Abstract
We describe the clinicopathologic findings, relative prevalence, and pathogens associated with infectious keratoconjunctivitis in mule deer (Odocoileus hemionus) in Wyoming. Seventeen cases with ocular lesions were identified among 1,036 mule deer postmortem submissions (1.6%) in an ~16 y period. Sixteen cases were observed in winter and most were in male (15 cases) and juvenile (13 cases) deer. Blindness was the most commonly reported clinical sign (10 cases). A herpesvirus was detected only in the 4 cases of bilateral necrotizing bulbar conjunctivitis. Phylogenetic analysis of glycoprotein amino acid sequences consistently identified this virus as a novel alphaherpesvirus. In 2 of these herpesvirus-positive cases, Actinomyces sp. and Moraxella ovis were also identified. Trueperella pyogenes was identified in 4 cases of unilateral ulcerative keratitis, keratoconjunctivitis, and panophthalmitis. M. ovis was cultured from 3 cases of bilateral conjunctivitis and keratoconjunctivitis. In the remaining cases, isolates included Moraxella bovis (1 case), Staphylococcus sp. and Streptococcus sp. (2), Flavobacterium sp. and Pseudomonas sp. (2), Escherichia coli and Enterobacter sp. (1), and bovine viral diarrhea virus 1 (1). No pathogens were identified in 2 cases. The relative prevalence of keratoconjunctivitis in mule deer in Wyoming appears to be low, and this disease is most commonly associated with infection by a novel alphaherpesvirus, T. pyogenes, and M. ovis.
Keywords: Alphaherpesvirus, infectious keratoconjunctivitis, Moraxella sp., mule deer
Introduction
Mule deer (Odocoileus hemionus) are an iconic and common cervid species in the western United States. Based on field records and observations, it was estimated that Wyoming harbored ~578,000 mule deer in 1991, and despite ample habitat and regulated hunting activities, mule deer populations decreased to ~353,000 animals (39% population decline) by 2013 (Wyoming Game and Fish Department, The Wyoming Mule Deer Initiative, v.02/22/2018, https://wgfd.wyo.gov/WGFD/media/content/PDF/Habitat/Mule%20Deer%20Initiative/Mule-Deer-Initiative.pdf). Overall, major attributable causes of mule deer decline include starvation, habitat degradation, urbanization and intensive land uses, extreme weather events, interspecies habitat competition, and infectious diseases (The Wyoming Mule Deer Initiative). Mule deer are susceptible to a wide variety of infectious agents,24,34,36 but chronic wasting disease accounts perhaps for most mule deer mortalities, more than any other infectious disease in the state of Wyoming (TE Creekmore, pers. comm., 2017). Nevertheless, the specific impact of infectious disease on mule deer population decline is unclear. Documentation of the prevalence and cause of infectious disease occurring in mule deer and other wildlife species is important not only to assess disease frequency, but also to modify current management strategies that will ultimately enhance species conservation and help to evaluate the status of such disease at the livestock–wildlife interface.
One infectious disease reported in mule deer is infectious keratoconjunctivitis (IKC), a multifactorial disease that was first documented in Wyoming in 1944.23 IKC can progress from impaired vision to blindness, which increases the likelihood of predation, fatal accidents, and starvation.8,15 To our knowledge, there are no large retrospective studies regarding the prevalence, clinicopathologic features, and pathogens associated with IKC in mule deer. Documentation of IKC in mule deer has been limited to case reports of ocular lesions associated with infection by Moraxella ovis,7,26 Chlamydia sp.,26 Thelazia californiensis,26 Yersinia pestis,9 and poxvirus.35 Thus, we 1) determined the relative prevalence of IKC in Wyoming mule deer submitted to the Wyoming State Veterinary Laboratory (WSVL, Laramie, WY), 2) documented the gross and microscopic lesions in the cases identified, and 3) determined the most common infectious agents associated with IKC in mule deer.
Materials and methods
Animals
Mule deer postmortem submissions to the WSVL from November 2000 through February 2016 were considered for our study and examined retrospectively. Cases with a diagnosis of ocular lesions, including keratitis, conjunctivitis, keratoconjunctivitis, and panophthalmitis, were collated. Cases included whole carcasses and heads submitted by veterinarians, wildlife biologists, and/or hunters for diagnostic testing.
Gross examination and histopathology
In all cases, macroscopic examination of eyelids, conjunctiva, and cornea was completed. Histopathology of globes was performed in 12 of 17 submissions (cases 1–3, 5–8, 10, 12–14, 17). Briefly, globes were dissected along with eyelids and were placed in Davidson13 solution for 24–48 h at room temperature. After fixation, globes were cross-sectioned perpendicularly to the long posterior ciliary artery and adjacent to the optic nerve for routine histology processing and hematoxylin and eosin staining.
Ancillary tests
Swabs and fresh conjunctival and corneal samples were collected aseptically from all of the cases of our study and submitted and processed for bacterial culture, viral isolation, and immunofluorescence for Chlamydia spp. For cases with unilateral gross lesions (cases 2, 3), cross-sections of both globes were examined microscopically. However, only the globe with gross lesions was sampled for ancillary testing. For cases with different lesions in each globe, both globes were sampled. Each sample was plated for isolation on Columbia blood agar with 5% sheep blood and MacConkey agar (Hardy Diagnostics, Santa Maria, CA) and incubated at 37°C in 10% CO2 for 48 h. Initial plate readings occurred at 24 h by the same technician, and if colonies were present at 24 or 48 h, the colonies were subcultured for additional biochemical testing and identification. In some (cases 2, 3, 14, 16), samples were tested (per prosector request) by PCR for ovine herpesvirus 2 (Ovine gammaherpesvirus 2), bovine viral diarrhea virus (BVDV) 1 and 2 (Pestivirus A and B), and bovine herpesvirus 1 (Bovine alphaherpesvirus 1). Fresh samples were tested by virus isolation using bovine embryonic testicle, white-tailed deer umbilical vein, or bovine arterial endothelium cells. Briefly, cells were grown to 60–70% confluence, drained of medium, inoculated with swabs or conjunctival and/or corneal macerates diluted 1:10 in medium, and incubated for 1 h at 37°C. The inoculum was then removed and replaced with minimal essential medium (Cellgro, Corning, Manassas, VA), supplemented with 4% fetal bovine serum (VWR Seradigm, Avantor, Providence, UT) and 1× penicillin–streptomycin (Lonza, Walkersville, MD; 100 units/mL and 100 μg/mL), and cells were incubated at 37°C with 5% CO2. Cultures were monitored on a daily basis, and the supernatant of those with obvious cytopathic effect was further examined for the presence of virions with negative staining with 1.5% phosphotungstic acid and transmission electron microscopy.
Viral re-isolation, next-generation sequencing, and phylogenetic analysis
Viral re-isolation
Stock isolates from 3 submissions (cases 5, 7, 8) were used to inoculate Madin–Darby bovine kidney (MDBK) cells, and expanded for DNA extraction and next-generation sequencing. Briefly, virus from second or third passage was grown in 150-cm flasks to ≥90% cytopathic effect, frozen and thawed, and clarified by centrifugation at 5,000 × g for 20 min prior to centrifugation through a 20% sucrose cushion at 107,000 × g for 12 h. Pellets were resuspended in 400 μL of PBS, and DNA was extracted using commercial filter columns (Blood and tissue kit, Qiagen, Valencia, CA) according to the manufacturer’s directions.
Next-generation sequencing and sequence assembly
Shotgun libraries were prepared (Hyper Library Construction kit, Kapa Biosystems, Wilmington, MA) and sequenced (Nano V2 kit, Illumina MiSeq; Oxford Nanopore sequencing; High-Throughput Sequencing and Genotyping Unit, Roy J. Carver Biotechnology Center/WM Keck Center, University of Illinois at Urbana-Champaign). Oxford Nanopore sequences generated long reads with median lengths of >1,400 bp, capable of bridging repeat regions. These data were assembled using Canu Assembly v.1.314 to generate a full genome length backbone. The draft assembly was corrected using the MiSeq paired-end sequence data using PILON v.1.18.30 Assembly was also performed using the Oxford data-generated backbone with MIRA 4.0.2 (https://sourceforge.net/projects/mira-assembler/) and produced similar results.
Phylogenetic analysis
Phylogenetic relationship of the herpesvirus isolated from cases 5, 7, and 8 with other related ruminant alphaherpesviruses was estimated using amino acid sequences of surface glycoproteins B (gB), C (gC), and D (gD). Ruminant alphaherpesviruses included: bovine herpesvirus 1 and 5 (BoHV-1, -5; Bovine alphaherpesvirus 1 and 5), water buffalo herpesvirus (BuHV; Bubaline alphaherpesvirus 1), caprine herpesvirus 1 (CapHV-1; Caprine alphaherpesvirus 1), cervid herpesvirus 1 and 2 (CerHV-1, -2; Cervid alphaherpesvirus 1 and 2), and elk herpesvirus (ElkHV). In addition, equine herpesvirus 1 (EHV-1; Equid alphaherpesvirus 1) and pseudorabies virus (PRV; Suid alphaherpesvirus 1) were included as outliers. Multiple sequence alignment was performed with the Clustal Omega method25 (MegAlign Pro v.13, DNASTAR, Madison, WI) based on protein coding amino acid sequences for gB (UL27), gC (UL44), and gD (US6). The reliability of phylogenetic trees was tested by bootstrapping using neighbor-joining methodology with 1,000 trials of 111 random seed. Sequence distance was calculated as uncorrected pairwise distance with global gap removal between the compared amino acid sequences.
Sanger sequencing
Sanger sequencing was used to verify whole genome sequences in the glycoprotein coding regions that were used in phylogenetic analysis. Primers were used to amplify at least 500 bp of each gene (Supplementary Table 1). PCR reactions included 0.5 μM primer with Apex 2.0× master mix (Genesee Scientific, San Diego, CA), 0.1 volume DNA template, and 0.2 volume Q reagent (Qiagen). PCR conditions were 94ºC for 2 min, 40 cycles of 94ºC for 30 s and 72ºC for 45 s, then a final 72ºC for 2 min and 4ºC hold. Amplicons were purified using spin columns (DNA clean and concentrator, Zymo, Irvine, CA) according to the manufacturer’s instructions, and then sequenced (GENEWIZ, South Plainfield, NJ). Glycoprotein nucleotide sequences have been deposited in GenBank as accessions KY748207 (MDHV_gB), KY748208 (MDHV_gC), and KY748209 (MDHV_gD).
Results
Animals and signalment
A total of 1,036 mule deer postmortem submissions were identified at the WSVL within the study period. Seventeen cases with ocular lesions were found (Supplementary Table 2). These cases were received for diagnostic workup during the months of November (6 cases), December (4 cases), January and February (3 cases each), and June (1 case). Thirteen cases corresponded to carcasses submitted for postmortem examination; in 4 cases, only the head was available for gross examination. Fifteen cases were male, 1 case was female, and the sex was not reported for 1 case (case 11). Thirteen cases corresponded to juvenile mule deer, 3 cases were adults, and the approximate age was not reported for 1 case (case 11). Carcasses and heads were collected from the following Wyoming counties: Sweetwater and Fremont (5 cases each); Carbon (3 cases); Lincoln, Albany, and Sheridan (1 case each). The location was not reported for 1 case (case 11). The clinical history was known for all cases and included blindness (10 cases), lesions suggestive of eye infection (5 cases), and circling (2 cases). Ten animals were euthanized, and 1 was found dead. For the remaining cases, it was not reported whether animals were euthanized or found dead.
Gross lesions
Bilateral purulent conjunctivitis (Fig. 1; cases 7, 9, 10, 12, 13, 16, 17) and keratoconjunctivitis (Fig. 2; cases 3–6, 8, 11, 15) were observed in 7 cases each (Table 1). Corneal ulceration (Fig. 3) was unilateral (cases 2, 3, 9) and bilateral (cases 10, 11, 14) in 3 cases each. Unilateral purulent panophthalmitis with contralateral proliferative keratitis (Fig. 4) was seen in 1 case (case 1). Corneal opacity (Fig. 1) was observed in 4 cases of conjunctivitis (cases 7, 10, 16, 17), 1 case of keratoconjunctivitis (case 3), and 1 case of corneal ulceration (case 14).
Figure 1.
Purulent conjunctivitis with corneal opacity of the right globe of a mule deer (case 7). Abundant purulent exudate is adhered to the eyelids and conjunctiva. The cornea is diffusely opaque. This lesion was bilateral and was associated with infection by herpesvirus and Actinomyces sp. Figure 2. Purulent keratoconjunctivitis of the left globe of a mule deer (case 3). The cornea is diffusely thickened by purulent exudate, fibrin, and neovascularization with hyperemia. Purulent exudate is adhered to the lower eyelid and medial canthus. This lesion was unilateral and associated with infection by Trueperella pyogenes. Figure 3. Focal corneal ulceration with purulent conjunctivitis of the right globe of a mule deer (case 10). The cornea is diffusely opaque with a central area of ulceration flanked by a rim of neovascularization. Purulent exudate is seen along the eyelids. Moraxella ovis was cultured from this lesion, which was bilateral. Figure 4. Proliferative keratitis of the left globe of a mule deer (case 1). The center of the cornea is markedly thickened by a focally extensive, raised lesion composed of granulation tissue. No pathogens were cultured from this lesion; severe panophthalmitis associated with Trueperella pyogenes was seen in the contralateral globe.
Table 1.
Ocular lesions and pathogens identified in mule deer (Odocoileus hemionus) postmortem submissions to the Wyoming State Veterinary Laboratory from November 2000 through February 2016.
| Case | Gross and/or microscopic lesions | Pathogen(s) identified |
|---|---|---|
| 1 | Chronic panophthalmitis (R) Chronic and ulcerative keratitis (L) |
Trueperella pyogenes (R) |
| 2 | Ulcerative keratitis with anterior synechia (L) | T. pyogenes |
| 3 | Ulcerative keratitis with anterior synechia (L) | T. pyogenes |
| 4 | Bilateral, purulent keratoconjunctivitis | T. pyogenes |
| 5 | Bilateral, necrotizing, and fibrinosuppurative, bulbar conjunctivitis | Herpesvirus |
| 6 | Bilateral, necrotizing, and fibrinosuppurative, bulbar conjunctivitis | Herpesvirus |
| 7 | Bilateral, necrotizing, and fibrinosuppurative, bulbar conjunctivitis | Herpesvirus and Actinomyces sp. |
| 8 | Bilateral, necrotizing, and fibrinosuppurative, bulbar conjunctivitis | Herpesvirus and Moraxella ovis (L) |
| 9 | Bilateral, purulent conjunctivitis Corneal ulceration (L) |
M. ovis and M. bovis |
| 10 | Bilateral, chronic keratoconjunctivitis | M. ovis (L) |
| 11 | Bilateral, purulent, and ulcerative keratoconjunctivitis | Pestivirus, Staphylococcus sp., and Streptococcus sp. |
| 12 | Bilateral, fibrinosuppurative, and ulcerative keratoconjunctivitis | Staphylococcus sp. and Streptococcus sp. |
| 13 | Bilateral, fibrinosuppurative, and ulcerative keratoconjunctivitis | Flavobacterium sp. and Pseudomonas sp. |
| 14 | Chronic keratitis (L) Ulcerative keratitis with anterior uveitis (R) |
E. coli (L) |
| 15 | Bilateral, purulent keratoconjunctivitis | Enterobacter sp. |
| 16 | Bilateral, purulent conjunctivitis with corneal opacity | None |
| 17 | Bilateral, fibrinosuppurative, and ulcerative keratoconjunctivitis | None |
L = left globe; R = right globe.
Microscopic lesions
Histopathology was completed in 12 of 17 cases (cases 1–3, 5–8, 10, 12–14, 17; Table 1). Keratoconjunctivitis was observed in 5 cases and was either ulcerative and fibrinosuppurative (cases 12, 13, 17), necrosuppurative (case 7), or chronic and ulcerative with anterior synechia (Fig. 5; case 10). Ulcerative keratitis was observed in 4 cases (Fig. 6; cases 1–3, 14); this lesion was unilateral in 3 (cases 1–3) and bilateral in 1 (case 14). Anterior synechia was seen in 2 of the aforementioned cases of unilateral keratitis (cases 2, 3). Bilateral, necrotizing, and fibrinosuppurative bulbar conjunctivitis with lymphoplasmacytic perivasculitis was observed in 4 cases (Fig. 7; cases 5–8). In 1 case (case 8), intranuclear inclusions were observed in a few remaining epithelial cells of the bulbar conjunctiva (Fig. 8). Unilateral chronic panophthalmitis was seen in 1 case (case 1).
Figure 5.
Keratoconjunctivitis with anterior synechia in a mule deer (case 10). Large collections of neutrophils along with edema and fibrin obscure and infiltrate the corneal epithelium (arrow) and stroma (CS). The Descemet membrane is focally disrupted (asterisk), and the iris is adhered to the inner aspect of the cornea (anterior synechia, AS). H&E. Moraxella ovis was cultured from this lesion. Figure 6. Chronic ulcerative keratitis in a mule deer (case 2). A full-thickness, perforating ulcer disrupts the cornea, and inflammatory cells infiltrate the anterior chamber. The corneal epithelium and stroma are replaced and expanded by inflammatory cells and granulation tissue. H&E. Trueperella pyogenes was cultured from this lesion. Figure 7. Necrotizing and fibrinosuppurative, bulbar conjunctivitis in a mule deer (case 5). The epithelium of the bulbar conjunctiva is ulcerated and replaced by large numbers of macrophages and neutrophils admixed with fibrin and necrotic cell debris (asterisk). Inflammatory cells infiltrate the subepithelial connective tissue. Many blood vessels (arrows) are flanked by lymphocytes, plasma cells, and macrophages. H&E. A herpesvirus was isolated from this lesion. Figure 8. Necrotizing bulbar conjunctivitis in a mule deer (case 8). A few remaining epithelial cells of the bulbar conjunctiva contain intranuclear, amphophilic, inclusion bodies with margination of chromatin (arrows). H&E. Figures 9 and 10. Supernatant from tissue-inoculated bovine embryonic testicle cells (cases 7 and 8). A single, 100–120 nm diameter, enveloped, and icosahedral virion is depicted. Symmetrically arranged capsomeres are visible in Figure 10. Transmission electron micrograph, negative staining with 1.5% phosphotungstic acid. Bar = 100 nm.
Ancillary tests
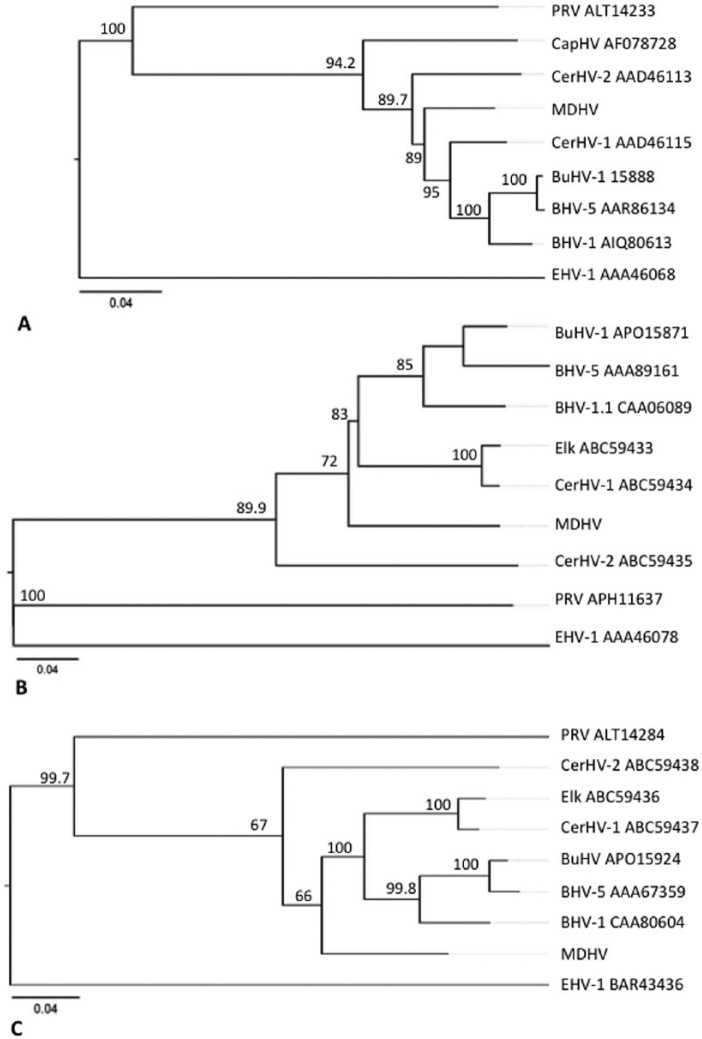
Trueperella pyogenes was cultured from 4 cases (cases 1–4). A herpesvirus was identified in 4 cases (cases 5–8) through isolation and electron microscopy (Figs. 9, 10). Testing for BoHV-1 using fluorescent antibody assay and PCR was negative in these cases. Viral whole genome sequences were obtained from 3 of these cases (cases 5, 7, 8). Conserved glycoprotein gene sequences (gB, gC, gD) were confirmed, and the deduced amino acid sequences were used to determine ancestry with related ruminant alphaherpesviruses. The glycoprotein sequences of mule deer–derived herpesviruses were identical to each other, but unique from previously described ruminant alphaherpesviruses. The nearest ancestors based on the highly conserved gB protein was CerHV-1 with 7% divergence; BoHV-1, BoHV-5, BuHV, and CerHV-2 with 9–10% divergence; and CapHV with 14% divergence (Supplementary Table 3; Fig. 11A). For gC and gD sequences, divergence from other related viruses was greater; the closest ancestors included CerHV-1, BoHV-1, BoHV-5, BuHV, and ElkHV (19–23% divergence), and CerHV-2 with divergence of 30% and 25% for gC and gD, respectively (Supplementary Table 3; Fig. 11B, 11C). Sanger sequencing was used to confirm glycoprotein gene sequences in all herpesviruses isolated (including the virus isolated from case 6).
Figure 11.
Phylogenetic trees of the herpesvirus surface glycoprotein amino acid sequences A. gB, B. gC, and C. gD. Neighbor-joining phylogenetic trees illustrate ancestry estimated using Clustal Omega in MegAlign Pro, and show the phylogenetic relationship between herpesviral isolates (cases 5, 7, 8; mule deer herpesvirus, MDHV) and related ruminant alphaherpesvirus. Reference strains included: bovine herpesvirus 1 and 5 (BoHV-1, -5), cervid herpesvirus 1 and 2 (CerHV-1, -2), water buffalo herpesvirus (BuHV), and caprine herpesvirus (CapHV). Equine herpesvirus 1 (EHV-1) and pseudorabies virus (PRV; suid herpesvirus) were included as alphaherpesvirus outliers. Reference strains with complete cds were retrieved from GenBank, and accession identifiers are shown. Reliability of the inferred trees was tested by bootstrapping (bootstrap trials = 1,000; seed = 111). Bars indicate amino acid substitution per site.
Moraxella ovis was cultured from 3 cases (cases 8–10). Polymicrobial infections were seen in 6 cases, which included: M. ovis and M. bovis (case 9); M. ovis and herpesvirus (case 8); Actinomyces sp. and herpesvirus (case 7); BVDV-1, Staphylococcus sp., and Streptococcus sp. (case 11); Flavobacterium sp. and Pseudomonas sp. (case 13), and Staphylococcus sp. and Streptococcus sp. (case 12). Escherichia coli and Enterobacter sp. were cultured from 1 case each (cases 14, 15, correspondingly). Ancillary tests failed to identify pathogens in 2 cases (cases 16, 17). Immunofluorescence for Chlamydia spp. was negative in all cases. PCR for BoHV-1 and OvHV-2 was negative in 4 cases tested (cases 2, 3, 14, 16).
Discussion
We described the relative prevalence, clinicopathologic features, and pathogens associated with IKC in mule deer in Wyoming. Although the precise prevalence of infectious disease in wildlife is difficult or impossible to determine, our results suggest that the prevalence of IKC in mule deer is low (1.6% of the postmortem submissions to the WSVL in an ~16 y period). In addition, the relative prevalence of IKC in mule deer in Wyoming is lower than that reported for cases of bovine IKC.16,31 Moreover, the impact of IKC on mule deer population decline within the state of Wyoming is likely low.
In our study, most cases with ocular lesions were observed in male and juvenile mule deer, blindness was the most common clinical sign, and all cases but one were diagnosed during the winter season. Therefore, it could be speculated that the harsh winter conditions (e.g., blowing snow and ice crystals) within the region of study may cause ocular abrasions and increase the susceptibility of juvenile mule deer to infection with IKC pathogens. The reason for male mule deer overrepresentation is unclear. Male mule deer frequently rub their face on trees or shrubs to mark their territory during the rut.11 Thus, it could be that ocular lesions develop secondary to abrasions sustained during the rut or that transfer of pathogens between males occurs after they rub their face on the same structure. Additionally, if adult mule deer carry this novel herpesvirus, it could be that rut-associated stress may allow reactivation of this virus with potential spread to immunologically naive, juvenile deer. The seroprevalence of this herpesvirus is currently being investigated. In our study, IKC cases derived from 6 different counties with only 1 possible IKC outbreak in Sweetwater, which involved 5 mule deer in November 2000 and included 2 cases of herpesviral infection. According to the Wyoming Game and Fish Department, there are no established feeding stations or programs for mule deer in Wyoming that could represent areas with higher animal density and that therefore contribute to the spread of IKC in these deer.
IKC is regarded as a complex infectious disease, in which different microorganisms have been incriminated as causative agents. In domestic ruminants, IKC is primarily associated with infection by Moraxella sp., Chlamydia sp., and Mycoplasma sp.,5,6,16,31 but in farmed red deer, IKC is most commonly associated with CerHV-1.28 It is important to note that many of the aforementioned pathogens have been isolated from healthy animals,1 and that environmental factors (e.g., ultraviolet radiation, dust, and flies)20 are thought to play a role in the development of IKC. We identified one or multiple potential pathogens in 15 of 17 cases with ocular lesions. T. pyogenes and a novel herpesvirus were the most commonly detected pathogens. T. pyogenes was primarily associated with unilateral ulcerative keratitis. T. pyogenes is largely considered to be an opportunistic microorganism; its entry and colonization of tissues require local injury (e.g., penetrating wound) or stress (e.g., polymicrobial infection).17 Thus, it is likely that, in these cases, corneal ulceration caused by trauma was followed by infection with commensal T. pyogenes. Noninfectious factors that may contribute to corneal ulceration in mule deer include dust, tall grass, sunlight, nutritional deficiencies, and territorial marking behavior.11,15,24–26 It is unclear, however, whether T. pyogenes resides in the conjunctival mucosa, periorbital haired skin, or both.
The list of viral agents associated with IKC in domestic animals and wildlife is relatively short. BoHV-1,21,27 OvHV-2,19 adenovirus,32,33 and CerHV-128 can cause ocular lesions in various ruminant species. In our study, a novel herpesvirus was consistently detected in cases of bilateral necrotizing and fibrinosuppurative bulbar conjunctivitis and was not found in other cases of mule deer IKC. Although cattle with the respiratory form of infectious bovine rhinotracheitis often have conjunctivitis with profuse ocular discharge, no respiratory gross lesions were reported for our cases of herpesviral conjunctivitis in mule deer. Microscopically, acute hemorrhage was reported for one (case 6), but no microscopic lesions in the lungs were reported for other cases (cases 5, 8). The trachea from any of these cases was not examined microscopically. Our results from phylogenetic analyses of surface glycoproteins gB, gC, and gD indicate that this is a novel alphaherpesvirus (tentatively named mule deer herpesvirus). Further research is required to establish a causal relationship between herpesviral infection and bulbar conjunctivitis in mule deer, and to determine the prevalence and distribution of this novel herpesvirus among mule deer populations in Wyoming and other states.
BVDV-1 was detected in a single case of polymicrobial IKC along with Staphylococcus sp. and Streptococcus sp. BVDV-1 and -2 have been identified in tissues of mule deer and other cervid species.22,29 However, to our knowledge, there are no reports in the literature regarding BVDV infection associated with IKC. Unfortunately, no archived tissues were available for microscopic examination and immunohistochemistry from this case.
Moraxella bovis is generally recognized as the most common and economically significant cause of IKC in cattle.16,18,31 Other Moraxella spp. (M. ovis and M. bovoculi) have been isolated from cases of bovine IKC, and these isolates produce exotoxins capable of inducing cytotoxicity in vitro.2–4 However, experimental inoculation of cattle with M. ovis and M. bovoculi, and fawns with M. ovis, has failed to reproduce lesions in multiple studies,7,10,12 suggesting that these Moraxella spp. are not primary ocular pathogens and that other co-factors (e.g., environmental or infectious agents) are required for lesion development. In our study, Moraxella spp. were identified in 2 cases of conjunctivitis and 1 of keratoconjunctivitis. These findings are consistent with previous reports of ocular disease associated with M. ovis in mule deer.7 M. ovis was identified alone in 1 case, and in conjunction with M. bovis and herpesvirus in 1 case each. Based on the aforementioned understanding of IKC related to Moraxella spp., it could be that either coinfection with other microorganisms (i.e., herpesvirus and M. bovis) or the effect of environmental co-factors enhances colonization by M. ovis along the conjunctiva and cornea of mule deer. Further investigation is required to determine if infection with M. ovis alone is sufficient to reproduce conjunctivitis and/or keratoconjunctivitis in mule deer, and if mule deer–derived Moraxella spp. produce virulence factors similar to those reported for cattle-derived Moraxella spp.
Other bacterial species identified in cases of IKC in mule deer include Staphylococcus sp., Streptococcus sp., Flavobacterium sp., Pseudomonas sp., and E. coli. All of these bacterial species have been isolated from the conjunctiva of healthy mule deer8 and, in our study, were predominantly identified in cases of ulcerative keratitis and keratoconjunctivitis. Therefore, it is possible that, in these cases, trauma to the cornea and/or conjunctiva resulted in infection by these commensal bacteria.
Supplemental Material
Supplemental material, DS1-3_JVDI_10.1177_1040638718787862 for Infectious keratoconjunctivitis in free-ranging mule deer in Wyoming: a retrospective study and identification of a novel alphaherpesvirus by Juan F. Muñoz Gutiérrez, Kerry S. Sondgeroth, Elizabeth S. Williams, Donald L. Montgomery, Terry E. Creekmore and Myrna M. Miller in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Mark Rutherford Davidson (database search), Rebecca Ashley (histology), Marce Vasquez and David Berry (virology), Kylie Sinclair and Hank Edwards (photograph database search), and Megan Dillon (electron microscopy) for their excellent technical assistance.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This material is based upon work that is supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under awards WYO-568-16 and WYO-564-16.
ORCID iD: Terry E. Creekmore  https://orcid.org/0000-0002-4793-5976
https://orcid.org/0000-0002-4793-5976
References
- 1. Akerstedt J, Hofshagen M. Bacteriological investigation of infectious keratoconjunctivitis in Norwegian sheep. Acta Vet Scand 2004;45:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelos JA, et al. Identification and characterization of complete RTX operons in Moraxella bovoculi and Moraxella ovis. Vet Microbiol 2007;125:73–79. [DOI] [PubMed] [Google Scholar]
- 3. Angelos JA, et al. Moraxella bovoculi sp. nov., isolated from calves with infectious bovine keratoconjunctivitis. Int J Syst Evol Microbiol 2007;57(Pt 4):789–795. [DOI] [PubMed] [Google Scholar]
- 4. Cerny HE, et al. Effects of Moraxella (Branhamella) ovis culture filtrates on bovine erythrocytes, peripheral mononuclear cells, and corneal epithelial cells. J Clin Microbiol 2006;44:772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dagnall GJ. An investigation of colonization of the conjunctival sac of sheep by bacteria and mycoplasmas. Epidemiol Infect 1994;112:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagnall GJ. The role of Branhamella ovis, Mycoplasma conjunctivae and Chlamydia psittaci in conjunctivitis of sheep. Br Vet J 1994;150:65–71. [DOI] [PubMed] [Google Scholar]
- 7. Dubay SA, et al. Association of Moraxella ovis with keratoconjunctivitis in mule deer and moose in Wyoming. J Wildl Dis 2000;36:241–247. [DOI] [PubMed] [Google Scholar]
- 8. Dubay SA, et al. Bacteria and nematodes in the conjunctiva of mule deer from Wyoming and Utah. J Wildl Dis 2000;36:783–787. [DOI] [PubMed] [Google Scholar]
- 9. Edmunds DR, et al. Ocular plague (Yersinia pestis) in mule deer (Odocoileus hemionus) from Wyoming and Oregon. J Wildl Dis 2008;44:983–987. [DOI] [PubMed] [Google Scholar]
- 10. Elad D, et al. Moraxella ovis in cases of infectious bovine keratoconjunctivitis (IBK) in Israel. Zentralbl Veterinarmed B 1988;35:431–434. [DOI] [PubMed] [Google Scholar]
- 11. Geist V. Mule Deer Country. Minocqua, WI: NorthWord Press, 1990:141–151. [Google Scholar]
- 12. Gould S, et al. Randomized blinded challenge study to assess association between Moraxella bovoculi and infectious bovine keratoconjunctivitis in dairy calves. Vet Microbiol 2013;164:108–115. [DOI] [PubMed] [Google Scholar]
- 13. Humason GL. Animal Tissue Techniques. 2nd ed. San Francisco, CA: WH Freeman, 1967:432. [Google Scholar]
- 14. Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res 2017;27:722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kreeger TJ, et al. Field Guide to Diseases of Wyoming Wildlife. 1st ed Cheyenne, WY: Wyoming Game and Fish Department, 2011:129–131. [Google Scholar]
- 16. Loy JD, Brodersen BW. Moraxella spp. isolated from field outbreaks of infectious bovine keratoconjunctivitis: a retrospective study of case submissions from 2010 to 2013. J Vet Diagn Invest 2014;26:761–768. [DOI] [PubMed] [Google Scholar]
- 17. Nagaraja TG. Arcanobacterium. In: McVey DS, et al., eds. Veterinary Microbiology. 3rd ed. Ames, IA: Wiley-Blackwell, 2013:203–205. [Google Scholar]
- 18. O’Connor AM, et al. Descriptive epidemiology of Moraxella bovis, Moraxella bovoculi and Moraxella ovis in beef calves with naturally occurring infectious bovine keratoconjunctivitis (Pinkeye). Vet Microbiol 2012;155:374–380. [DOI] [PubMed] [Google Scholar]
- 19. O’Toole D, Li H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet Pathol 2014;51:437–452. [DOI] [PubMed] [Google Scholar]
- 20. Postma GC, et al. Moraxella bovis pathogenicity: an update. Comp Immunol Microbiol Infect Dis 2008;31:449–458. [DOI] [PubMed] [Google Scholar]
- 21. Rebhun WC, et al. An outbreak of the conjunctival form of infectious bovine rhinotracheitis. Cornell Vet 1978;68:297–301. [PubMed] [Google Scholar]
- 22. Ridpath JF, Neill JD. Challenges in identifying and determining the impacts of infection with pestiviruses on the herd health of free ranging cervid populations. Front Microbiol 2016;7:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenfeld I, Beath OA. Contagious panophthalmitis in deer. J Wild Manage 1944;8:247–250. [Google Scholar]
- 24. Roug A, et al. Serosurveillance for livestock pathogens in free-ranging mule deer (Odocoileus hemionus). PLoS One 2012;7:e50600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sievers F, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol Syst Biol 2011;7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taylor SK, et al. Infectious keratoconjunctivitis in free-ranging mule deer (Odocoileus hemionus) from Zion National Park, Utah. J Wildl Dis 1996;32:326–330. [DOI] [PubMed] [Google Scholar]
- 27. Timoney PJ, O’Connor PJ. An outbreak of the conjunctival form of infectious bovine rhinotracheitis virus infection. Vet Rec 1971;89:170. [DOI] [PubMed] [Google Scholar]
- 28. Tryland M, et al. Cervid herpesvirus 2, the primary agent in an outbreak of infectious keratoconjunctivitis in semidomesticated reindeer. J Clin Microbiol 2009;47:3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Campen H, et al. Isolation of bovine viral diarrhea virus from a free-ranging mule deer in Wyoming. J Wildl Dis 2001;37:306–311. [DOI] [PubMed] [Google Scholar]
- 30. Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 2014;9:e112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Webber JJ, Selby LA. Risk factors related to the prevalence of infectious bovine keratoconjunctivitis. J Am Vet Med Assoc 1981;179:823–826. [PubMed] [Google Scholar]
- 32. Wilcox GE. The aetiology of infectious bovine keratoconjunctivitis in Queensland. 2. Adenovirus. Aust Vet J 1970;46:415–420. [DOI] [PubMed] [Google Scholar]
- 33. Wilcox GE. Isolation of adenoviruses from cattle with conjunctivitis and kerato-conjunctivitis. Aust Vet J 1969;45:265–270. [DOI] [PubMed] [Google Scholar]
- 34. Williams ES. Chronic wasting disease. Vet Pathol 2005;42:530–549. [DOI] [PubMed] [Google Scholar]
- 35. Williams ES, et al. Spontaneous poxviral dermatitis and keratoconjunctivitis in free-ranging mule deer (Odocoileus hemionus) in Wyoming. J Wildl Dis 1985;21:430–433. [DOI] [PubMed] [Google Scholar]
- 36. Woods LW, et al. Systemic adenovirus infection associated with high mortality in mule deer (Odocoileus hemionus) in California. Vet Pathol 1996;33:125–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1-3_JVDI_10.1177_1040638718787862 for Infectious keratoconjunctivitis in free-ranging mule deer in Wyoming: a retrospective study and identification of a novel alphaherpesvirus by Juan F. Muñoz Gutiérrez, Kerry S. Sondgeroth, Elizabeth S. Williams, Donald L. Montgomery, Terry E. Creekmore and Myrna M. Miller in Journal of Veterinary Diagnostic Investigation