Abstract
In late summer 2017, we observed acute, fatal cases of bovine viral diarrhea in captive Rocky Mountain bighorn sheep (Ovis canadensis canadensis) in Colorado following use of a contaminated modified-live bluetongue virus vaccine. Following vaccination, at least 14 of 28 (50%) vaccinated bighorn sheep developed hemorrhagic diarrhea, and 6 of 28 (21%) vaccinated bighorn sheep died. Autopsy findings were predominantly necroulcerative-to-necrohemorrhagic gastrointestinal lesions. Less frequent lesions included suffusive hemorrhages of serosal surfaces of abdominal viscera, and lymphoid necrosis in gut-associated lymphoid tissues. All of the 6 bighorn sheep that died were positive on real-time PCR (rtPCR) for bovine viral diarrhea virus (BVDV) in multiple tissues. Seroconversion to BVDV-1 and immunohistochemistry for BVDV in affected tissues confirmed rtPCR results. Next-generation sequencing confirmed a match between the infecting strain of BVDV-1b and the contaminated vaccine.
Keywords: Bighorn sheep, bovine viral diarrhea virus 1, Ovis canadensis canadensis, vaccine contamination
Bovine viral diarrhea (BVD) is a common disease of domestic cattle with a wide range of clinical signs. Acute, transient infections vary in severity, including subclinical cases, immune suppression that predisposes to other illnesses, and severe enteric and/or hemorrhagic diseases.5 Transiently infected animals shed variable amounts of bovine viral diarrhea virus (BVDV) over short periods of time with limited horizontal transmission of disease. However, transient infections of naive pregnant animals can be transmitted vertically, giving rise to persistently infected offspring that lack an immune response to the virus and shed virus at high concentrations. Persistently infected animals are a significant source of BVDV, and transmission occurs either through direct contact with bodily excretions, or indirectly through fomites or contaminated biological products (e.g., semen).4 Modified-live virus vaccines that require growth of virus in cell culture may be compromised by use of BVDV-contaminated bovine fetal serum. Outbreaks of BVD following vaccination with BVDV-contaminated vaccines have been described1,3 and may be associated with introduction of virulent strains of BVDV to previously naive populations.
Wild populations of ruminants frequently demonstrate exposure or seroconversion to BVDV,14 although reports of natural disease are limited. In populations in which BVDV was suspected to be endemic, BVDV-1 was isolated from lungs of Rocky Mountain bighorn sheep (Ovis canadensis canadensis)16 and mountain goats (Oreamnos americanum)7 that died from bacterial respiratory disease, and from the spleen of a mountain goat that died of bacterial respiratory disease.16 Experimental infections of deer (Odocoileus hemionus, Odocoileus virginianus) and elk (Cervus elaphus nelsoni) with BVDV have demonstrated viremia, shedding of virus, and seroconversion, although clinical disease was absent or mild.11–13 Persistent infections have been produced experimentally in white-tailed deer (Odocoileus virginianus),9 and natural occurrences have been detected using BVDV antigen enzyme-linked immunosorbent assays (AgELISAs), immunohistochemistry (IHC), and virus isolation from tissues of free-ranging white-tailed deer.8,10
From August 3–9, 2017, we vaccinated 28 captive Rocky Mountain bighorn sheep at a wildlife facility in Colorado using a modified-live virus against bluetongue virus serotype 10 (BTV-10; Colorado Serum Company, Denver, CO). The vaccine was used according to the manufacturer’s instructions, with vaccine reconstituted with the provided diluent and 2 mL given subcutaneously to each of the 28 sheep. A sterile syringe and needle were used for each injection. In addition to bluetongue vaccination, animals were also treated with a killed rabies vaccine (Rabvac3, Boehringer Ingelheim Vetmedica, St. Joseph, MO; n = 11), a 7-way clostridial vaccine (Vision7 with Spur, Merck Animal Health, Omaha, NE; n = 11), an injectable eprinomectin dewormer (LongRange, Merial, Duluth, GA; n = 19), a pour-on permethrin insecticide (Permectrin CDS, BayerLivestock, Shawnee Mission, KS; n = 26), and a research Pasteurellaceae vaccine (proprietary; n = 5). Vaccinated animals included 20 ewes and 8 rams. Two ewes were yearlings; all other ewes and rams were adults.
From August 14–29, 2017, at least 14 of the vaccinated bighorn sheep were observed with clinical signs of hemorrhagic diarrhea. Onset of diarrhea was 11–18 d following vaccination, with an average onset of diarrhea 14 d after vaccination. Other clinical signs observed included lethargy, decreased food intake, and mild dyspnea. One ram (case 4) demonstrated neurologic signs including star-gazing, head rolling, and head pressing, although infection by BTV-10 in this ram may have contributed to clinical signs. Leukograms were not obtained, and serum chemistry was nonspecific (hypoproteinemia and mild elevations in liver enzyme activity). Animals with diarrhea were treated every other day with flunixin meglumine and florfenicol (Resflor, Merck Animal Health, Madison, NJ). Transient responses to treatment included improved attitude and appetite. One unvaccinated bighorn lamb was in contact with 2 vaccinated, diarrheic bighorn yearlings. This in-contact lamb did not develop clinical signs of disease.
Serum was collected at the time of vaccination and again 13–25 d post vaccination from 11 bighorn sheep including 5 of the 6 animals that died. All (11 of 11) tested bighorn sheep seroconverted to BVDV-1 by virus neutralization (Table 1). Serum AgELISA for BVDV was consistently negative. Agar gel immunodiffusion serology results for BTV demonstrated detectable antibodies in 7 of 11 bighorn sheep pre-vaccination and 9 of 11 bighorn sheep post-vaccination (Table 1).
Table 1.
Pre- and post-vaccination serology for bovine viral diarrhea virus 1 (BVDV-1) and bluetongue virus, and PCR results for BVDV for captive bighorn sheep.
| ID | VN BVDV-1 |
AGID BTV |
BTV PCR spleen | BVDV |
|||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | PCR+ | PCR− | ||
| 1* | <1:8 | 1:32 | − | + | + | cecum (17.5) spleen (27.4) RLN (27.7) brain (31.7) liver (31.9) |
NA |
| 2* | ND | ND | ND | ND | − | spleen (25.1) cecum (35.5) |
NA |
| 3* | <1:8 | 1:16 | − | + | − | ILN (27.0) lung (27.5) cecum (29.1) spleen (31.0) brain (31.5) liver (35.0) |
NA |
| 4* | <1:8 | 1:16 | + | + | − | ILN (24.0) cecum (26.6) lung (26.8) spleen (29.3) brain (29.6) liver (32.8) |
NA |
| 5* | <1:8 | 1:128 | − | + | − | RLN (26.9) spleen (30.6) lung (30.6) brain (33.2) |
cecum liver |
| 6* | <1:8 | 1:256 | + | + | − | RLN (25.5) spleen (29.1) lung (29.2) liver (33.7) |
cecum |
| 7 | <1:8 | 1:128 | + | − | ND | ND | ND |
| 8 | <1:8 | 1:512 | − | − | ND | ND | ND |
| 9 | <1:8 | 1:64 | + | + | ND | ND | ND |
| 10 | <1:8 | 1:64 | + | + | ND | ND | ND |
| 11 | <1:8 | 1:64 | + | + | ND | ND | ND |
| 12 | <1:8 | 1:64 | + | + | ND | ND | ND |
AGID = agar gel immunodiffusion; BTV = bluetongue virus; cecum = cecal tissue or contents; ILN = ileocecal lymph node; NA = not applicable; ND = not done; Pre = pre-vaccination serology (date 8/3/2017 or 8/4/2017); Post = post-vaccination serology (date 8/16/2017, 8/20/2017, 8/23/2017, or 8/29/2017); RLN = retropharyngeal lymph node; VN = virus neutralization. Numbers in parentheses are PCR cycle threshold values.
Died.
Six vaccinated bighorn sheep died 16–25 d after vaccination. One animal (case 1) was euthanized at 13 d after vaccination because of neurologic signs. Preliminary testing of all dead bighorn sheep included autopsy and histopathology (fixation of tissues in 10% buffered formalin and routine processing to produce 4–6 µm thick sections stained with hematoxylin and eosin), fecal culture, fecal parasite screen, and real-time PCR (rtPCR) for epizootic hemorrhagic disease virus (EHDV) and BTV (Texas A&M Veterinary Medical Diagnostic Laboratory, College Station, TX), and BVDV (Colorado State University Veterinary Diagnostic Laboratories, Fort Collins, CO [CSU-VDL]). Fecal culture and parasite screening demonstrated mild clostridial overgrowth and mild coccidiosis, considered incidental findings for animals at this facility. All bighorn sheep tested negative by PCR for EHDV in spleen. A spleen sample from case 1 tested positive for BTV-10 by rtPCR (Table 1). All dead bighorn sheep were positive for BVDV by reverse-transcription rtPCR (RT-rtPCR) in multiple tissues (Table 1; Thermo Fisher Scientific, Applied Biosystems, Foster City, CA). Lymphoid tissue provided the lowest cycle threshold (Ct) values. Three of the BVDV-positive samples were typed using primers to detect the 5’ untranslated region (UTR) of BVDV (CSU-VDL). The 5’-UTR PCR products were sequenced using Sanger sequencing, and all 3 were consistent with BVDV-1 (results not shown). Virus isolation for BVDV on bovine nasal turbinate cells using BVDV PCR-positive cecal contents was unsuccessful.
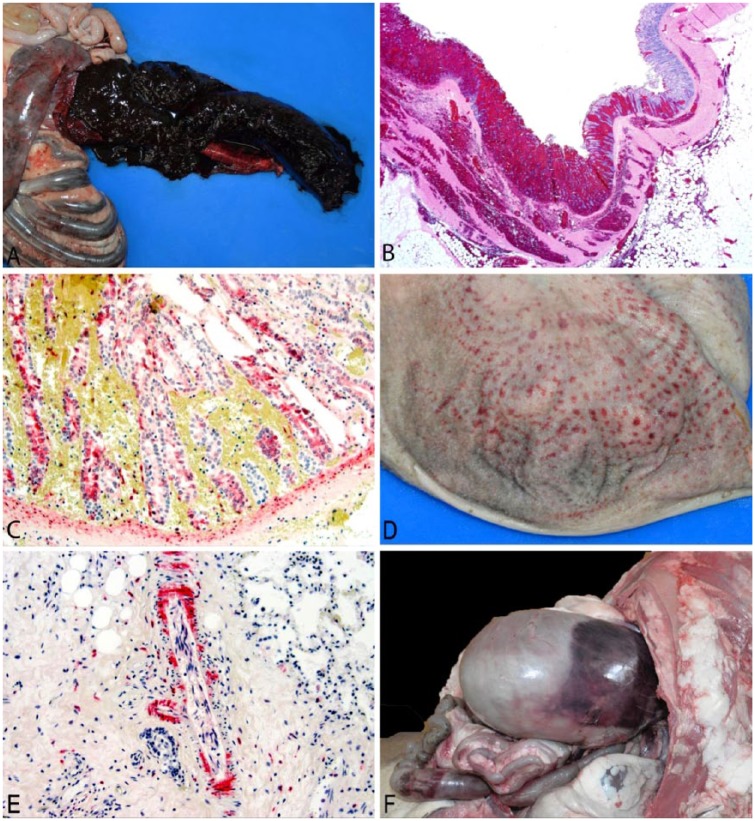
Autopsy findings were predominantly hemorrhagic typhlocolitis (Figs. 1A, 1B; Table 2) of variable severity in all 6 bighorn sheep that died. Histologically, typhlocolitis varied from acute necrohemorrhagic lesions (n = 3) to subacute necroulcerative lesions with infiltration of the submucosa by neutrophils, lymphocytes, and plasma cells (n = 3). IHC for BVDV (CSU-VDL) localized to necrotizing epithelial lesions of typhlocolitis in one case; positive staining was observed within mucosal epithelial cells and diffusely distributed within fibroblasts of the submucosa (Fig. 1C). Lymphoid necrosis of submucosal gut-associated lymphoid tissue was observed histologically in 2 cases, and lymphoid necrosis of mesenteric lymph node was observed in 1 case. Immunohistochemical staining for BVDV was localized to lesions of lymphoid necrosis in all 3 instances. IHC for B- and T-lymphocytes (PAX5 and CD3 respectively; CSU-VDL) did not demonstrate significant lymphoid depletion in the spleen when subjectively compared to spleen from a healthy control bighorn sheep. Necrohemorrhagic rumenitis was observed in 2 cases (Fig. 1D), with scant positive staining for BVDV observed in rumen epithelium adjacent to a region of hemorrhage and necrosis in 1 case. BVDV IHC also occasionally localized to scattered lymphocytes and macrophages of the lymph node (n = 2) or spleen (n = 2). In one case, antigen was diffusely localized to endothelial cells, smooth muscle, and fibroblasts associated with vessels in all tissues examined (Fig. 1E).
Figure 1.
Autopsy and histopathology from captive bighorn sheep with bovine viral diarrhea. A. Cecum opened to show necrohemorrhagic typhlitis, case 4. B. Histopathology of colon demonstrating hemorrhagic typhlocolitis from case 4 with full-thickness hemorrhage and mucosal necrosis. H&E. 20×. C. Immunohistochemistry (IHC) of panel B; positive staining for bovine viral diarrhea virus (BVDV) in mucosal epithelium and submucosal fibroblasts. BVDV IHC. 200×. D. Rumen opened to show necrohemorrhagic rumenitis, case 5. E. Immunohistochemistry of lung from case 6 showing diffuse staining of perivascular smooth muscle and fibroblasts, as well as a few scattered lymphocytes and macrophages. BVDV IHC. 200×. F. Suffusive hemorrhage on ruminal serosa, case 2.
Table 2.
Pathology and immunohistochemistry associated with bovine viral diarrhea infection in captive bighorn sheep.
| ID | DPV | Lesions | BVDV IHC distribution |
|---|---|---|---|
| 1 | 13* | Acute necrohemorrhagic typhlocolitis; ecchymotic-to-suffusive serosal hemorrhages of cecum and/or colon; acute multifocal hemorrhages of caudal thalamus and brainstem; endocardial hemorrhages; pulmonary edema; perivascular edema of brain. | Mucosal epithelial cells and submucosal fibroblasts associated with necrotizing typhlocolitis; rare cortical neurons of brain. |
| 2 | 16† | Hemorrhagic typhlocolitis; ecchymotic-to-suffusive serosal hemorrhages of cecum, colon, and rumen; autolysis prevented histopathology interpretation. | Not done. |
| 3 | 17† | Necroulcerative typhlocolitis with submucosal infiltrates of neutrophils, lymphocytes, and plasma cells; ecchymotic serosal hemorrhages of cecum and/or colon; endocardial hemorrhages; pulmonary edema; scattered pulmonary hemorrhages; minimal fibrinous alveolitis; perivascular edema of brain. | Scattered lymphocytes and macrophages in spleen. |
| 4 | 17† | Subacute necrohemorrhagic typhlocolitis with submucosal infiltrates of neutrophils, lymphocytes, and plasma cells, and crypt abscesses; necrohemorrhagic rumenitis; ecchymotic-to-suffusive serosal hemorrhages of cecum and/or colon; endocardial hemorrhages; mild lymphoplasmacytic portal hepatitis; perivascular edema of brain. | Scant staining of rumen epithelium associated with necrotizing rumenitis; scattered lymphocytes and macrophages in mesenteric lymph node. |
| 5 | 19† | Necroulcerative typhlocolitis; necrohemorrhagic rumenitis; lymphoid necrosis of GALT in cecum and/or colon; ecchymotic-to-suffusive serosal hemorrhages of cecum, colon, and rumen; endocardial hemorrhages; pulmonary edema; scattered pulmonary hemorrhages; mild fibrinous alveolitis and multifocal suppurative pneumonia; perivascular edema of brain. | Lymphocytes associated with necrosis of GALT. |
| 6 | 25† | Necroulcerative typhlocolitis; lymphoid necrosis of GALT and mesenteric lymph node; endocardial hemorrhages; pulmonary edema; mild fibrinous alveolitis; necrosuppurative hepatitis; mild portal lymphoplasmacytic hepatitis; perivascular edema of brain. | Lymphocytes associated with necrosis of GALT and mesenteric lymph node; scattered lymphocytes and macrophages in mesenteric lymph node and spleen; endothelial cells, smooth muscle, and fibroblasts of vasculature in all tissues examined; fibroblasts of gastrointestinal submucosa. |
BVDV = bovine viral diarrhea virus; DPV = days post-vaccination; GALT = gut-associated lymphoid tissue; IHC = immunohistochemistry.
Euthanized.
Died.
In 5 of 6 bighorn sheep that died, ecchymotic-to-suffusive hemorrhages on the serosal surfaces of the cecum, colon, and/or rumen (Fig. 1F) suggested a hemorrhagic disorder, although whole blood was not available to evaluate for thrombocytopenia. Infection with BTV may have contributed to hemorrhagic lesions in case 1. Autopsy findings that were attributed to nonspecific signs of systemic disease (likely associated with compromise of the gastrointestinal mucosal barrier) included endocardial hemorrhages (n = 6), pulmonary edema (n = 4), pulmonary hemorrhages (n = 2), mild fibrinous alveolitis (n = 3), necrosuppurative hepatitis (n = 1), mild portal lymphoplasmacytic hepatitis (n = 2), and multifocal suppurative pneumonia (n = 1). Aerobic culture of lung with suppurative pneumonia yielded heavy growth of Escherichia coli, supporting a hematogenous route of infection from the gastrointestinal tract. Aerobic culture of liver with necrosuppurative hepatitis yielded no significant growth, although the animal had been treated repeatedly with antibiotics prior to death, and antibiotics may have interfered with bacterial isolation.
Case 1 was euthanized because of neurologic signs and had severe multifocal acute hemorrhages within the caudal thalamus and brainstem, which were not observed in any other cases. Although rare cortical neurons in the brain of this animal did stain positively by BVDV immunohistochemical staining, case 1 was the only animal found to be positive for BTV in spleen by rtPCR. We suspect that hemorrhages in the brain and clinical neurologic signs were attributable to BTV infection in this animal, and that BTV infection was likely the result of vaccination. BTV from spleen of the affected bighorn sheep was identified as BTV-10 (the vaccine serotype) by rtPCR; the predominant circulating serotype of BTV in Colorado at that time was BTV-17. Coinfection with BVDV may have contributed to BTV pathogenicity.
To determine the source of BVDV in this outbreak, we screened the BTV-10 vaccine, rabies vaccine, 7-way clostridial vaccine, and injectable dewormer for BVDV using the same RT-rtPCR assay that was used to detect BVDV in autopsy samples. Only the BTV-10 vaccine was positive for BVDV (Ct = 27.5), and Sanger sequencing demonstrated 100% homology between the vaccine and autopsy samples for the 5’-UTR fragment. Virus isolation for BVDV was unsuccessful using the BTV-10 vaccine applied to bovine nasal turbinate cells.
To further investigate a possible match between BVDV detected in the vaccine and in the autopsy samples, we used RT-PCR and Sanger sequencing to examine the NPro region2,15 (Supplementary Data) of BVDV from cecal contents of 2 dead bighorn sheep (cases 3 and 4) and the BTV-10 vaccine. The NPro regions (365 nucleotides in length) shared 100% identity between the autopsy samples and the vaccine, with 95.1% and 96.4% identity to the next closest GenBank sequences, KT355592 and KC963967, respectively. Sequencing of NPro regions indicated that the vaccine strain and virus detected in bighorn samples were highly homologous and were most closely related to other BVDV-1b viruses.
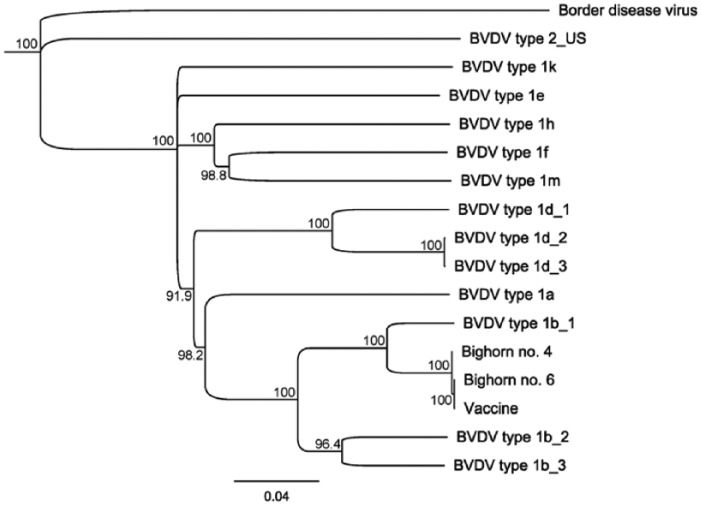
Based on highly homologous 5’-UTR and Npro BVDV sequences detected in bighorn cases and the BTV-10 vaccine, we pursued next-generation sequencing (NGS) to evaluate homology across the BVDV genome (Supplementary Data). Whole genomes of 3 samples (vaccine strain and lymph nodes from cases 4 and 6) were sequenced via NGS. Lymph nodes from cases 4 and 6 had the lowest Ct values of all tissues tested via RT-rtPCR, making these samples the best candidates for whole-genome sequencing. NGS produced single, near full-length BVDV consensus sequences from the vaccine strain and case 6. Case 4 attained lower coverage, resulting in 8 non-overlapping contigs covering 97% of the reference sequence. Sequence alignments showed that the vaccine strain and virus from cases 4 and 6 had >99.9% similarity across the sequenced portions of the BVDV genome. The sequences from all 3 samples clustered together with other BVDV-1b isolates with high bootstrap support when compared to diverse BVDV sequences (Fig. 2). When assembled and aligned to all BVDV sequences in GenBank, the vaccine strain had greatest similarity (94.1% identity) to a non-cytopathic BVDV-1b virus identified as a cell culture contaminant in RK-13 cells (GenBank accession KT355592.1).6 These findings validated the high sequence similarity between the vaccine strain and the virus detected in the bighorn sheep, in addition to demonstrating that the virus belongs to the BVDV-1b subgenotype.
Figure 2.
Phylogenetic tree including next-generation sequencing results for cases 4 (Bighorn no. 4), 6 (Bighorn no. 6), and BTV-10 vaccine (vaccine). Neighbor-joining tree based on HKY genetic distance of near full-length (open reading frame: 11,696 bp) bovine viral diarrhea virus (BVDV) sequences with 1,000 bootstrapping replicates. Nodes with >70% support are shown. The vaccine strain and bighorn samples cluster together and with other BVDV-1b subgenotypes.
Our diagnostic investigation was greatly enhanced by NGS, a tool that may be underutilized in wildlife disease investigations. Definitively determining the source of virus in this disease event facilitated management of the outbreak. There was no evidence that the virus was transmitted horizontally, and subsequent RT-rtPCR of buffy coat from whole blood suggested that BVDV infection was transient in the surviving animals (data not shown). The herd was not bred in the fall, and BVDV testing will be repeated prior to any future breeding. We found lymphoid tissues to be the sample of choice for PCR detection.
Interestingly, we were unable to demonstrate BVDV replication, by standard virus isolation methods, from PCR-positive cecal contents and the BTV-10 vaccine. Virus isolation from the vaccine demonstrated cell death in bovine nasal turbinate cells; however, RT-rtPCR of the cell culture supernatant yielded a lower Ct value for BVDV than the pure vaccine (supernatant Ct = 34.1 vs. vaccine Ct = 27.5), and we suspect cell death was caused by BTV infection. Virus isolation from cecal contents that were found to be positive for BVDV by PCR showed no cytopathic effects on bovine nasal turbinate cells. Despite our inability to demonstrate infection in vitro from the vaccine or autopsy samples, infection in vivo was demonstrated by development of BVD-type lesions, detection of BVDV antigen within these lesions, widespread IHC detection of intracellular BVDV antigen in non-phagocytic cells, seroconversion to BVDV, and apparent replication in the host. Using RT-rtPCR to detect BVDV, many of the autopsy samples from bighorn sheep that died yielded Ct values lower than the Ct value acquired from the vaccine (Table 1). The negative virus isolation results are concerning however, given that screening processes in production may rely on virus isolation to detect contamination. This disease outbreak raises awareness of animal safety risks when using vaccines, particularly modified-live virus vaccines, in free-ranging and captive wildlife.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638718814583 for Bovine viral diarrhea in captive Rocky Mountain bighorn sheep associated with administration of a contaminated modified-live bluetongue virus vaccine by Karen A. Fox, Jennifer H. Kopanke, Justin S. Lee, Lisa L. Wolfe, Kristy L. Pabilonia and Christie E. Mayo in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Michael Miller, Todd Bass, Ivy LeVan, Shari Green, Sierra Amundson, Mark Fisher, Christina Weller, and Kirsten Reed for their assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Barkema HW, et al. [Outbreak of bovine virus diarrhea on Dutch dairy farms induced by a bovine herpesvirus 1 marker vaccine contaminated with bovine virus diarrhea virus type 2]. Tijdschr Diergeneeskd 2001;126:158–165. Dutch. [PubMed] [Google Scholar]
- 2. Deng M, et al. Prevalence study and genetic typing of bovine viral diarrhea virus (BVDV) in four bovine species in China. PLoS One 2015;10:e0121718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falcone E, et al. Bovine viral diarrhea disease associated with a contaminated vaccine. Vaccine 1999;18:387–388. [DOI] [PubMed] [Google Scholar]
- 4. Khodakaram-Tafti A, Farjanikish GH. Persistent bovine viral diarrhea virus (BVDV) infection in cattle herds. Iran J Vet Res 2017;18:154–163. [PMC free article] [PubMed] [Google Scholar]
- 5. Lanyon SR, et al. Bovine viral diarrhoea: pathogenesis and diagnosis. Vet J 2014;199:201–209. [DOI] [PubMed] [Google Scholar]
- 6. Nam B, et al. Complete genome sequence of noncytopathic bovine viral diarrhea virus 1 contaminating a high-passage RK-13 cell line. Genome Announc 2015;3:e01115-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson DD, et al. Evidence for persistent bovine viral diarrhea virus infection in a captive mountain goat (Oreamnos americanus). J Vet Diagn Invest 2008;20:752–759. [DOI] [PubMed] [Google Scholar]
- 8. Passler T, et al. Bovine viral diarrhea virus (BVDV) in white-tailed deer (Odocoileus virginianus). Front Microbiol 2016;7:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Passler T, et al. Transmission of bovine viral diarrhea virus among white-tailed deer (Odocoileus virginianus). Vet Res 2010;41:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pogranichniy RM, et al. Prevalence and characterization of bovine viral diarrhea virus in the white-tailed deer population in Indiana. J Vet Diagn Invest 2008;20:71–74. [DOI] [PubMed] [Google Scholar]
- 11. Ridpath JF, et al. Febrile response and decrease in circulating lymphocytes following acute infection of white-tailed deer fawns with either a BVDV-1 or a BVDV-2 strain. J Wildl Dis 2007;43:653–659. [DOI] [PubMed] [Google Scholar]
- 12. Tessaro SV, et al. Viremia and virus shedding in elk infected with type 1 and virulent type 2 bovine viral diarrhea virus. J Wildl Dis 1999;35:671–677. [DOI] [PubMed] [Google Scholar]
- 13. Van Campen H, et al. Experimental infection of deer with bovine viral diarrhea virus. J Wildl Dis 1997;33:567–573. [DOI] [PubMed] [Google Scholar]
- 14. Vilcek S, et al. Pestiviruses in wild animals. Vet Microbiol 2006;116:1–12. [DOI] [PubMed] [Google Scholar]
- 15. Vilcek S, et al. Bovine viral diarrhoea virus genotype 1 can be separated into at least eleven genetic groups. Arch Virol 2001;146:99–115. [DOI] [PubMed] [Google Scholar]
- 16. Wolff PL, et al. Evidence of bovine viral diarrhea virus infection in three species of sympatric wild ungulates in Nevada: life history strategies may maintain endemic infections in wild populations. Front Microbiol 2016;7:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638718814583 for Bovine viral diarrhea in captive Rocky Mountain bighorn sheep associated with administration of a contaminated modified-live bluetongue virus vaccine by Karen A. Fox, Jennifer H. Kopanke, Justin S. Lee, Lisa L. Wolfe, Kristy L. Pabilonia and Christie E. Mayo in Journal of Veterinary Diagnostic Investigation