Abstract
Real-time PCR (rtPCR) tests have become a method of choice in many diagnostic settings, both animal and human. A concern remains, however, regarding rtPCR assay inhibition during nucleic acid extraction and/or rtPCR reaction process that may result in false-negative results. The use of an internal positive control, either endogenous or exogenous, to mitigate this issue has become more commonplace. We identified and standardized an endogenous internal positive control that can be utilized in rtPCR assays targeting canine-specific pathogens in either a singleplex or multiplex format. The target chosen for the endogenous internal positive control (EIPC-K9) was a highly conserved region in canine mitochondrial DNA. Samples from 240 dogs and 11 other species were screened with EIPC-K9; all canine samples were detected, and no cross-amplification with other species tested was observed. Additionally, no inhibition was noted when comparing singleplex to multiplex rtPCR formats.
Keywords: Dogs, quality control, real-time PCR
To ensure proper performance of real-time PCR (rtPCR) assays, diagnostic laboratories utilize internal positive controls (IPCs) to monitor nucleic acid extraction and subsequent test results.2,3 Traditional IPC methodologies utilize exogenous controls (XIPC) for monitoring rtPCR assays but feature limitations that may not fully mitigate false-negative results. There are many types of XIPCs, such as plasmids and phages. In general, XIPCs are utilized by spiking a known quantity of control material along with the biological sample undergoing DNA and/or RNA purification (purNA).4,5 Although utilizing an XIPC is usually sufficient, cases exist in which an endogenous IPC (EIPC) is necessary.
The aim of our study was to identify a genetic marker that could be standardized to specifically amplify a conserved gene region located within canine mitochondrial DNA (mtDNA) for use as an endogenous internal control. The methodology of an EIPC mitigates the need to spike a specific amount of an XIPC into an extracted sample, and alleviates the necessity to rely on the lysis of a phage for monitoring the purNA extraction process. The proposed canine-specific EIPC (EIPC-K9) control could potentially work as an internally regulated genetic marker that removes the need for additional external control reagents to the purNA extraction process. The control would theoretically amplify within a rtPCR reaction only if the host (Canis sp.) cell has fully lysed. A standardized endogenous internal control could benefit future research studies by limiting the time and reagents necessary for developing such a control for experiments, as well as provide a means for uniform rtPCR testing across studies. Although alternative endogenous internal housekeeping genes have been utilized (e.g., GAPDH), the emphasis has been placed on targeting these genes as a reference for gene expression analysis.6 We focused on targeting a region of mtDNA for identifying the species regardless of the sample type being extracted in a veterinary laboratory environment.
All available domestic dog (Canis lupus familiaris) mtDNA nucleotide sequences available in GenBank (n = 1,129; query Nov 2016) were sorted and independently aligned (CLC Main Workbench v.7.7, Qiagen Aarhus, Aarhus, Denmark) for gene sequence alignments and nucleotide conservation identification. Subsequent analysis identified the most conserved sequence regions in the canine mtDNA complete genomes that featured nucleotide mismatches with respect to heterologous species mtDNA complete genomes commonly screened in veterinary laboratories (e.g., horse, cat, goat, sheep, white-tailed deer, skunk, cow, pig, chicken, raccoon, and red fox; represented by n = 441, 176, 184, 102, 13, 2, 290, 196, 103, 14, and 6, respectively) for primers–probe selection (data not shown). Sequence analysis also included region homology to closely related Canis species to ensure adaptive usage of the internal control for future studies aimed at utilizing wildlife samples such as gray wolf (Canis lupus, n = 112; data not shown) and coyote (Canis latrans, n = 8; data not shown).
Primers and probes were positioned over aforementioned sequence regions and further analyzed (Primer Express v.3.0, Applied Biosystems, Carlsbad, CA). Sequences were then evaluated on the basis of the following criteria: predicted cross-reactivity with closely related organisms, internal primer binding properties for hairpin and primer–dimer (cross-dimerization) potential, physical attributes of primers for extended base-pair repeats and runs, length of the desired amplicon, GC-content, melting temperatures (Tm) of primers and probes, and desired distance between 3′-end of primer and 5′-end of the probe. Oligonucleotide sequences were then searched using BLAST and the C. lupus familiaris genome to confirm specificity.
Sequence analysis revealed a 96-bp region targeting the NADH:ubiquinone oxidoreductase core subunit 5 (MT-ND5) gene within canine mtDNA as the ideal and specific internal control location. Amplifying the selected region with the rtPCR assay proved efficacious by performing the reaction using primers EIPC.K9mt.12942F and EIPC.K9mt.13018R, and dual-labeled probe (BioSearch, Novato, CA) EIPC.K9mt.12980P (Table 1). The rtPCR assay was evaluated in both singleplex and multiplex formats with a final reaction volume of 25.0 µL, by following optimal reaction conditions consisting of 12.5 µL of 2× rtPCR Path-ID buffer (Thermo Fisher Scientific, Waltham, MA), 1.0 µL of 25× primer–probe mix (singleplex: working 25× concentration of primers EIPC.K9mt.12942F, EIPC.K9mt.13018R, and probe EIPC.K9mt.12980P-TAMRA at 31.25 nM each; multiplex: the aforementioned EIPC-K9 oligonucleotides at the same concentration and up to 3 additional unique pathogen primer sets and probe labels), 8.0 µL of template DNA, and 3.5 µL of RNase-free water (Applied Biosystems 7500 fast real-time PCR system, Thermo Fisher Scientific). Recommended cycling conditions include initial activation of the DNA polymerase at 95°C for 10 min, 40 cycles of 1 s denaturation at 95°C, followed by a 30 s annealing-extension step at 60°C.
Table 1.
Primers and probe used for the detection of Canis lupus familiaris.
| Oligo ID | Sequence (5′–3′) | Amplicon |
|---|---|---|
| EIPC.K9mt.12942F | GGATTCTACTCCAAAGACCTGATCA | 96 bp |
| EIPC.K9mt.13018R | GGTTAGGGATGTGGCAACGA | |
| EIPC.K9mt.12980P | TAM-CACGTCGAATACCAACGCCTGAGCC-BHQ2 |
BHQ = black hole quencher; TAM = TAMRA (N,N,N′,N′-tetramethyl-6-carboxyrhodamine).
A positive amplification control (PAC) and standard for the quantitation of the rtPCR assay was developed by cloning a 96-bp segment of the MT-ND5 region that spans the coordinates 12942 and 13037 of the complete mtDNA genome (GenBank accession EU408254.1). This segment was amplified with the designed primers (Table 1) from a template of genomic DNA extracted and purified from C. lupus familiaris blood (MagMax total nucleic acid extraction kit, KingFisher Flex purification system, Thermo Fisher Scientific), as described previously.5 The PCR products were then cloned in Escherichia coli following manufacturer’s recommendations (TOPO TA cloning kit, Invitrogen, Carlsbad, CA).
To evaluate the analytical sensitivity of the assay, a 10-fold dilution series was conducted with the constructed plasmid PAC to determine a cycle threshold (Ct) cutoff for the rtPCR assay, as well as provide the limit of detection (LOD) of gene copies. An efficiency of 92% and an R2 value of 0.999 were calculated based on the 10-fold dilution series.1 A LOD of 2 plasmid copies at a Ct cutoff of 38.0 was determined based on the data collected from the generated standard curve.
The diagnostic specificity of the EIPC-K9 control was assessed by demonstrating in duplicate the failure to amplify DNA extracted from opportunistically collected blood samples originating from species routinely tested at the Texas A&M Veterinary Medical Diagnostic Laboratory (TVMDL; College Station, TX). The species tested included: gray wolf, coyote, horse, cat, goat, sheep, white-tailed deer, skunk, cow, pig, chicken, raccoon, and red fox (Fig. 1). Specificity analysis revealed that a positive EIPC-K9 result indicates that the tested specimen originates from 1 of 3 Canis species (C. lupus familiaris, C. lupus, and C. latrans).
Figure 1.
Oligonucleotide analysis of the canine-specific endogenous internal positive control (EIPC-K9) assay in respect to homologous and heterologous species’ mitochondrial DNA target sequences. The second column indicates qualitative results (positive or negative) in respect to EIPC-K9 specificity to 2 independent blood samples from homologous and heterologous species (dog, gray wolf, coyote, horse, cat, goat, sheep, white-tailed deer, skunk, cow, pig, chicken, raccoon, and red fox). Mismatch analysis of base-pair positions that are not conserved against EIPC-K9 primers and probe are highlighted in gray.
To test the detection and sample specificity utility of the EIPC-K9 assay in canine samples, DNA was screened from 240 (123 from blood, 71 from urine, 21 from nasal swabs, and 25 from tissues biopsies) domestic canine samples, collected opportunistically at TVMDL. EIPC-K9 results of the specimens yielded a detection rate of 100%, and their mean Ct values were noted (Fig. 2). One-way ANOVA statistical analysis revealed that each specimen type’s mean Ct value was significantly different from every other specimen (p values of <0.0001 for blood–urine, <0.01 for blood–nasal swab, <0.0001 for blood–tissue, <0.001 for urine–nasal swab, <0.0001 for urine–tissue, and <0.0001 for nasal swab–tissue). This utility may be extrapolated to act as a quality control method, in which quality of DNA extracted from a canine sample can be monitored by its subsequent EIPC-K9 Ct value. EIPC-K9 was not tested against other sample types derived from dogs, given that we focused on the evaluation of the internal control in respect to commonly tested laboratory specimens. Further studies to determine the efficacy of EIPC-K9 as a standard with other sample types, and to evaluate the control under various inhibitory effects, will be needed for complete analysis.
Figure 2.
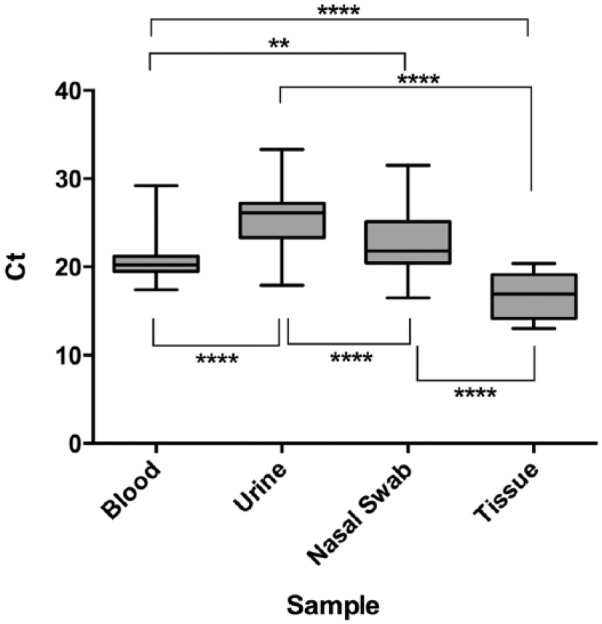
Mean cycle threshold (Ct) values ± standard deviation for sample types screened with the canine-specific endogenous internal positive control (EIPC-K9): 16.8, 20.5, 22.5, 25.5, respectively. Samples originated from archived TVMDL cases. “Tissue” denotes variety of tissue samples used for testing (e.g., lung, kidney, cerebrum, brain). One-way ANOVA statistical analysis of sample types in respect to other sample types is represented by asterisks (** = p < 0.01, **** = p < 0.0001).
The utility of EIPC-K9 for use in a multiplex format was also tested and proved useful (Fig. 3). The rtPCR results revealed no significant inhibition regardless of multiplex reaction format in respect to Ct value production by one-way ANOVA analysis for EIPC-K9 and the 3 select pathogen assays (Borrelia sp., Ehrlichia sp., and Babesia sp.). Although initial analysis using the listed pathogens revealed no significant difference for pathogen Ct values, it is important that future use of the control with other targets include verifying that no inhibition is observed in a multiplex format with the intended assays.
Figure 3.

Mean cycle threshold (Ct) values ± standard deviation from performing duplicate real-time PCR runs using the canine-specific endogenous internal positive control (EIPC-K9) assay and various pathogen-specific assays (Borrelia burgdorferi [Bb], Ehrlichia canis [Ec], and Rickettsia rickettsii [Rr]) under various plex reaction formats. Multiplex assays consisted of duplex (EIPC-K9, Bb), triplex (EIPC-K9, Bb, Ec), and quadriplex (EIPC-K9, Bb, Ec, Rr). EIPC-K9 Ct of ~22.0 represents genomic DNA (gDNA) purified from canine blood and spiked with each pathogen (gDNA at Ct of ~31.0). Concentration of EIPC-K9 and each pathogen is estimated to be 150,000 and 2,000 gene copies per plasmid serial-dilution calculations, respectively.
Limitations of the assay include Ct value irregularity within sample types that may be influenced by the way in which specimens are collected. For example, DNA samples derived from blood featured the least variability in respect to produced Ct values, whereas urine and nasal swabs experienced higher variability. Blood collection may feature the least inconsistency from animal to animal, whereas a urine or nasal swab sample may be collected from animals experiencing various health conditions (hematuria or epistaxis) and subsequently influence the cellularity of the sample, resulting in a higher or lower Ct value. In addition, sample collection methodology among veterinarians may differ, resulting in further irregularities. Additional limitations include potential inhibition by EIPC-K9 on low levels of target pathogens in a tested sample, and the lack of true analytical specificity evaluations for the control. However, the specificity of the control for only amplifying canine targets should be concluded based on the extensive diagnostic specificity analysis, including the use of homologous and heterologous species, conducted on the control. Finally, as the EIPC-K9 control is limited to detecting genomic DNA, it cannot verify the fidelity of the reverse-transcription process in assays intended for RNA targets.
The EIPC proved efficacious when supplemented into rtPCR assays that screen canine samples for pathogens of interest. The control may be incorporated into similar assays that feature a melting temperature of 60°C, and may be utilized in multiplex rtPCR panels of up to 4 detectors. Although limited, obtained data indicate the potential use of this assay to verify cell lysis and DNA extraction, as well as species verification.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed financial support from the Texas AgriLife Research seed grant TEXV 6579 (project I-9524) and the Texas A&M Department of Veterinary Pathobiology.
ORCID iD: Joseph J. Modarelli  https://orcid.org/0000-0002-5543-7133
https://orcid.org/0000-0002-5543-7133
References
- 1. D’Haene B, et al. . Accurate and objective copy number profiling using real-time quantitative PCR. Methods 2010;50:262–270. [DOI] [PubMed] [Google Scholar]
- 2. Goncalves-de-Albuquerque Sda C, et al. Tracking false-negative results in molecular diagnosis: proposal of a triplex-PCR based method for leishmaniasis diagnosis. J Venom Anim Toxins Incl Trop Dis 2014;20:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huhn GD, et al. Early development of non-Hodgkin lymphoma following initiation of newer class antiretroviral therapy among HIV-infected patients—implications for immune reconstitution. AIDS Res Ther 2010;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mikel P, et al. Methods for preparation of MS2 phage-like particles and their utilization as process control viruses in RT-PCR and qRT-PCR detection of RNA viruses from food matrices and clinical specimens. Food Environ Virol 2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schroeder ME, et al. Development and performance evaluation of a streamlined method for nucleic acid purification, denaturation, and multiplex detection of Bluetongue virus and Epizootic hemorrhagic disease virus. J Vet Diagn Invest 2013;25:709–719. [DOI] [PubMed] [Google Scholar]
- 6. Van Rijn SJ, et al. Expression stability of reference genes for quantitative RT-PCR of healthy and diseased pituitary tissue samples varies between humans, mice, and dogs. Mol Neurobiol 2014;49:893. [DOI] [PubMed] [Google Scholar]



