedgehog reported from Japan to have SkAdV-1 tracheitis died of cardiomyopathy, and the incidental adenovirus-associated lesions were not considered causative.11 In contrast, we report clinically evident infection in a group of hedgehogs, with histology performed on a single infected animal. No significant cardiomyopathy was detected in our case. The noted, mild individual fiber degeneration and associated mild fibrosis were not consistent with the well-documented cardiomyopathy in APHs, which primarily affects the left ventricle.11,13 It is possible that portions of cardiac muscle not represented in the examined sections had more severe lesions that could have contributed to clinical disease.
The diagnostic investigation in our case started with histopathology, which was followed by degenerate herpesvirus PCR given the intranuclear inclusion bodies and epitheliotropism (Fig. 1A, 1B).8,15 The PCR result was negative, so TEM followed. The visualized capsid, dimensions, and paracrystalline array (Fig. 1C) facilitated testing by degenerate adenovirus PCR and sequencing that identified SkAdV-1.10,11,16 This timeline highlights the value of a multimodal investigation in cases with potential novel viruses. It is probable that the 2 other hedgehogs that died in temporal proximity to the one submitted for autopsy were infected with SkAdV-1. This probability is supported by the absence of other mortalities at the facility prior to or after the outbreak of respiratory disease. Tissues from these 2 animals were not submitted, hence no laboratory testing was undertaken to confirm this possibility.
Adenoviral respiratory disease has been described in calves, Arabian foals with severe combined immune deficiency, dogs, and bats, although, unlike in our case, the viruses involved are specific for the affected species.1,3,6,7,10,12 SkAdV-1 was named for the first species in which it was discovered; however, no work has been reported on host adaptation, to our knowledge. The prevalence of SkAdV-1 in skunks and APHs is unknown. Our report raises the possibility that a species other than skunk and/or hedgehogs could be the adapted host. We favor a skunk-to-hedgehog transfer, based on the history that the introduced hedgehogs had been exposed to skunks, although it is unclear why the introduced hedgehogs, or the skunks, were not affected. Although most adenoviruses cause subclinical infections and are host-specific, adenoviruses of bats, dogs, and primates are capable of infecting other closely related species, which may also have occurred with SkAdV-1 in our case and in the previously reported case.2,5,6,9
Based on respiratory infection of APHs with SkAdV-1 in unrelated reports from Japan and the United States, SkAdV-1 should be considered an emerging respiratory pathogen of APHs.11 Our report highlights the potential downside of mixed-species husbandry. The pathogenicity of SkAdV-1 in skunks and APHs, as well as its capability to colonize and infect other animals, require additional investigation.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638718812123 for Fatal bronchopneumonia caused by skunk adenovirus 1 in an African pygmy hedgehog by David B. Needle, Martin K. Selig, Kenneth A. Jackson, Eric Delwart, Ellyn Tighe, Stephen L. Leib, Torsten Seuberlich and Patricia A. Pesavento in Journal of Veterinary Diagnostic Investigation
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David B. Needle  https://orcid.org/0000-0002-7215-5306
https://orcid.org/0000-0002-7215-5306
References
- 1. Balboni A, et al. Investigation of the presence of canine adenovirus (CAdV) in owned dogs in Northern Italy. Res Vet Sci 2014;97:631–636. [DOI] [PubMed] [Google Scholar]
- 2. Balboni A, et al. Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Vet Microbiol 2013;162:551–557. [DOI] [PubMed] [Google Scholar]
- 3. Bell SA, et al. Equine adenovirus 1 infection of hospitalised and healthy foals and horses. Equine Vet J 2006;38:379–381. [DOI] [PubMed] [Google Scholar]
- 4. Bennett JA, et al. Giant cell tumor of the uterus. Int J Gynecol Pathol 2015;34:340–350. [DOI] [PubMed] [Google Scholar]
- 5. Chen EC, et al. Cross-species transmission of a novel adenovirus associated with a fulminant pneumonia outbreak in a new world monkey colony. PLoS Pathog 2011;7:e1002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Decaro N, et al. Canine adenoviruses and herpesvirus. Vet Clin North Am Small Anim Pract 2008;38:799–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubovi EJ, Maclachlan JN. Adenoviridae. In: Fenner’s Veterinary Virology. 4th ed Tokyo: Academic Press, 2011:203–214. [Google Scholar]
- 8. Ehlers B, et al. Detection of new DNA polymerase genes of known and potentially novel herpesviruses by PCR with degenerate and deoxyinosine-substituted primers. Virus Genes 1999;18:211–220. [DOI] [PubMed] [Google Scholar]
- 9. Kohl C, et al. Genome analysis of bat adenovirus 2: indications of interspecies transmission. J Virol 2012;86:1888–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozak RA, et al. Characterization of a novel adenovirus isolated from a skunk. Virology 2015;485:16–24. [DOI] [PubMed] [Google Scholar]
- 11. Madarame H, et al. Detection of skunk adenovirus 1 (SkAdV-1) in an African pigmy hedgehog (Atelerix albiventris). Vet Rec Case Reports 2016;4:e000321. [Google Scholar]
- 12. Narita M, et al. Bovine adenovirus type 3 pneumonia in dexamethasone-treated calves. Vet Pathol 2003;40:128–135. [DOI] [PubMed] [Google Scholar]
- 13. Raymond JT, Garner MM. Cardiomyopathy in captive African hedgehogs (Atelerix albiventris). J Vet Diagn Invest 2000;12:468–472. [DOI] [PubMed] [Google Scholar]
- 14. Rowe WP, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Exp Biol Med 1953;84:570–573. [DOI] [PubMed] [Google Scholar]
- 15. VanDevanter DR, et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol 1996;34:1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wellehan JFX, et al. Detection and analysis of six lizard adenoviruses by consensus primer PCR provides further evidence of a reptilian origin for the atadenoviruses. J Virol 2004;78:13366–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638718812123 for Fatal bronchopneumonia caused by skunk adenovirus 1 in an African pygmy hedgehog by David B. Needle, Martin K. Selig, Kenneth A. Jackson, Eric Delwart, Ellyn Tighe, Stephen L. Leib, Torsten Seuberlich and Patricia A. Pesavento in Journal of Veterinary Diagnostic Investigation