Abstract
Nine of 23 (39%) research ewes with severe diarrhea and weight loss had histologic lesions consistent with Eimeria gilruthi infection in their abomasa. Gross anatomic lesions included hundreds of opaque 1-mm nodules in abomasal mucosa that corresponded microscopically to 200–300 µm diameter organisms surrounded by areas of necrosis. Analysis of fecal samples from 4 ewes demonstrated oocysts from typical ovine Eimeria species, none of which were E. gilruthi. Two separate PCR reactions were performed on abomasal tissue from 4 sheep to amplify the 18S ribosomal DNA (rDNA) and internal transcribed spacer (ITS) region of the rDNA, respectively. The resultant 18S rDNA nucleotide sequences shared 99% homology with multiple Eimeria species in GenBank. The ITS region shared 77% homology with E. ellipsoidalis in GenBank. Further studies are needed to understand the life cycle and pathogenicity of E. gilruthi. Our results underscore the inclusion of E. gilruthi in the differential diagnosis of diarrhea and weight loss in sheep.
Keywords: Abomasum, coccidiosis, Eimeria gilruthi, ovine
Coccidiosis, caused by Eimeria species in ruminants, is an economically important disease, characterized by severe enteritis, diarrhea, dehydration, anorexia, weight loss, and potentially death.3,6 Eimeria species generally affect young animals and are host specific.1,3 Both sexual and asexual stages of life cycle occur intracellularly within the host followed by sporogony in the environment.2,9
Abomasal coccidiosis is described as an infection of sheep and goats caused by Eimeria (Globidium) gilruthi.9,13 Although E. gilruthi has been reported sporadically,5–7,9 taxonomic classification is incomplete given the scarce data available on the parasite.9 There is a large knowledge gap in the life cycle and pathogenicity of this parasite, including typical definitive host(s), how ruminants are infected, potential transport hosts, and control options. E. gilruthi has been found incidentally on postmortem examination.6,8 Gross anatomic lesions typically consist of edema with multiple white, raised foci in the abomasum.6 Microscopically, the main findings are giant schizonts within the abomasal mucosa.9
Twenty-three, ~15-mo-old, Suffolk ewes were part of a nutrition study assigned to 1 of 2 experimental diets: 1) fed to meet maintenance requirements, or 2) 30% feed restriction of maintenance requirements. The ewes were purchased through a livestock order buyer (Tennessee Livestock Producers, Columbia, TN) that grouped the ewes from different sources, such that the principal investigators were unaware of the origin and background of the ewes. To ensure animal health, all 23 ewes were vaccinated on arrival for Clostridium perfringens types C and D and tetanus, and administered 10 mL of an oral anthelmintic (Cydectin, moxidectin 5 mg/mL, Bayer Animal Health, Whippany, NJ). Upon arrival, all ewes were weighed and assigned a body condition score (BCS). Throughout the study, all ewes were weighed twice weekly, and BCS was performed every other week. Based on the clinical presentation of these ewes, including diarrhea, weekly FAMACHA scoring was performed to assess anemia.11 Approximately 2 wk after the initial anthelmintic dose, the ewes were administered a second 10-mL oral dose of the same anthelmintic. Over the next month, FAMACHA scoring continued for the entire group.
Two ewes (ewes 3 and 5) in the group exhibited little-to-no improvement with the anthelmintic treatments. One ewe was in the maintenance feed group, and the other was in the 30% feed-restricted group. Less than 1 mo after the second dose of anthelmintic, ewes 3 and 5 had rectal temperatures >40.5°C (105.0°F; normal range: 38–40°C [102–104°F]) anorexia, anemia (category 4 FAMACHA), and decreased rumen contractions. Affected ewes were lethargic and separated themselves from the rest of the group. Diarrhea and anorexia followed within ~1 d. Initial FAMACHA scores for ewes 3 and 5 were scores of 2 and 1, respectively. Additionally, initial body weights were 45.7 kg for ewe 3 and 44.1 kg for ewe 5. Over the next several days, these ewes received oral boluses of sulfadimethoxine (Albon, Zoetis, Parsippany, NJ) and oxytetracycline, as well as intramuscular injections of flunixin meglumine (Banamine, Merck Animal Health, Madison, NJ). Ewes 3 and 5 each experienced >10% body weight loss in <2 wk, which, in combination with continuation of elevated body temperature, diarrhea, dehydration, and anorexia, led to the decision to euthanize the ewes. All surviving ewes were euthanized at the end of the experiment, and abomasal samples were collected in 10% buffered formalin, processed, sectioned, and stained with H&E. Fecal samples were collected from ewes 3 and 5 at autopsy, as well as from 2 other ewes in the group, and submitted to the University of Tennessee parasitology laboratory (Knoxville, TN) for fecal flotation examination using Sheather sugar solution.
Significant autopsy findings from ewes 3 and 5 included diffusely thickened and finely nodular abomasal mucosae with many opaque white 1-mm foci (Fig. 1). Gastrointestinal contents were watery with no formed fecal pellets. Microscopically, the lamina propria of the abomasum in 9 of 23 (39%) ewes contained many 200–300 µm diameter protozoal schizonts with a thick eosinophilic wall surrounding many elongate, 10 × 12 µm merozoites with nuclei arranged in circular blastophores (Figs. 2, 3). Large parasitophorous vacuoles surrounded the schizonts, separating them from the host tissue. Occasional schizonts were ruptured and infiltrated by lymphocytes and neutrophils and surrounded by hemorrhage and necrosis. The mucosa was diffusely thickened by hyperplasia of mucous neck cells with loss of chief and parietal cells. Attenuation of the epithelium was diffuse with extensive areas of necrosis and karyorrhectic debris. There were areas of epithelial hemorrhage, with a few free merozoites on the surface. No sexual forms of the organism or oocysts were found. The abomasal samples from the remaining 14 ewes had multinodular lymphocytic and eosinophilic inflammation with diffuse mucous neck cell hyperplasia. Loss of chief and parietal cells was variably present. Three of 14 abomasal sections contained variably sized concentric mineralized structures within the mucosa.
Figure 1.
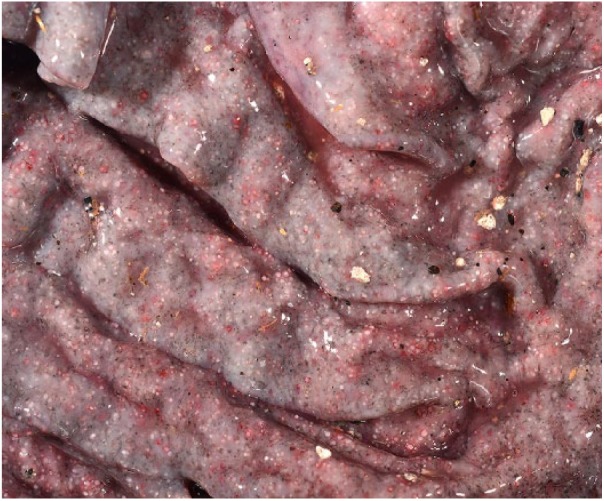
Opaque white-to-tan, 1-mm nodules throughout the abomasal mucosa of a sheep are sometimes surrounded by hemorrhage.
Figure 2.
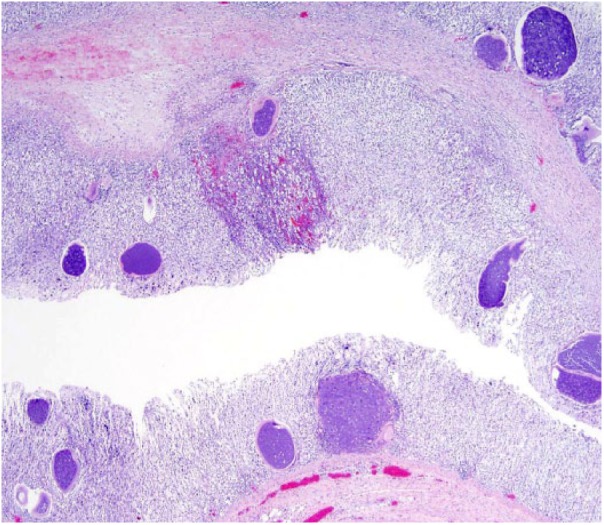
Numerous 200–300 μm diameter protozoal schizonts are throughout the abomasal mucosa. Areas of hemorrhage are within the mucosa. H&E.
Figure 3.
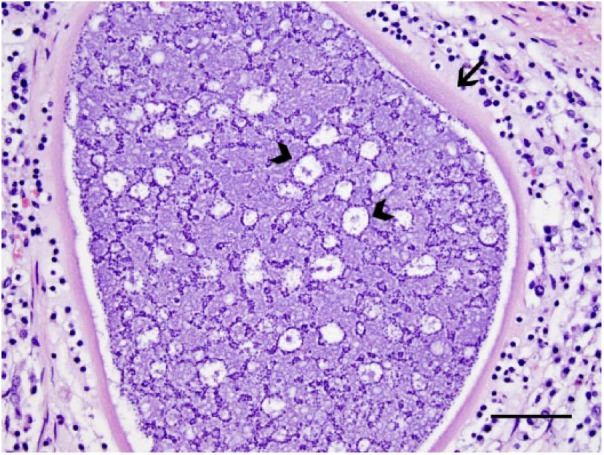
Protozoal schizonts within the abomasum have a thick eosinophilic wall (arrow). Thousands of elongate, 10 × 2 μm merozoites are within the schizont. Nuclei are often arranged in circular blastophores (arrowheads). H&E. Bar = 50 µm.
DNA was extracted from formalin-fixed, paraffin-embedded abomasal sections from 1 ewe and fresh abomasum tissue samples from 3 other ewes (DNeasy blood and tissue kit, Qiagen, Valencia, CA) according to the manufacturer’s instructions. Negative controls were included to ensure no contamination during the DNA extraction and PCR. Initial PCR was performed using the Eimeria-wide primers Ei18Sr1 (5’-CTAGTCGGCATAGTTTATGG-3’) and Ei18Sf1 (5’-AGCTCGTAGTTGGATTTCTG-3’) that amplify 18S ribosomal DNA (rDNA).10 To further analyze the phylogenetic relationship of this coccidia species, a secondary PCR amplifying the internal transcribed spacer (ITS) region of the rDNA was performed using primers WW1 (5’-AAGTTGGGTAAATAGAGCCC-3’) and WW3r (5’-CAAGACATCCATTGCTGAAA-3’).14 Amplicons were separated on 1.5% agarose gels, stained with ethidium bromide, and detected by ultraviolet transillumination. The 18S rDNA PCR products were purified (QIAquick PCR purification kit, Qiagen) and submitted to the sequencing laboratory at the University of Tennessee (Knoxville, TN). Resultant forward and reverse complementary sequences were aligned (Sequencher 5.1 software, Gene Codes, Ann Arbor, MI). The consensus nucleotide sequence was subjected to a BLAST search (https://blast.ncbi.nlm.nih.gov/Blast.cgi) in GenBank.
The purified ITS region of DNA was cloned into the pGEM-T-easy vector (Promega, Madison, WI) according to the manufacturer’s instructions. The plasmid was then transformed into chemically competent DH5α Escherichia coli cells (Invitrogen, Waltham, MA). White colonies were selected, and the plasmid was sequenced using the primers SP6 and T7 at the sequencing laboratory at the University of Tennessee. The sequences were subject to BLAST analysis after removing the plasmid sequences. Phylogenetic analysis and bootstrapping distances were conducted for both 18S and ITS sequences (MEGA v.7, http://www.megasoftware.net).
Using Eimeria spp.–specific primers, 18S rDNA sequences of E. gilruthi were obtained for 4 sheep, and ITS sequences of E. gilruthi were obtained for 2 of these 4 sheep. The 18S rDNA sequences were identical for all 4 samples and shared 99% homology with E. ahsata, E. zuernii, E. bovis, and E. crandallis. The ITS sequences shared 77% homology with E. ellipsoidalis and 76% homology with E. bovis. We deposited sequences in GenBank (accessions MH062791 for 18S rDNA; MH086966, MH086967, and MH086968 for ITS).
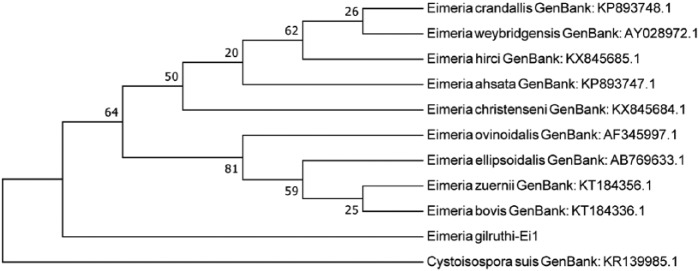
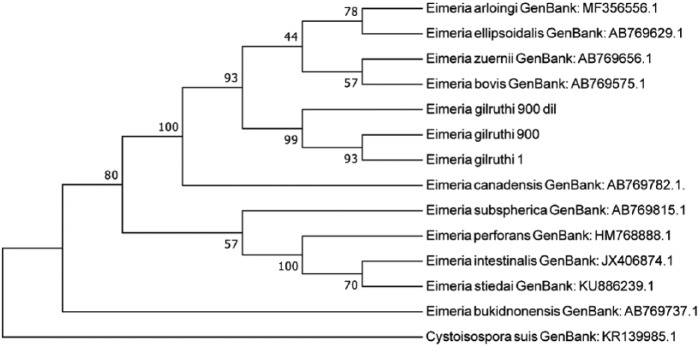
The phylogenetic tree comparing 18S rDNA between E. gilruthi samples and other Eimeria species shows close relationship among the 4 samples in our study, suggesting a common origin. Samples from our study are closely related to a cluster that contained E. ellipsoidalis, E. bovis, E. zuernii, and E. illinoisensis (Fig. 4). Phylogenetic analysis of the ITS1 region indicates close relationship between the 2 samples from our study. Sample 1 had 2 different ITS1 sequences (Fig. 5), suggesting divergent copies of ITS1 regions, which has been reported in apicomplexan parasites.5 The E. gilruthi ITS1 sequences are closely related to E. ellipsoidalis.
Figure 4.
Phylogenetic analysis of 18S rDNA sequences of Eimeria gilruthi DNA extracted from ewe abomasum from our study and other related Eimeria species. The tree was constructed using 477 aligned nucleotide positions and a neighbor-joining algorithm with 500 replications in a Kimura 2-parameter model with Cystoisospora canis as an outgroup. Bootstrap values for neighbor-joining are shown on the nodes.
Figure 5.
Phylogenetic analysis of internal transcribed spacer 1 region sequences of Eimeria DNA extracted from ewe abomasum from our study and other related Eimeria species. The tree was constructed using 267 aligned nucleotide positions and a neighbor-joining algorithm with 500 replications in a Kimura 2-parameter model with Cystoisospora suis as an outgroup. Bootstrap values for neighbor-joining are shown on the nodes.
The gross and microscopic features of schizonts in the abomasum are consistent with previous studies of E. gilruthi.6,8,9 Infrequently, patchy hemorrhages in the abomasal wall have also been reported.6 Previously reported findings ranged from mild abomasitis with no clinical signs8 to severe inflammation extending from the superficial mucosa to the muscularis of the abomasum.5 The lymphocytic and eosinophilic abomasitis, loss of parietal and chief cells, and mucous neck cell hyperplasia in 14 of the 23 examined sheep may also represent infection with E. gilruthi; however, in the absence of characteristic schizonts, diagnosis could not be confirmed. In the 7 confirmed E. gilruthi cases in the 21 ewes euthanized after the initial diagnosis of E. gilruthi, necrotic protozoal schizonts were often mineralized. The mineralized structures present in 3 of the 14 examined cases may likewise represent necrotic schizonts. The histologic appearance of the abomasa of these 21 sheep may be indicative of chronic E. gilruthi infection.
There is little known about this parasite, and further research is needed to elucidate the transmission of this protozoan. Although lesions have been reported in sheep and goats, these ruminants may not be the definitive host of E. gilruthi. The parasite develops one or more generations of schizonts and is then unable to continue to gametogony.9 In a previous study, a structure similar to a zygote was seen in histologic section, but no further information was provided.8
To elucidate the life cycle of this parasite, an attempt has been made to infect cell cultures with the merozoites from sheep abomasum globidia, which are membranous cysts filled with the parasite.12 Highly motile merozoites did not develop in cultured cells. Cells used in the in vitro study included goat esophagus cells, sheep primary lung cells, and dog kidney cells. It may be useful to attempt to infect ovine or caprine gastric origin cells rather than the types used in the earlier study.12
Diarrhea, anorexia, and weight loss are common signs of infection by various enteric pathogens, including parasitic, viral, and bacterial agents. The gross finding of the finely nodular abomasal mucosa caused by E. gilruthi is similar to the lesion caused by Teladorsagia spp. The nodular abomasal mucosa in E. gilruthi–infected sheep differs in that the opaque white 1-mm spots represent giant schizonts. E. gilruthi should be included in the differential diagnosis in small ruminants with the aforementioned clinical signs, especially if abomasal lesions are present.9
Qualitative flotation of feces performed in a study of E. gilruthi–infected lambs from Saudi Arabia showed a moderate number of Nematodirus eggs but no Eimeria oocysts.9 In our study, fecal analysis by double centrifugation flotation with Sheather sugar solution detected Eimeria ovinoidalis, E. ovina, E. faurei, and E. ahsata. None of the fecal analyses detected the larger E. intricata oocysts. Two ewes had moderate numbers of trichostrongyle-type eggs and a few eggs consistent with Strongyloides papillosus (Table 1).
Table 1.
Results of fecal flotation analysis of 4 ewes in a nutrition study.
| Sheep ID | Result of fecal flotation |
|---|---|
| 1 | Eimeria oocyst, trichostrongyle-type eggs, Strongyloides papillosus eggs |
| 2 | Trichostrongyle-type eggs, adult mites and mite eggs, Strongyloides papillosus eggs, Eimeria ovina and E. faurei oocysts |
| 3 | Eimeria oocysts, including E. ovinoidalis, E. faurei, and E. ovina |
| 4 | Eimeria oocysts, including E. ovinoidalis, E. faurei, E. ahsata, and E. ovina |
Mixed Eimeria spp. infections are common in sheep and goats, and a few of these oocysts may be present without causing any clinical signs, especially in adult sheep.15 Trichostrongyle eggs can be present in the feces at low numbers and often represent subclinical infections. When present in large numbers, trichostrongyles can cause debilitating watery diarrhea. Although a few trichostrongyle eggs were found in the feces of sheep in our study, no worms were detected in the gastrointestinal tract.2,6 Teladorsagia is a trichostrongyle that can cause similar gross anatomic lesions in the abomasum, but the 2 lesions can be distinguished microscopically.4 S. papillosus infections are usually moderate and asymptomatic, except in lambs or immunocompromised animals; in adult females, infection is confined to the intestines.2
Given the absence of oocysts and the inability to find any other stages except for schizonts, PCR may be useful in further investigations of this pathogen. Molecular epidemiology, in conjunction with classical parasitology techniques, should be used to determine the natural definitive host(s) and life cycle, and understand transmission dynamics.
Acknowledgments
We thank Brian K. Whitlock with assistance on obtaining the samples and history. We also thank Amanda Hand for assistance with manuscript preparation.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Andrews A. Some aspects of coccidiosis in sheep and goats. Small Rumin Res 2013;110:93–95. [Google Scholar]
- 2. Bowman D. Georgis’ Parasitology for Veterinarians. 10th ed. London: Elsevier, 2014:98–108. [Google Scholar]
- 3. Chartier C, Paraud C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res 2012;103:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elsheikha H. Essentials of Veterinary Parasitology. 1st ed Wymondham, UK: Caister Academic, 2011:91–112. [Google Scholar]
- 5. El-Sherry S, et al. Divergent nuclear 18S rDNA paralogs in a turkey coccidium, Eimeria meleagrimitis, complicate molecular systematics and identification. Int J Parasitol 2013;43:679–685. [DOI] [PubMed] [Google Scholar]
- 6. Fox M, et al. A case of Eimeria gilruthi infection in a sheep in northern England. Vet Rec 1991;129:141–142. [DOI] [PubMed] [Google Scholar]
- 7. Hermosilla C, et al. Fatal Eimeria gilruthi-induced abomasal coccidiosis: a still neglected parasitosis? J Vet Med Res 2016;3:1055. [Google Scholar]
- 8. Hilali M. Studies on globidial schizonts in the abomasum of Norwegian sheep. Acta Vet Scand 1973;14:22–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahmoud O. Eimeria gilruthi infection in Nadji lambs in Gassim region of central Saudi Arabia. Trop Anim Health Prod 1997;29:249–250. [DOI] [PubMed] [Google Scholar]
- 10. Maratea K, Miller M. Abomasal coccidiosis associated with proliferative abomasitis in a sheep. J Vet Diagn Invest 2007;19:118–121. [DOI] [PubMed] [Google Scholar]
- 11. Nishida T, et al. Intranuclear coccidiosis in a calf. J Vet Med Sci 2009;71:1109–1113. [DOI] [PubMed] [Google Scholar]
- 12. Reynecke DP, et al. Validation of the FAMACHA(c) eye colour chart using sensitivity/specificity analysis on two South African sheep farms. Vet Parasitol 2011;177:203–211. [DOI] [PubMed] [Google Scholar]
- 13. Sénaud J, et al. Three new types of globidium of sheep: an in vivo and in vitro investigation. Z Parasitenkd 1984;70:721–729. [DOI] [PubMed] [Google Scholar]
- 14. Soliman K. Globidium infections in the Sudan with special reference to Globidium gilruthi (Chatton, 1910) in sheep and goats. J Parasitol 1960;46:29. [PubMed] [Google Scholar]
- 15. Woods W, et al. High-resolution electrophoretic procedures for the identification of five Eimeria species from chickens, and detection of population variation. Electrophoresis 2000;21:3558–3563. [DOI] [PubMed] [Google Scholar]